Original Article
Remote ischemic preconditioning protects the kidney
against injury after ischemia and reperfusion through
activation of hypoxia-inducible factor 1
Lin Wu*, Mingyu Chen*, Jingfeng Zhu, Jun Qian, Changying Xing
Department of Nephrology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China. *Equal contributors.
Received May 24, 2016; Accepted June 25, 2016; Epub August 1, 2016; Published August 15, 2016
Abstract: Clinical studies suggest that remote ischemic preconditioning (RIPC) in the arm or leg may protect the heart, brain and kidney against injury after ischemia and reperfusion. However, the detailed mechanism remains unclear. A recent study indicates that Hypoxia-Inducible Factor 1 (HIF-1) activates IL-10 gene transcription and is required for remote ischemic preconditioning of the heart. In this study, we investigate the role of HIF-1 in the protec-tion effect of RIPC in the kidney after ischemia and reperfusion injury (IRI). RIPC was induced by blocking unilateral femoral artery for 5 min followed by 5 min of reperfusion for a total of five cycles. IRI was induced by blocking bilat-eral renal artery for 45 min followed by 120 min of reperfusion. The HIF specific inhibitor YC-1 was injected through tail vein with the dose of 2 mg/kg before surgery operation. Results showed that renal functions including serum creatinine, cystatin C and tubular injury score after IRI in rats with RIPC pretreatment were significantly better than in those without RIPC treatment. Renal expression of SOD and p-AKT were also elevated after RIPC. Our study also showed that RIPC treatment itself obviously induced the accumulation of HIF-1 in rat renal tubular epithelial nucleus and further induced upregulation of mRNA levels of target genes of HIF, such as EPO, VEGF and HO-1. Taken togeth-er, these data indicate that the protective effect of RIPC against IRI might be achieved through the activation of HIF.
Keywords: Remote ischemic preconditioning (RIPC), ischemia and reperfusion injury (IRI), hypoxia-inducible factor 1 (HIF-1)
Introduction
Compelling evidence indicates that the inci-dence of acute kidney injury (AKI) is rapidly increasing. With the greater recognition of AKI and the improvement of medical technology, AKI-associated mortality rate is decreasing slightly, but remains unacceptably high [1]. Therefore, prevention and treatment of AKI is still an urgent problem. Cardiovascular surgery is one of the important causes of AKI. As report-ed by Bastin et al. [2], the incidence of AKI after cardiac surgery is up to about 25%. Although recently the discovery of various biomarkers makes early diagnosis of cardiovascular sur-gery-related acute kidney injury (CSA-AKI) become possible, there is no effective protec-tion and management of AKI [3, 4]. For elective cardiovascular surgery, prevention of postop-erative AKI is of great importance.
Ischemic preconditioning (IPC) was first pro -posed by Murry et al. [5]. It is a phenomenon in which previous application of a short, tempo-rary ischemic pretreatment can protect an organ against subsequent prolonged ischemia injury. It was found afterwards that remote isch-emic preconditioning (RIPC) with brief episodes of ischemia-reperfusion applied in distant tis-sues or organs results in the protective effect in the heart and render the myocardium resistant to a subsequent sustained episode of ischemia [6], which makes it possible in clinical applica-tion. Clinical observations indicate that RIPC has the same protective effect as IPC in isch-emia injuries of the heart, brain, kidney and other vital organs [7-10]. In clinical applications,
it is a simple, economic and efficient method to
protec-tive effect of RIPC in renal ischemia injury. Research by Zimmerman et al. [11] showed that RIPC prevented acute kidney injury in patients undergoing cardiopulmonary bypass-assisted cardiac surgery. RIPC was accom-plished by an automated thigh tourniquet con-sisting of three 5-min intervals of lower extrem-ity ischemia separated by 5-min intervals of
reperfusion. After surgery AKI occurred signifi -cantly lower in remote ischemic preconditioned patients than in control group. Another study stated that in patients with a non-ST-segment elevation myocardial infarction undergoing Percutaneous Coronary Intervention (PCI),
those who received RIPC by serial balloon infla
-tions and defla-tions were conferred protection
against AKI with lower AKI incidence and the 30-day rate of death or re-hospitalization than the control group [12]. However, it was also
reported that there was no benefit of RIPC
which protected renal function and reduced the incidence of AKI [13]. Thus, it is necessary to further valuate the clinical feasibility and effec-tiveness of RIPC. The mechanism how RIPC exerts effect on kidney in ischemia situation is very complex and still remains unclear.
Hypoxia-inducible factor (HIF) plays a pivotal role in the transcriptional response to changes in inadequate oxygen availability. HIF is a het-erodimer transcription factor which consists of
an unstable α subunit and a stable β subunit.
Under hypoxic conditions, members of the pro-lyl hydroxylase domain (PHD) family, the key
factor involved in the process of α subunit deg
-radation, are deactivated, resulting in HIFα accumulation, dimerization with a HIFβ subunit,
translocation to the nucleus, and transcription-al activation of targeted genes, including genes involved in erythropoiesis, angiogenesis, au- tophagy, and energy metabolism [14, 15]. In AKI, HIF activation by various factors has strong renoprotective effect, including relieving de- crease of renal functions and reducing histo-pathological damage. Pharmacological activa-tion of HIF by small molecules that inhibit HIF hydroxylases protected mouse kidneys against ischemia-reperfusion injury [16]. HIF induction by cobalt chloride administration led to reno-protective gene expression and thereby amelio-rated ischemic injury of the kidney in rats [17]. It has been demonstrated that HIF-1 plays an essential role in cardioprotection that is
induced by RIPC [18]. However, the role of HIF in protection of renal IRI by RIPC is almost blank. In this study, we investigated whether RIPC had protective effect on rat kidney injury induced by ischemia and reperfusion and try to explore the role of HIF in this process.
Materials and methods
Animals and study design
Male SD rats weighting 160-180 g were pur-chased from Shanghai Jiesijie Laboratory Animal Co., Ltd (Shanghai, China). All rats were maintained in a temperature- and light-con-trolled environment in accordance with the Principles of Laboratory Animal Care and Use. All animal experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. Rats were randomly divided into four groups: 1. SHAM group (n=10): After opening the abdominal cavity, separate perirenal adipose tissue, expose both kidneys but without any treatment and suture abdomi-nal incision after 45 min; 2. IRI group (n=10): Rat kidneys received ischemia-reperfusion inju-ry. The detailed process was described after-ward; 3. RIPC group (n=10): Rats received RIPC which was immediately followed by ischemia-reperfusion injury. The detailed RIPC treatment
was described afterward; 4. YC-1 group (n=10): The specific HIF-1 inhibitor YC-1 (Sigma, 2 mg/
kg) was intravenously administered before rats received RIPC and the following IRI. Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (5 mL/kg). Body tempera-ture was maintained at 37°C by performing
sur-gery on a heating pad (Yuyan Instruments Co.,
Ltd., Shanghai, China). 24 h after induction of IRI, blood samples were obtained from the heart and levels of serum creatinine and cys-tatin C were determined by an automatic bio-chemical analyzer (Olympus AU5400 analyzer, Olympus Diagnostics). Kidney tissue was obtained after saline infusion for histologic analysis.
Induction of IRI
After confirmation of anesthesia, rats were
tis-sue was gently pulled out and put on saline-soaked gauze to expose the kidney. Perirenal fat tissue was separated by blunt dissection. The renal pedicles were exposed and a
micro-vascular clamp (Yuyan Instruments Co., Ltd.,
Shanghai, China) was applied to clip bilateral
renal artery. The kidneys were confirmed to be
completely clipped by observing the exterior color change. After 45 min, the clamps were removed and reperfusion was applied. After reperfusion, Roxithromycin (5 mg/kg) and Diclofenac sodium (1 mg/kg) was given in the peritoneal cavity and the abdomen incision was closed in layers.
Induction of RIPC
RIPC was induced in advance of IRI. Hair of the thigh region was removed and povidone-iodine was applied for sterilization. After incision, the subcutaneous tissue and fascia were separat-ed by blunt dissection to expose femoral artery and vein. Microvascular clamps were applied to clip bilateral femoral artery for 5 minutes isch-emia followed by a 5-minute reperfusion (clamps open). The RIPC stimulus consisted of 5 clip-release cycles (total duration 50 min-utes). In animals randomized to the RIPC
con-trol group, five 5-minute of clip-release cycles
were also applied, but without the following IRI treatment.
Real-time PCR
Total RNA was isolated from rat kidney tissue with Trizol (Invitrogen) according to the manual.
cDNA was synthesized from 1 μg of total RNA
using a reverse transcription kit (Thermo
Scientific). 18S rRNA was served as an internal
control. Primer sequences were as follows:
HIF-1α forward: 5’-AGCAATTCTCCAAGCCCTCC-3’, HIF-1α reverse: 5’-TTCATCAGTGGTGGCAGTTG-3’, EPO forward: 5’-CGGAACTGTAATCCACGC-CA-3’, EPO reverse: 5’-CATTCTCCAGGCCCTGT-GTT-3’, VEGF forward: 5’-TGGACCCTGGCTTTA-CT-GCTG-3’, VEGF reverse: 5’-GGCAATAGCTGC-GCTGGTAGA-3’, HO-1 forward: 5’-AGGTGCACAT-CCGTGCAGAG-3’, HO-1 reverse: 5’-TCCAGGG-CCGTATAGATATGGTACA-3’; 18 S forward: 5’-CA-TGATTAAGAGGGACGGC-3’, 18 S reverse: 5’-TT-CAGCTTTGCAACCATACTC-3’. Quantitative
real-time PCR was performed in duplicate with 0.2
μM primers, 1 μL cDNA and the SYBR Green
Real-time PCR Master Mix (Roche Applied
Science) in a total volume of 10 μL. Reactions
were run at 95°C for 60 s, followed by 40 cycles of 15 s at 95°C, 15 s at 60°C and 45 s at 72°C. The StepOne Plus Real-time PCR system from ABI was used for analysis. Results were expressed as cycle threshold (Ct) and
calculat-ed as ∆Ct, which were normalizcalculat-ed to endoge -nous control 18S rRNA.
Western blot
Rat kidney tissue was homogenized and lysed in RIPA buffer (KeyGEN BioTECH, China). A liquots of tissue lysates containing 30 µg of total proteins were subjected to SDS-PAGE, transferred to a PVDF membrane and immuno-blotted with anti-total AKT, p-AKT, SOD, and GAPDH antibodies. Antibodies were all pur-chased from Abcam Shanghai Trade Co., LTD. The ECL plus western blotting detection system (Bio-Rad, USA) was used for signal detection. Densitometric analysis was performed with Image J software.
Histological examination
Rat kidney tissue was fixed with formalin and embedded in paraffin. Sections in 3 μm thick
were stained with hematoxylin-eosin (HE). Histological injury was mainly evaluated by quantitative measurement of tubular injury by
assessment of specific changes in 10 individu
-al and random selected fields with light micros -copy. The number of necrotic cells, loss of brush border, cast formation, and tubule dila-tion were estimated. A percentage of the area affected was used for damage scoring[19]. All evaluations were made without knowledge of the sample identity. Three pathologists respec-tively made the histological assessments of injury and the average score was obtained as
the final result.
Immunohistochemistry
Sections in 3 μm thick were grilled, dewaxed,
rehydrated and heated in microwave oven with high power for 12 min in antigen retrieval citrate solution (pH 6.0). Sections were then incubated
with polyclonal rabbit anti-HIF1α antibody
stained with HE, dehydrated, and mounted in neutral baisam with coverslips.
Statistics
Data are presented as mean ± SEM from at
least three experiments. Statistical significance was evaluated using student’s t-test for two
groups, or ANOVA for multiple groups. A value of p < 0.05 was considered statistically signifi -cant. Data analysis was carried out using GraphPad Prism 5.0.
Results
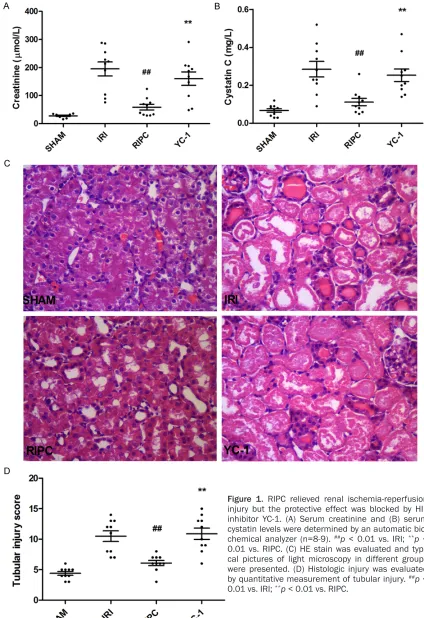
RIPC relieved renal ischemia-reperfusion injury and the protective effect was blocked by HIF inhibitor YC-1
In RIPC group, five RIPC ([5 × I5R5]fem) was sub-jected to rats before renal ischemia-reperfu-sion injury. After 24 h, blood and renal tissue were obtained for further analysis. As shown in
Figure 1A and 1B, serum creatinine level and
cystatin C level were both significantly
de-creased in RIPC pretreated rats than in those without RIPC pretreatment. Pathology analysis showed that in RIPC group, renal tissue
dam-ological injury score was reduced (Figure 1D). These results demonstrated that RIPC relieved tissue injury caused by renal IRI and protected
renal functions. However, when the specific HIF inhibitor YC-1 was administratered in advance
of RIPC, the protective effect of RIPC disap-peared. Serum creatinine level and cystatin C
level in YC-1 group were significantly increased
compared with RIPC grpup (Figure 1A and 1B).
The pathologic damage in YC-1 group was also
more severe than in RIPC group (Figure 1C and
1D). The above results indicated that RIPC treatment obviously relieved tissue injury induced by renal IRI and protected renal
func-tions, while the protective effect was signifi -cantly weakened when the in vivo HIF system was suppressed, suggesting that RIPC may exert its protective effect by activating the HIF system.
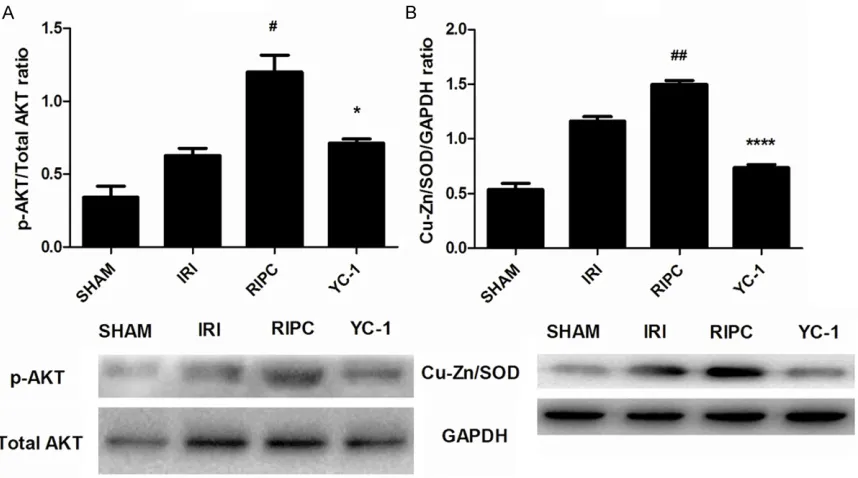
AKT activation in renal tissue after RIPC treat-ment
Activation of AKT signaling pathway is an impor-tant part through which RIPC exerts organ pro-tective effect in ischemia-reperfusion injury [20]. In this study, phosphorylation of AKT in
[image:5.612.93.522.72.311.2]serine 473 was significantly enhanced in renal
ly repressed by the specific HIF inhibitor YC-1
(Figure 2A).
Expression of SOD in renal tissue after RIPC treatment RIPC
In renalIRI, SOD is an important protective fac-tor which can effectively eliminate oxygen free radicals and reduce the direct tissue damage caused by reactive oxygen species (ROS). Under IRI condition, transient production of a large number of SOD was induced due to acute hypoxia. As shown in Figure 2B, RIPC treatment
could yield a significant increase in SOD
production. However, this effect was also
repressed by the specific HIF inhibitor YC-1.
Accumulation of HIF in kidney after RIPC treat-ment
Previous studies have shown that after RIPC
treatment the kidney has a significant ability to
resist IRI, and the effect is dependent on acti-vation of HIF system [21]. As a transcript factor, HIF enters into the nucleus to regulate the expression of many target genes. Cu/Zn-SOD is one of the adjustable target genes regulated by
HIF. In this study, it was observed that
expres-sion of SOD was significantly increased in renal
tissue after RIPC, which was down-regulated by
the HIF inhibitor YC-1. Based on these results,
we speculate that RIPC may directly induce activation of HIF system through femoral artery ischemia-reperfusion cycles in rat kidney so as to play a protective role in IRI. Results of
immu-nohistochemical analysis showed that HIF-1α
accumulated in renal tubular epithelial cell cytoplasm (Figure 3B) in renal tissue of RIPC group. As IRI created a hypoxic environment in the kidney which induced the production of HIF
and 24 hours later HIF-1α in renal tissue could
be degraded by ubiquitination of the activity-regained prolyl hydroxylase (Phd), rats were
treated with RIPC ([5 × I5R5]fem) only, after which the renal tissue were immediately obtained and
expression of HIF-1α was assayed by immuno -histochemical analysis. It was observed in
Figure 3C that HIF-1α aggregated in nucleus of
tubule epithelial cells, suggesting that a sepa-rate treatment of RIPC in the femoral artery could induce the activation of HIF in renal tis-sue and play a role in renal protection.
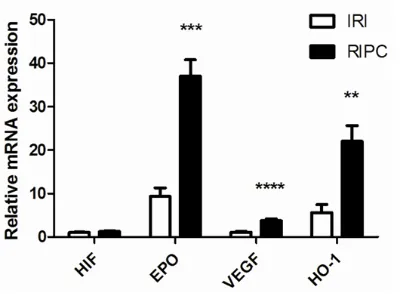
mRNA expression of target genes of HIF in kidney after RIPC treatment
The activated HIF dimer which is released into cytoplasm can adjust multiple downstream tar-get genes and play a role in resistance to isch-emic injury, protecting renal functions. As shown in Figure 4, mRNA expression of target genes of HIF in kidney after RIPC treatment was assayed by real-time PCR. Although there was no prominent change in mRNA level of HIF itself after RIPC treatment, mRNA levels of the main target genes adjusted by HIF such as EPO,
VEGF, and HO-1 was significantly upregulated
(p < 0.001), further demonstrating that RIPC treatment might activate HIF system and its transcriptional adjustable activity.
Discussion
It has been thirty years since the concept of RIPC came into being. In animal experiments, RIPC exhibits strong protective effect on isch-Figure 3. RIPC activated HIF-1α Expression in renal tubular epithelial cells. Kidney sections were subjected to im-munohistochemistry using anti-HIF1α antibody. Typical pictures in different groups were presented. A. There was no expression of HIF-1α in SHAM group. B. 24 h after RIPC and IRI treatment, HIF1α could be observed in renal tubular epithelial cell plasma. C. Immediately after a single RIPC treatment( [5 × I5R5]fem) without IRI, kidney tissue was ob-tained and treated for immunohistochemistry. HIF1α was obviously accumulated in nucleus of renal tubular epithe-lial cells. D. HIF inhibitor YC-1 blocked production of HIF1α in renal tubular epitheepithe-lial cell. Left: 400×; Right: 100×.
[image:7.612.90.290.157.304.2]emia induced damage in organs. However, RIPC remains controversial in clinical validity and so far has not been widely used. In this study, we
confirmed that RIPC could significantly protect
the kidney against renal IRI and conserve renal functions. Administration of RIPC treatment in advance of renal IRI could effectively reduce the increase of serum creatinine and cystatin C levels, increase production of SOD, alleviate pathological injury in renal tissue. Although the mechanism that IPC and RIPC protect ischemia organs still remains unclear, the activation of AKT signal pathway is widely accepted to be the central link [22]. In this study, phosphorylation
of AKT was significantly enhanced in renal tis -sue after RIPC treatment, while was obviously
repressed by the specific HIF inhibitor YC-1, giv -ing a hint that HIF plays a key role in protection of AKI by RIPC treatment. Further study demon-strated that the activated HIF dimmers could be detected in nucleus early after a separate
RIPC treatment ([5 × I5R5]fem) towards unilateral femoral artery in rats. Results of RT-PCR showed that the HIF system activated by RIPC treatment possessed the transcriptional
regu-latory activity, with a significant up-regulation of
mRNA levels of target genes of HIF, including
EPO, VEGF and HO-1, confirming the important
role of HIF in RIPC- mediated protection against renal IRI. In RIPC induced hypoxic conditions, although mRNA level of HIF itself was not altered, the accumulation of active HIF in the nucleus due to less degradation further acti-vated the upexpression of downstream genes. HIF is a heterodimeric transcription factor which plays an important role in the process of hypoxia adjustment. It is well known that pre-activation of HIF system in kidney can protect the kidney against AKI induced by various fac-tors. Previous studies proposed that HIF played an important role in the protective mechanism of RIPC in myocardial ischemia [18, 23]. Our study also demonstrated that HIF participated in the process of protection against renal IRI by RIPC. Although activation of HIF provides a powerful protective effect in AKI, selection of HIF activation manner is still a problem in clini-cal. And whether excessive HIF activation may
cause tumor growth and fibrosis is inconclusive
currently. The commonly used HIF agonist cobalt dichloride has strong toxicity and is not
applicable to humans. New specific prolyl
hydroxylase inhibitors are still under
develop-ment and not yet available in the market. Aanesthetic xenon is one of the more promising options, with a strong role in activating HIF and is very suitable for prevention of cardiovascular postoperative AKI [24]. However, the high price limits its clinical application. In contrast, RIPC treatment by simply pressing the cuff on patient
limbs has significant advantages before cardio -vascular surgery. It is non-invasive and does
not increase the financial burden of patients.
Further, it does not induce the potential side effects caused by overexpression of HIF. Therefore, RIPC might has great applicable value in clinical.
RICP is an exciting future strategy, but more work is needed before wide application. In ani-mal experiments the role of RIPC is unanimous-ly approved, but its effect is controversial in clinical studies. Some researchers reported that RIPC could not reduce the incidence of car-diovascular postoperative AKI [25, 26]. The rea-son for this phenomenon is varied. In addition to the different experimental conditions and methods of each research team, the anatomi-cal differences between humans and rats are inevitable. In the process of femoral artery liga-tion and reperfusion in mice, a transient
decrease in renal vascular flow can be moni -tored, which causes the renal tissue in tran-sient ischemia [27], suggesting that short-term hypoxic state may activate the HIF system in the kidney and thus result in a series of protec-tive effect. In animal experiments the common-ly used RIPC treatment is to expose femoral artery and clamp it using vascular clips. In terms of human bodies, body weight, kidney volume and distance between peripheral artery
and renal vessels are significantly higher than
the size of rats. Therefore, changes in renal
plasma flow and so-causing HIF activation in
renal tissue after human limbs cuff pressure may be used as a reference to value whether RIPC treatment could play a protective role. Moreover, whether increasing cuff pressure and extending blocking time and cycles can enhance the protective effect of RIPC remains to be explored in more clinical studies.
Acknowledgements
nology Projects of Jiangsu Province (BL2012- 032, BL2014080) and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
Address correspondence to: Changying Xing, De- partment of Nephrology, The First Affiliated Hos- pital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, China. Tel: 86-25-68136039; Fax: 86-25-83724420; E-mail: cyxing62@126.com
References
[1] Rewa O and Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014; 10: 193-207.
[2] Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ and Evans TW. Acute kidney injury after cardiac surgery according to Risk/Injury/ Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 2013; 28: 389-396.
[3] Thiele RH, Isbell JM and Rosner MH. AKI asso-ciated with cardiac surgery. Clin J Am Soc Nephrol 2015; 10: 500-514.
[4] Gaffney AM and Sladen RN. Acute kidney inju-ry in cardiac surgeinju-ry. Curr Opin Anaesthesiol 2015; 28: 50-59.
[5] Murry CE, Jennings RB and Reimer KA. Preconditioning with ischemia: a delay of le-thal cell injury in ischemic myocardium. Circulation 1986; 74: 1124-1136.
[6] Przyklenk K, Bauer B, Ovize M, Kloner RA and Whittaker P. Regional ischemic ‘precondition-ing’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993; 87: 893-899.
[7] Jensen HA, Loukogeorgakis S, Yannopoulos F, Rimpilainen E, Petzold A, Tuominen H, Lepola P, Macallister RJ, Deanfield JE, Makela T, Alestalo K, Kiviluoma K, Anttila V, Tsang V and Juvonen T. Remote ischemic preconditioning protects the brain against injury after hypo-thermic circulatory arrest. Circulation 2011; 123: 714-721.
[8] Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM and Davidson BR. Remote isch-emic preconditioning: a novel protective meth-od from ischemia reperfusion injury--a review. J Surg Res 2008; 150: 304-330.
[9] Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T, Benzing T, Erdmann E, Burst V and Gassanov N. Ischemic precondi-tioning for prevention of contrast
medium-in-duced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012; 126: 296-303.
[10] Przyklenk K and Whittaker P. Remote ischemic preconditioning: current knowledge, unre-solved questions, and future priorities. J Cardiovasc Pharmacol Ther 2011; 16: 255-259.
[11] Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL and Kramer RS. Ischemic preconditioning at a re-mote site prevents acute kidney injury in pa-tients following cardiac surgery. Kidney Int 2011; 80: 861-867.
[12] Deftereos S, Giannopoulos G, Tzalamouras V, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, Karageorgiou S, Avramides D, Toutouzas K, Hahalis G, Pyrgakis V, Manolis AS, Alexopoulos D, Stefanadis C and Cleman MW. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon infla-tions in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2013; 61: 1949-1955.
[13] Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR and Kwak YL. Effect of remote isch-emic preconditioning on renal dysfunction af-ter complex valvular heart surgery: a random-ized controlled trial. J Thorac Cardiovasc Surg 2011; 142: 148-154.
[14] Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-induc-ible transcription factors, and O2-regulated gene expression. FASEB J 2002; 16: 1151-1162.
[15] Kaelin WG Jr and Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hy-droxylase pathway. Mol Cell 2008; 30: 393-402.
[16] Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P and Maxwell PH. Inhibition of hy-poxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 2008; 19: 39-46.
[17] Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T and Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kid-ney in rats. J Am Soc Nephrol 2003; 14: 1825-1832.
[18] Cai Z, Luo W, Zhan H and Semenza GL. Hypoxia-inducible factor 1 is required for re-mote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A 2013; 110: 17462-17467.
cat-echolamine homeostasis and protection against heart failure during embryonic devel-opment. Genes Dev 1998; 12: 3320-3324. [20] Cai ZP, Parajuli N, Zheng X and Becker L.
Remote ischemic preconditioning confers late protection against myocardial ischemia-reper-fusion injury in mice by upregulation interler-kin-10. Basic Res Cardiol 2012; 107: 277-283. [21] Nordquist L, Friederich-Persson M, Fasching A,
Liss P, Shoji K, Nangaku M, Hansell P and Palm F. Activation of hypoxia-inducible factors pre-vents diabetic nephropathy. J Am Soc Nephrol 2015; 26: 328-338.
[22] Hausenloy DJ and Yellon DM. Survival kinases in ischemic preconditioning and postcondition-ing. Cardiovasc Res 2006; 70: 240-253. [23] Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot
K and Darshan MS. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection in-duced by ischemic preconditioning. Proc Natl Acad Sci U S A 2012; 109: 10504-10509. [24] Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H,
Hossain M, Maxwell PH and Maze M. Xenon preconditioning protects against renal isch-emic-reperfusion injury via HIF-1alpha activa-tion. J Am Soc Nephrol 2009; 20: 713-720.
[25] Young PJ, Dalley P, Garden A, Horrocks C, La Flamme A, Mahon B, Miller J, Pilcher J, Weatherall M, Williams J, Young W and Beasley R. A pilot study investigating the effects of re-mote ischemic preconditioning in high-risk car-diac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol 2012; 107: 256-265.
[26] Murphy N, Vijayan A, Frohlich S, O’Farrell F, Barry M, Sheehan S, Boylan J and Conlon N. Remote ischemic preconditioning does not af-fect the incidence of acute kidney injury after elective abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 2014; 28: 1285-1292.