DOI: 10.1534/genetics.109.102079
Transcriptional Silencing and Reactivation in Transgenic Zebrafish
Mary G. Goll,* Ryan Anderson,
†Didier Y. R. Stainier,
†Allan C. Spradling*
and Marnie E. Halpern*
,1*Department of Embryology, Carnegie Institution for Science, Baltimore, Maryland 21218,†Department of Biochemistry and Biophysics, Programs in Developmental Biology, Genetics and Human Genetics, University of California, San Francisco, California 94158
Manuscript received February 21, 2009 Accepted for publication April 30, 2009
ABSTRACT
Epigenetic regulation of transcriptional silencing is essential for normal development. Despite its importance, in vivosystems for examining gene silencing at cellular resolution have been lacking in developing vertebrates. We describe a transgenic approach that allows monitoring of an epigenetically regulated fluorescent reporter in developing zebrafish and their progeny. Using a self-reporting Gal4-VP16 gene/enhancer trap vector, we isolated tissue-specific drivers that regulate expression of the green fluorescent protein (GFP) gene through a multicopy, upstream activatorsequence (UAS). Transgenic larvae initially exhibit robust fluorescence (GFPhigh); however, in subsequent generations,gfpexpression is
mosaic (GFPlow) or entirely absent (GFPoff), despite continued Gal4-VP16 activity. We find that
transcriptional repression is heritable and correlated with methylation of the multicopy UAS. Silenced transgenes can be reactivated by increasing Gal4-VP16 levels or inDNA methyltransferase-1(dnmt1) mutants. Strikingly, indnmt1homozygous mutants, reactivation ofgfpexpression occurs in a reproducible subset of cells, raising the possibility of different sensitivities or alternative silencing mechanisms in discrete cell populations. The results demonstrate the power of the zebrafish system forin vivomonitoring of epigenetic processes using a genetic approach.
T
HE ability to constrain transcription is fundamental to genome maintenance. Underscoring its impor-tance, several conserved mechanisms maintain repres-sive chromatin at transposons, repetitive sequences, and gene promoters (Hsiehand Fire2000; Bernsteinet al. 2007; Girard and Hannon 2008). Histone modifica-tions, small RNAs, and chromosome organization within the nucleus contribute to transcriptional silencing (Strahland Allis 2000; Heardand Bickmore 2007; Girardand Hannon2008). Although basic components of the silencing machinery are conserved, evolution of transposons has driven organisms to evolve specialized responses. For example, DNA methylation is required for silencing in plants, vertebrates, and some fungi, but is absent from other species, including many invertebrates (Golland Bestor2005).Epigenetic regulation of transcription is essential for normal development. Mouse embryos lacking DNA methyltransferase or histone-modifying enzymes are abnormal (Liet al.1992; Peterset al.2001; Tachibana et al. 2002). Loss of methylation leads to H2AX
phosphorylation, ATM activation, and initiation of the G2/M checkpoint in cell culture (Chen et al. 2007). Similarly, p53-dependent apoptosis is observed in embryos lacking DNA methyltransferase-1 (Dnmt1) ( Jackson-Grusby et al. 2001; Stancheva et al. 2001). Although these observations implicate programmed cell death as an underlying cause of lethality in dnmt1 mutants, some evidence suggests that not all tissues are similarly affected by the loss of DNA methylation. For example, in zebrafish, methylation of DNA and histone H3K9 appear to be required for terminal differentiation of the intestine, exocrine pancreas, and retina, but not of the endocrine pancreas (Rai et al. 2006). Similarly, hypomethylation-induced cell death was observed only in some Xenopus tissues at early stages of development (Stancheva et al. 2001). Tissue-specific differences in expression of chromatin-associated factors have been reported in mice and zebrafish, suggesting that gene requirements for silencing may not be equivalent in all cells ( Jarviset al.1996; Bourc’hiset al.2001; Raiet al. 2006; Sunet al. 2008).
Transcriptional silencing and methylation of trans-genes have been studied in many organisms (for exam-ple: Sapienzaet al.1989; Stuartet al.1990; Weberet al. 1990; Kilbyet al.1992; Matzkeet al.1994; and references in Dorer 1997). Transgenes containing the LacZ re-porter allow transcriptional silencing to be visualized in dissected tissues and fixed embryos (Allen et al.1990; Supporting information is available online athttp://www.genetics.org/
cgi/content/full/genetics.109.102079/DC1.
1Corresponding author:Department of Embryology, Carnegie Institution
for Science, 3520 San Martin Dr., Baltimore, MD 21218. E-mail: halpern@ciwemb.edu
Sutherlandet al.2000; Chevalier-Marietteet al.2003; Strathdeeet al.2008). In the mouse, a dominant screen for new genes involved in epigenetic regulation relied on fluorescence-activated cell sorting of blood cells ex-pressing a greenfluorescent protein (GFP) transgene (Blewittet al.2005; Asheet al.2008). While such ap-proaches have yielded useful information, transgenic tools to monitor silencing in live developing embryos have been lacking.
The zebrafish is highly amenable to such an in vivo strategy. The transparency of the embryo permits visual-ization of fluorescent reporters at the level of individual cells throughout development and over multiple gener-ations. In addition, a short generation time and large clutches of embryos facilitate unbiased genetic screens.
A gene/enhancer trap system was previously developed that integrates a self-reporting Gal4-VP16; 143upstream
activator sequence (UAS):GFP vector into the zebrafish genome by Tol2 transposition (Kawakami2004; Davison et al.2007). Although recovered transgenic lines initially exhibit robust tissue-specific patterns of GFP labeling, mosaic expression is observed in subsequent generations. In this report, we systematically characterize the onset and progression of GFP silencing using a newly identi-fied gene trap insertion that shows brain-specific ex-pression. Through the generation of independent epiallelic lines with high (GFPhigh), reduced (GFPlow),
or no GFP (GFPoff) labeling, we demonstrate that the
143 UAS is subject to DNA methylation, which corre-lates with the heritable loss of gfp expression. We examine the impact of Gal4-VP16 activity on methyla-tion and demonstrate that mutamethyla-tion of thednmt1gene overrides transcriptional repression. Reactivation ofgfp expression indnmt1mutants occurs only in a stereotypic region of the brain, raising the possibility that discrete cell populations possess different sensitivities to silenc-ing mechanisms. The results highlight the value of an in vivosystem for monitoring epigenetic regulation of transcription in developing tissues.
MATERIALS AND METHODS
Zebrafish strains and lines: Zebrafish were raised under standard conditions at 27° (Westerfield2000). The splice
acceptor-Gal4-VP16, UAS:GFP (SAGVG) construct (Davison
et al.2007) and Tol2 transposase mRNA (Kawakami 2005)
were injected into one-cell embryos of the AB strain (Walker
1999), and injected G0larvae were raised to adulthood. Adults were mated with AB fish, and the resultant F1progeny were screened for restricted patterns of GFP labeling. One F1 founder male generated larvae with GFP labeling in the forebrain, midbrain retina, and rhombomeres 5–6 (r5–6) and was designated as carrying the c269 allele. Transgenic F2 adults carrying a single-gene trap insertion were used to establish the c269 epiallelic lines. Additional Gt(Gal4-VP16; UAS:EGFP) gene/enhancer trap lines and Tg(UAS-E1b:NfsB-mCherry)c264/1
were previously described (Davisonet al.2007)
and renamed according to nomenclature conventions (http://
zfin.org/zf_info/nomen.html). The N-ethyl-N-nitrosourea
(ENU)-induced mutationdnmt1s872(mixed AB, TU, and TL
background) was identified in a mutagenesis screen and is a G-to-A transition at nucleotide 4376 (R. Anderson and D.
Stainier, unpublished results). To identify dnmt1s872
homo-zygotes, genomic DNA was isolated from individual larvae and polymerase chain reaction (PCR) amplified using the primers GCCTACATGCCATATGCTGA and TCTCCTGCT TCACAGGCTCT.
Assessment of transgene copy number: Genomic DNA (2.5mg) was isolated from fin clips of c269 F2adults that had been sorted for GFPhighor GFPlowlabeling at larval stages and
reared separately. DNA was digested with BamHI and sub-jected to Southern blotting (Southern 1975). Membranes
were hybridized with radiolabled GFP coding sequence. Transmission of epialleles: Progeny from the c269 F1 founder male were scored for high, low, or no GFP labeling at 3 days post fertilization (dpf). Sorted groups of F2larvae were raised separately to adulthood and mated to AB adults. Resultant F3 larval progeny were scored for GFP labeling. GFPoff larvae that maintained the c269 transgene insertion
were identified by mating F2 GFPlow adults with 143 UAS: mCherry transgenic fish and raised to adulthood to establish the stable, nonexpressing c269 GFPoffepiallelic line.
Methylation-sensitive Southern blots:Genomic DNA (5mg) from pools of20 GFPhighor GFPofflarvae was digested with
SacI and then divided, with one-half further digested byHpaII. Following digestion, samples were subjected to Southern blotting (Southern 1975). Membranes were probed with
radiolabeled 143UAS coding sequence amplified by PCR from SAGVG (Davison et al.2007) using the primers CGGCCAT
CAAGCTTAGGCTC and TCAAAGTGAGGCGAGACGC. Bisulfite sequencing: Individual larval heads and bodies were dissected by cutting anterior to the yolk at 3 dpf. DNA isolation and bisulfite conversion were achieved using the EZ DNA Methylation Direct kit (Zymo Research). Bisulfite con-verted DNA was amplified using the primers TTTTATGTTT TAGGTTTAGGG and CCCTTACTCACCATAATAAC followed by TATGTTTTAGGTTTAGGGGGA and CCCTTACTCACCA TAATAAC. The resultant fragment was purified and se-quenced. Analysis and statistical comparison of bisulfite data was performed using QUMA (Kumakiet al. 2008). P-values
were calculated using both Fisher’s exact test and the Mann– Whitney U-test.
Gal4-VP16 overexpression:Plasmid DNA (25 pg) encoding Gal4-VP16 under the control of the EF1a promoter was injected into one-cell embryos from GFPoff intercrosses. At
1 dpf, larvae were assessed for GFP-labeled cells under a fluorescent stereomicroscope (Leica MZ16F). DNA was col-lected from injected larvae that showed the highest levels ofgfp reactivation or mock-injected controls and analyzed by bi-sulfite sequencing.
Quantification of fluorescence intensity in c269 larvae: Larvae showing the highest number of GFP-labeled cells in r5–6 were imaged for GFP and mCherry fluorescence under a Zeiss Axio Imager.D1 with the ApoTome system using AxioVision digital imaging software. GFP- and mCherry-integrated fluorescent intensities were independently as-sessed in the mid/forebrain region and in r5–6 using MetaMorph software (Version 7, Molecular Devices). The ratio of fluorescence was calculated for each region by dividing the integrated intensity of GFP by the integrated intensity of mCherry.
RESULTS
UAS:GFP gene/enhancer trap insertions exhibit ro-bust tissue-specific patterns of GFP labeling in the F1 generation, but some showed variegated expression (GFPlow) in subsequent generations (Figure 1A). To
assess the origins of the variegation systematically, expression from a newly identified insertion was tracked over multiple generations. F1larvae carrying the c269
insertion showed robust expression ofgfpin the retina, forebrain, and midbrain and in a broad stripe in the hindbrain corresponding to rhombomeres (r) 5–6. This pattern was evident at 1 dpf and persisted until at least 7 dpf (data not shown and Figure 1B). In the F2
generation, two classes of transgenic larvae were de-tected: those with robust GFP labeling that recapitu-lated the F1pattern (GFPhigh) and those showing only a
small subset of GFP-positive cells spatially restricted within the F1pattern of labeling (GFPlow) (Figure 1C).
Using linker-mediated PCR, the c269 insertion was mapped within the fourth intron of the zebrafish homolog of theodd Oz/ten-m homolog 4(odz4) gene (data not shown). Brain-specific fluorescence in GFPhighlarvae
corresponded with the previously described expression pattern for theodz4gene (Miedaet al.1999).
Differences in GFP labeling between c269 sibling larvae could result from the segregation of multiple independent insertions of the gene/enhancer trap vector, mutation within the transgene, or a decrease in the number of UAS sites due to DNA polymerase slippage. Tol2-mediated trangenesis results in dispersed single integration events rather than large concato-meric arrays (Kawakami et al. 2000). Southern blots revealed GFPhighand GFPlowindividuals that carried only
a single insertion of the gene/enhancer trap in theodz4 gene (supporting information,Figure S1). Therefore, differences in transgene copy number cannot account
for the mosaicism observed in GFPlow individuals. To
rule out mutation of the transgene, DNA fragments encoding Gal4-VP16, the 143 UAS, or GFP were amplified by PCR from GFPlowindividuals. Sequencing
of the amplified products failed to reveal any mutations and confirmed the presence of 14 intact UAS copies (data not shown). Therefore, GFPhighand GFPlowlarvae
have the identical transgene insertion yet are phenotyp-ically distinct, suggesting that the transgene is regulated epigenetically.
Heritability of GFP epialleles:In mammals, genome-wide erasure and reestablishment of epigenetic marks early in development frequently leads to metastable inheritance of silenced epialleles (Rakyanet al.2002). To test whether epialleles of the c269 transgene were stably inherited, a single c269 F1adult was mated to wild
type. The resultant F2GFPhighand GFPlowsibling larvae
were separated and independently raised to adulthood. All F2 c269 GFPhigh adults produced both GFPhigh and
GFPlowF
3progeny (Table 1). In contrast, F2c269 GFPlow
adults did not produce any GFPhighprogeny and GFPlow
F3 larvae were recovered at less than the expected
frequency of 50%. F2 GFPlow adults also produced F3
larvae that retained the transgene but did not show any GFP labeling (GFPoff). GFP-labeled cells were never
observed in progeny (n .5000) from GFPoffadults in
the F3or F4generations (Table 1 and data not shown).
Similar inheritance of GFP epialleles was found upon mating other transgenic lines and occurred irrespective of the sex of the parent carrying the transgene (Table 1;
Table S1; Table S2). The results indicate that gfp ex-pression is subject to silencing, which, once established, is stably inherited.
Strain-specific modifiers that affect transgene expres-sion have been previously reported (Sapienza et al.
Figure 1.—Variegated
expression of UAS-regu-lated GFP in zebrafish lar-vae. (A) Representative larva carrying unique inser-tions of the gene/enhancer trap vector designated by c numbers. For a given inser-tion, sibling F2 larvae ex-press gfp either in a pattern similar to the F1 generation (GFPhigh, left
panels) or in a smaller sub-set of cells within this pat-tern (GFPlow, right panels).
Larvae were imaged at 3 dpf. (B) Fluorescent label-ing of the forebrain, mid-brain, and hindbrain (r5–6, arrowhead) of c269 GFPhigh larvae at 2 dpf.
(C) GFPlowc269 sibling larvae show significantly fewer GFP-labeled cells, which are restricted to within the c269 GFPhighpattern
of expression. (D) Corresponding images of GFP (top) and mCherry (bottom) fluorescence in c269 GFPhigh, GFPlow, and GFPoff
1989; Allenet al.1990; Martinand McGowan1995). Although the c269 transgene was introduced and maintained in the AB background, this background is unlikely to be homogeneous. To assess whether genetic variation affected expression of the transgene, GFPoff
adults (AB) were mated to adults of the WIK, Tubingen (TU), and mixed AB, TU, and Tupfel long fin (TL) genetic backgrounds. Progeny expressinggfpwere never observed (data not shown), indicating that genetic background differences do not account for transcrip-tional silencing.
All transgenic lines express functional Gal4-VP16:
The bipartite nature of the gene trap construct meant that loss ofgfpexpression could result from the failure to produce functional Gal4-VP16 or of Gal4-VP16 to activate UAS sites. To distinguish between the two possibilities, c269 GFPhigh, GFPlow, and GFPoffadults were
mated to adults carrying a newly integrated 143 UAS-regulated mCherry reporter. Expression of mCherry depends on Gal4-VP16 from the trapped transgene. Therefore, if Gal4-VP16 is absent in GFPoff cells,
mCherry will not be expressed. Alternatively, if activa-tion of the 143 UAS:GFP is compromised, Gal4-VP16 should still transactivate UAS:mCherry in GFP-negative cells. Labeling of mCherry was observed in GFPhigh,
GFPlow, and GFPoffc269 larvae in a pattern characteristic
of endogenous odz4 expression (Figure 1D). Robust mCherry fluorescence was also observed in larvae from other transgenic lines that exhibited variegated expres-sion, irrespective of the degree of GFP labeling (Figure S2). Thus, Gal4-VP16 is produced in GFPoffcells at levels
sufficient for activation of a second UAS-regulated transgene. These results also suggest that epigenetic modification of the multicopy UAS regulating GFP prevents activation by Gal4-VP16. Further implicating the 143 UAS in silencing, in later generations, some larvae carrying the 143 UAS:mCherry transgene also exhibited mosaic labeling of mCherry (data not shown).
DNA methylation correlates with silencing: One mechanism for transcriptional silencing is DNA
meth-ylation.In vitromethylation of UAS sites was previously shown to prevent Gal4 activation of a luciferase reporter following transfection into cultured cells (Liet al.2007). The 143 UAS (CGG-N11-CCG) may be particularly
susceptible to methylationin vivobecause it is a CpG-rich tandem repeat (Ginigeret al.1985). Methylation occurs predominately at CpG dinucelotides in verte-brates, and tandem repeats are frequent targets of DNA methylation (Garricket al.1998).
To test for DNA methylation, genomic DNA was collected from pools of c269 GFPhighor GFPoff
heterozy-gous larvae and digested withSacI to release an 840-bp fragment corresponding to the 143 UAS upstream of gfp. One-half of each sample was further digested with HpaII, which cleaves only at unmethylated CCGG sites. To visualize the fragment containing the UAS, Southern blots were performed on digested DNA and hybridized using a radiolabeled 143UAS DNA fragment as probe. Full digestion at the six HpaII sites within the UAS results in small fragments that are difficult to detect. Therefore, decreased intensity of the 840-bp fragment was used as a measure of the extent ofHpaII digestion. Following digestion with HpaII, the 840-bp fragment from GFPhigh larvae was significantly decreased in
in-tensity when compared to the HpaII-digested sample from GFPoff larvae, suggesting a correlation between
methylation of the 143UAS and loss ofgfpexpression (Figure 2A).
To assess DNA methylation at higher resolution and to determine if differences in methylation could be detected between Gal4-VP16-expressing and -non-expressing tissues, sodium bisulfite sequencing was performed. Genomic DNA was separately isolated from dissected heads and bodies of individual c269 GFPhighor
GFPoff heterozygous larvae. Following bisulfite
treat-ment, all unmethylated cytosine residues were con-verted to thymidine while methylated cytosine residues remained unconverted. Differences in methylation were not observed when DNA from Gal4-VP16-express-ing tissues of the head were compared to nonexpressGal4-VP16-express-ing body tissues from the same individual (Figure 2B). However, consistent with the Southern blot data presented in Figure 2A, a statistically significant increase (P,0.001) in the number of methylated CpG dinucleotides was observed when GFPoff sequences were compared to
GFPhighsequences (Figure 2B). In addition, DNA from
GFPhigh larvae showed a higher number of clustered
unmethylated sites, generally including at least one fully unmethylated Gal4-binding site. Fully unmethylated Gal4-binding sites were not observed in GFPoff larvae
(Figure 2B). Consistent with the heritability of the GFPoff epiallele, DNA methylation patterns in sperm
from GFPoffadults were very similar to those in sperm
from GFPofflarvae (average of 88.5% of CpG’s
methyl-ated; Figure S3). Collectively, the bisulfite sequencing data and Southern blot data indicate a correlation between methylation and silencing of gfp expression TABLE 1
Inheritance of c269 epialleles
Class of progeny (%)
No. of progeny c269/1 3wild type GFPhigh GFPlow No GFP
F1GFPhighmale 29 23 48 225
F2GFPhighfemale 1 34 14 52 380
F2GFPhighmale 1 45 4 51 84
F2GFPhighmale 2 5 5 90 400
F2GFPlowfemale 1 0 11 89 163
F2GFPlowmale 1 0 10 90 122
F2GFPlowmale 2 0 1 99 108
F3GFPoffmale 1 0 0 100 215
and suggest that methylation directs the stable inheri-tance of GFPoff.
Reactivation of gfp by exogenous Gal4-VP16: The UAS remains methylated in GFPoff larvae despite the
presence of Gal4-VP16. In addition, differences in DNA methylation were not observed between Gal4-VP16-expressing and -nonGal4-VP16-expressing tissues in GFPhigh and
GFPlow individuals. However, previous reports indicate
that DNA-binding proteins can trigger demethylation of recognition sites (Matsuoet al.1998; Linet al.2000). To test whether higher levels of Gal4-VP16 could cause UAS demethylation and reactivation ofgfpexpression, GFPofflarvae were injected with plasmid DNA
contain-ing Gal4-VP16 under the control of a ubiquitous pro-moter. Labeled cells were not detected in mock-injected controls or in controls injected with an unrelated plasmid (Figure 3A). In contrast, GFP labeling was detected throughout the entire larva in the presence of excess Gal4-VP16 (Figure 3B). Reactivation ofgfpin Gal4-VP16-injected larvae correlated with a modest decrease in DNA methylation as assessed by bisulfite sequencing (Figure 3C). Therefore, increasing the levels of Gal4-VP16 can reactivategfpexpression, likely through partial demethylation of the UAS.
Mutation of dnmt1 reactivates selective gfp expres-sion: If methylation is responsible for suppression of UAS-regulated gene expression, then blocking the methylation process should lead to reactivation of gfp expression. Zebrafish homozygous for an ENU-induced G1459D amino acid change in the AdoMet-binding motif X of DNA methyltransferase-1 (dnmt1s872) show
morphological abnormalities and a reduction in global methylation (Figure S4A; R. AndersonandD. Stain-ier,unpublished results). To test for reactivation ofgfp, the dnmt1s872
mutation was introduced into the c269 GFPoff, UAS:mCherry transgenic background. Adults
heterozyogous for the mutation and the transgenes were intercrossed, and their progeny were examined for gfp expression. At 1 dpf, GFP-labeled cells were not detected despite transactivation ofUAS:mCherryin the complete c269 brain-specific pattern. The presence of maternal Dnmt1 activity likely accounts for transgene silencing at this stage. However, by 2 dpf, robust GFP labeling appeared in the midbrain, forebrain, and retina in 25% of larvae showing mCherry transactivation (Figure 4, D–F). Expression persisted until 7 dpf, when dnmt1 homozygous mutants are no longer viable. Genotyping confirmed that GFP-positive larvae were homozygous for thednmt1s872mutation (n¼10), while
those without GFP-labeled cells had one or no mutant alleles (n ¼ 6; Figure 4, A–C). Methylation-sensitive Southern blots revealed significant demethylation of the UAS in gfp-reactivated larvae compared to their GFPoffsiblings (Figure S4). These results indicate that
Dnmt1-mediated methylation is important for silencing of the UAS-regulated transgene.
While reactivation ofgfpwas reproducibly observed in the midbrain, forebrain, and retina, cells of the r5–6 hindbrain region were refractory to reactivation. Trans-activation of UAS:mCherry confirmed that cells in r5–6 express Gal4-VP16 in homozygous mutant larvae. How-ever, GFP-labeled cells were not detected in r5–6 in 65% of larvae (n¼135) that showed reactivation elsewhere in the brain (Figure 4, D–F). In the remaining 35%, two small groups of GFP-expressing cells were found at the lateral edges of the r5–6 stripe (Figure 4, G–I, arrow-head in I). The difference in reactivation between the midbrain/forebrain and the hindbrain stripe was quan-tified by determining the integrated fluorescent in-tensity of GFP and mCherry for each brain region using larvae that showed the greatest number of GFP-labeled cells in r5–6. The average ratio of GFP to Figure 2.—Methylation
of the multicopy UAS is correlated with decreased GFP labeling. (A) Genomic DNA from pooled GFPhigh
or GFPoff larvae was
di-gested withSacI to produce an 840-bpSacI fragment en-compassing the 143 UAS upstream of gfp. The 840-bp SacI fragment has in-creased sensitivity toHpaII cleavage in GFPhigh larvae
compared to GFPofflarvae.
Transactivation of a 143 UAS:mCherry transgene was used to identify GFPofflarvae that inherited the c269 transgene. The 143UAS probe also recognizes the 143UAS regulating
mCherry, althoughSacI cleavage releases a larger fragment (2.2 kb). This fragment, containing the 143 UAS upstream of mCherry and the mCherry coding sequence, is largely digested, indicating that it is unmethylated in GFPhighand GFPoffsamples.
(B) DNA from heads and bodies of individual GFPhighor GFPofflarvae was subjected to bisulfite sequencing. The methylation status
mCherry fluorescence was 0.6860.13 in the midbrain/ forebrain region and 0.05 6 0.07 in r5–6 (n ¼ 5), indicating at least a 13-fold difference in labeling. Cells in the hindbrain are therefore more refractory to reactivation upon loss of Dnmt1 activity.
Curiously, cells in r5–6 are often the first to be silenced in the c269 transgenic background, even when the forebrain and midbrain show high levels of GFP labeling (Figure 4, J–L). Among 112 GFPhigh F
2 progeny with
robustgfp expression in the forebrain, midbrain, and retina, only 16 had any labeled cells in r5–6. Cells in the r5–6 stripe are therefore more susceptible to silencing than those in the midbrain and forebrain regions.
DISCUSSION
In this study, we used a multicopy UAS and fluores-cent reporter genes to monitor transcriptional silencing in transgenic zebrafish. Independent GFPhigh, GFPlow,
and GFPoff epiallelic lines were established, which
allowed silencing of gfp expression to be followed in developing tissues and over multiple generations.
Com-plete heritable silencing of single-copy GFPoff
trans-genes was observed and correlated with increased methylation of the tandemly repeated UAS. Reactiva-tion of GFPofftransgenes was demonstrated by
increas-ing Gal4-VP16 levels and by mutation of DNA methyltransferase-1. In addition, we observed distinct profiles of gfp silencing and reactivation in different cell populations, underscoring the value of an in vivo system for monitoring epigenetic regulation of tran-scription. Collectively, these results demonstrate a power-ful system for studying transcriptional silencing during vertebrate development using genetic approaches.
Although prior work in zebrafish provided evidence for transgene silencing, integrated DNA existed in concatemeric arrays and lacked trackable fluorescent reporters (Gibbs et al. 1994; Martin and McGowan
Figure4.—Distinct regions of the brain show differential
silencing and reactivation. Corresponding images of GFP fluorescence (A, D, G, and J), mCherry fluorescence (B, E, H, and K), and an overlay of mCherry, GFP, and differential interference contrast (C, F, I, and L) from 3 dpf larvae. (A–C) c269 GFPofflarvae that are wild type or heterozygous for the
dnmt1s872 mutation do not show GFP labeling. (D–F) c269
GFPoff larvae that are homozygous dnmt1s872 mutants show
GFP labeling in the midbrain and forebrain. Despite transac-tivation of mCherry, GFP labeling is not observed in r5–6 (arrow in E and F). (G–I) The homozygousdnmt1s872mutant
withgfp reactivation in a small number of bilateral cells in r5–6. The insert in I is an enlargement of reactivated cells indi-cated by the arrowhead. ( J–L) Larvae from a c269 GFPhighadult
mated to wild type. Labeling of GFP is present in the forebrain and midbrain, but not in r5–6 mCherry-positive cells. Figure3.—Reactivation of GFP expression by exogenous
Gal4-VP16. (A) Mock-injected larvae from c269 GFPoff
inter-crosses show no GFP labeling (n¼100). (B) GFPoffsiblings
injected with 25 pg of plasmid DNA encoding the EF1a
1995). Silencing of a single-copy GFP transgene was described in one report, but only in adult tissues (Thummel et al.2006). Epiallelic transgenic lines were not previously established, and inheritance of a fully silenced transgene had not been followed over generations. In this study, transgenesis was mediated by Tol2 transposition, resulting in random, dispersed integrations rather than complex concatemers (Kawakami 2005). Single insertions were isolated, allowing epigenetic modifications to be compared at a short defined repeat, the multicopy UAS. Silencing may be triggered by the repetitive nature of the 143UAS. UAS-regulated transgenes are being used more widely in zebra-fish research (Halpern et al.2008); however, variegated expression could be detrimental in experiments where uniform transgene expression is required. Optimization of Gal4/UAS constructs may be necessary to increase their utility, and decreasing UAS copy number could potentially mitigate silencing.
Silencing appears to be a stochastic event because only some progeny from GFPhigh adults showed variegated
expression, and only a subset of transgenic progeny from GFPlowindividuals completely lacked GFP labeling. The
progression from GFPhighto GFPlowto GFPoffalso suggests
that more than one generation is required for extinction ofgfpexpression. Widespread methylation of sperm from GFPoffadults suggests that, once extensive methylation of
the transgene is achieved, it is propagated through the gametes. The heritable complete silencing observed at the UAS is in contrast to mouse transgenes, which frequently show metastable inheritance of silencing whereby restablishment of the epigenetic state in the next generation is a probabilistic event (Rakyanet al.2002).
Heritable silencing in GFPofflarvae provides a tool to
explore the mechanisms of silencing. Previous studies have shown that DNA-binding proteins can cause deme-thylation of their recognition sites (Matsuoet al.1998; Hsieh2000; Linet al.2000). It has been suggested that protein binding during replication may protect sites from methylation by Dnmt1, thereby demethylating genes designated for activation (Matsuo et al. 1998; Hsieh2000). We similarly found that Gal4-VP16 could cause demethylation of the UAS, leading to reactivation of gfp. However, reactivation was achieved using very high levels of Gal4-VP16. In contrast, methylation and silencing persist in GFPofflines that express lower
levels of Gal4-VP16, and differences in methylation were not observed between Gal4-VP16-expressing and -nonexpressing tissues. These data imply that physio-logical concentrations of DNA-binding proteins may not generally be sufficient to drive demethylation as a means of activating transcription.
Mutation ofdnmt1also resulted in reactivation ofgfp expression. Although transcriptional repression inde-pendent of methyltransferase activity has been reported for Dnmt1 (Milutinovic et al. 2004; Dunican et al. 2008), thednmt1S872
allele is a point mutation expected to compromise catalytic function without disrupting
N-terminal regulatory domains. Therefore, it is most likely that the methyltransferase activity of Dnmt1 is directly required for silencing at the UAS.
Reactivation of gfp expression in dnmt1S872 mutants
occurs in a cell-type-specific manner. Although strong GFP labeling was observed in the forebrain, midbrain, and retina, cells in r5–6 of the hindbrain showed limited or no reactivation of gfp. There are several potential explanations for this difference. It is possible that partially methylated UAS sites may be more sensitive to Gal4-VP16 levels. Transcription from the odz4 pro-moter appears similar in the forebrain, midbrain, and hindbrain as assessed by whole-mount RNA in situ hybridization (Miedaet al.1999); however, subtle differ-ences in expression between brain regions might not be detected by this method. Slightly lowerodz4promoter activity in the r5–6 stripe would selectively reduce Gal4-VP16 activity in this region, which could account for the differences in the onset of silencing and resistance to reactivation at a partially methylated UAS.
Another explanation for differences in reactivation between brain regions could be differences in the timing of neurogenesis. Reactivation ofgfpexpression in dnmt1 mutants was not observed at 1 dpf despite transactivation of UAS:mCherry. The delay in reactiva-tion likely reflects the presence of maternal Dnmt1 at this time. If neurons in r5–6 differentiate prior to depletion of maternal Dnmt1, they would retain meth-ylation, and reactivation would not be expected. How-ever, we do not favor this hypothesis. By 48 hours post fertilization (hpf), reactivation is observed in the fore-and midbrain, suggesting that maternal Dnmt1 activity is no longer present. At this time point, cell prolifera-tion is observed throughout the brain, including in r5–6
(Mueller and Wullimann2005). Moreover, another
1400 neurons are born in r4–5 after 48 hpf (Lyonset al. 2003). Finally, it is unclear how early maternal Dnmt1, which is not spatially restricted, could specifically in-fluencegfpexpression in r5–6 of GFPhighlarvae.
A third hypothesis is that different regions of the brain have different gene requirements for silencing. In addition to a single dnmt1 homolog, the zebrafish genome also contains sixDNA methyltransferase-3(dnmt3) homologs (Shimodaet al.2005). Although tissue-specific roles for thesede novomethyltransferases have not been reported, increased activity of Dnmt3 proteins might compensate for the loss of Dnmt1. Alternatively, over-expression of the histone methyltransferasesuv39h1 res-cues phenotypes caused by depletion of dnmt1 in zebrafish (Raiet al.2006). Althoughsuv39h1expression is higher in the forebrain compared to the hindbrain (Rai et al. 2006), it is possible that other histone-modifying enzymes or as-yet-unidentified molecular pathways could influence silencing in r5–6.
reactivation of expression in GFPofflarvae. It is unlikely
that the full complement of genes required for silencing in vertebrates has been identified, and cell-type-specific roles for known factors may have been overlooked. GFPofftransgenic lines provide an opportunity to probe
the mechanism of silencing through candidate testing, mutagenesis, or drug screening approaches. Because such experiments are performedin vivo, they have the potential to further our understanding of developmen-tal and tissue-specific requirements for gene silencing in a developing embryo.
We thank Michelle Macurak and Brian Hollenback for technical support and Steve Leach, Michael Parsons, and Courtney Akitake for useful discussions and reagents. This project was supported in part by the National Institute of Child Health and Human Development (grant no. R01HD058530) to M.E.H. M.G.G. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-#1945-07). A.C.S. is an investigator of the Howard Hughes Medical Institute.
LITERATURE CITED
Allen, N. D., M. L. Norrisand M. A. Surani, 1990 Epigenetic
con-trol of transgene expression and imprinting by genotype-specific modifiers. Cell61:853–861.
Ashe, A., D. K. Morgan, N. C. Whitelaw, T. J. Bruxner, N. K.
Vickaryouset al., 2008 A genome-wide screen for modifiers
of transgene variegation identifies genes with critical roles in development. Genome Biol.9:R182.
Bernstein, B. E., A. Meissnerand E. S. Lander, 2007 The
mamma-lian epigenome. Cell128:669–681.
Blewitt, M. E., N. K. Vickaryous, S. J. Hemley, A. Ashe, T. J.
Bruxner et al., 2005 An N-ethyl-N-nitrosourea screen for
genes involved in variegation in the mouse. Proc. Natl. Acad. Sci. USA102:7629–7634.
Bourc’his, D., G. L. Xu, C. S. Lin, B. Bollmanand T. H. Bestor,
2001 Dnmt3L and the establishment of maternal genomic im-prints. Science294:2536–2539.
Chen, T., S. Hevi, F. Gay, N. Tsujimoto, T. Heet al., 2007 Complete
inactivation of DNMT1 leads to mitotic catastrophe in human cancer cells. Nat. Genet.39:391–396.
Chevalier-Mariette, C., I. Henry, L. Montfort, S. Capgras, S.
Forlaniet al., 2003 CpG content affects gene silencing in mice:
evidence from novel transgenes. Genome Biol.4:R53. Davison, J. M., C. M. Akitake, M. G. Goll, J. M. Rhee, N. Gosseet al.,
2007 Transactivation from Gal4–VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev. Biol.
304:811–824.
Dorer, D. R., 1997 Do transgene arrays form heterochromatin in
vertebrates? Transgenic Res.6:3–10.
Dunican, D. S., A. Ruzov, J. A. Hackett and R. R. Meehan,
2008 xDnmt1 regulates transcriptional silencing in pre-MBT Xenopus embryos independently of its catalytic function. Devel-opment135:1295–1302.
Garrick, D., S. Fiering, D. I. Martin and E. Whitelaw,
1998 Repeat-induced gene silencing in mammals. Nat. Genet.
18:56–59.
Gibbs, P. D., A. Peekand G. Thorgaard, 1994 An in vivo screen for
the luciferase transgene in zebrafish. Mol. Marine Biol. Biotech-nol.3:307–316.
Giniger, E., S. M. Varnumand M. Ptashne, 1985 Specific DNA
binding of GAL4, a positive regulatory protein of yeast. Cell
40:767–774.
Girard, A., and G. J. Hannon, 2008 Conserved themes in
small-RNA-mediated transposon control. Trends Cell Biol.18:136–148. Goll, M. G., and T. H. Bestor, 2005 Eukaryotic cytosine
methyl-transferases. Annu. Rev. Biochem.74:481–514.
Halpern, M. E., J. Rhee, M. G. Goll, C. M. Akitake, M. Parsons et al., 2008 Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish5:97–110.
Heard, E., and W. Bickmore, 2007 The ins and outs of gene
regu-lation and chromosome territory organisation. Curr. Opin. Cell Biol.19:311–316.
Hsieh, C. L., 2000 Dynamics of DNA methylation pattern. Curr.
Opin. Genet. Dev.10:224–228.
Hsieh, J., and A. Fire, 2000 Recognition and silencing of repeated
DNA. Annu. Rev. Genet.34:187–204.
Jackson-Grusby, L., C. Beard, R. Possemato, M. Tudor,
D. Fambroughet al., 2001 Loss of genomic methylation causes
p53-dependent apoptosis and epigenetic deregulation. Nat. Genet.27:31–39.
Jarvis, C. D., T. Geiman, M. P. Vila-Storm, O. Osipovich, U. Akella et al., 1996 A novel putative helicase produced in early murine lymphocytes. Gene169:203–207.
Kawakami, K., 2004 Transgenesis and gene trap methods in
zebra-fish by using the Tol2 transposable element. Methods Cell Biol.
77:201–222.
Kawakami, K., 2005 Transposon tools and methods in zebrafish.
Dev. Dyn.234:244–254.
Kawakami, K., A. Shimaand N. Kawakami, 2000 Identification of a
functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebra-fish germ lineage. Proc. Natl. Acad. Sci. USA97:11403–11408. Kilby, N. J., H. M. Leyserand I. J. Furner, 1992 Promoter
meth-ylation and progressive transgene inactivation in Arabidopsis. Plant Mol. Biol.20:103–112.
Kumaki, Y., M. Odaand M. Okano, 2008 QUMA: quantification
tool for methylation analysis. Nucleic Acids Res.36:W170–W175. Li, E., T. H. Bestorand R. Jaenisch, 1992 Targeted mutation of the
DNA methyltransferase gene results in embryonic lethality. Cell
69:915–926.
Li, F., M. Papworth, M. Minczuk, C. Rohde, Y. Zhang et al.,
2007 Chimeric DNA methyltransferases target DNA methyla-tion to specific DNA sequences and repress expression of target genes. Nucleic Acids Res.35:100–112.
Lin, I. G., T. J. Tomzynski, Q. Ouand C. L. Hsieh, 2000 Modulation
of DNA binding protein affinity directly affects target site deme-thylation. Mol. Cell. Biol.20:2343–2349.
Lyons, D. A., A. T. Guyand J. D. Clarke, 2003 Monitoring neural
progenitor fate through multiple rounds of division in an intact vertebrate brain. Development130:3427–3436.
Martin, C. C., and R. McGowan, 1995 Genotype-specific modifiers
of transgene methylation and expression in the zebrafish, Danio rerio. Genet. Res.65:21–28.
Matsuo, K., J. Silke, O. Georgiev, P. Marti, N. Giovannini et al.,
1998 An embryonic demethylation mechanism involving binding of transcription factors to replicating DNA. EMBO J.17:1446–1453. Matzke, A. J., F. Neuhuber, Y. D. Park, P. F. Ambros and M. A.
Matzke, 1994 Homology-dependent gene silencing in
trans-genic plants: epistatic silencing loci contain multiple copies of methylated transgenes. Mol. Gen. Genet.244:219–229. Mieda, M., Y. Kikuchi, Y. Hirate, M. Aoki and H. Okamoto,
1999 Compartmentalized expression of zebrafish ten-m3 and ten-m4, homologues of the Drosophila ten(m)/odd Oz gene, in the central nervous system. Mech. Dev.87:223–227. Milutinovic, S., S. E. Brown, Q. Zhuangand M. Szyf, 2004 DNA
methyltransferase 1 knock down induces gene expression by a mechanism independent of DNA methylation and histone deace-tylation. J. Biol. Chem.279:27915–27927.
Mueller, T., and M. Wullimann, 2005 Atlas of Early Brain Develop-ment.Elsevier, Amsterdam/New York.
Peters, A. H., D. O’Carroll, H. Scherthan, K. Mechtler, S. Sauer et al., 2001 Loss of the Suv39h histone methyltransferases im-pairs mammalian heterochromatin and genome stability. Cell
107:323–337.
Rai, K., L. D. Nadauld, S. Chidester, E. J. Manos, S. R. Jameset al.,
2006 Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol. Cell. Biol.
26:7077–7085.
Rakyan, V. K., M. E. Blewitt, R. Druker, J. I. Preisand E. Whitelaw,
2002 Metastable epialleles in mammals. Trends Genet. 18:
348–351.
Sapienza, C., J. Paquette, T. H. Tran and A. Peterson,
Shimoda, N., K. Yamakoshi, A. Miyakeand H. Takeda, 2005
Iden-tification of a gene required for de novo DNA methylation of the zebrafish no tail gene. Dev. Dyn.233:1509–1516.
Southern, E. M., 1975 Detection of specific sequences among DNA
fragments separated by gel electrophoresis. J. Mol. Biol.98:503– 517.
Stancheva, I., C. Hensey and R. R. Meehan, 2001 Loss of the
maintenance methyltransferase, xDnmt1, induces apoptosis in Xenopus embryos. EMBO J.20:1963–1973.
Strahl, B. D., and C. D. Allis, 2000 The language of covalent
his-tone modifications. Nature403:41–45.
Strathdee, D., C. B. Whitelawand A. J. Clark, 2008 Distal
trans-gene insertion affects CpG island maintenance during differen-tiation. J. Biol. Chem.283:11509–11515.
Stuart, G. W., J. R. Vielkind, J. V. McMurrayand M. Westerfield,
1990 Stable lines of transgenic zebrafish exhibit reproducible patterns of transgene expression. Development109:577–584. Sun, X. J., P. F. Xu, T. Zhou, M. Hu, C. T. Fuet al., 2008
Genome-wide survey and developmental expression mapping of zebrafish SET domain-containing genes. PLoS ONE3:e1499.
Sutherland, H. G., M. Kearns, H. D. Morgan, A. P. Headley,
C. Morriset al., 2000 Reactivation of heritably silenced gene
expression in mice. Mamm. Genome11:347–355.
Tachibana, M., K. Sugimoto, M. Nozaki, J. Ueda, T. Ohtaet al.,
2002 G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev.16:1779–1791.
Thummel, R., S. Bai, M. P. Sarras, Jr., P. Song, J. McDermottet al.,
2006 Inhibition of zebrafish fin regeneration using in vivo elec-troporation of morpholinos against fgfr1 and msxb. Dev. Dyn.
235:336–346.
Walker, C., 1999 Haploid screens and gamma-ray mutagenesis.
Methods Cell Biol.60:43–70.
Weber, H., C. Ziechmannand A. Graessmann, 1990 In vitro DNA
methylation inhibits gene expression in transgenic tobacco. EMBO J.9:4409–4415.
Westerfield, M., 2000 The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio).Institute of Neuroscience, University of Oregon, Eugene, OR.
Supporting Information
http://www.genetics.org/cgi/content/full/genetics.109.102079/DC1
Transcriptional Silencing and Reactivation in Transgenic Zebrafish
Mary G. Goll, Ryan Anderson, Didier Y. R. Stainier, Allan C. Spradling and
Marnie E. Halpern
M. Goll et al.
2 SI
FIGURE S1.—A single transgene insertion event generates both c269 GFPhigh and GFPlow larvae (A)
Schematic of the enhancer gene trap construct. (B) Southern blot of BamH1 digested genomic DNA from individual GFPhigh (n=3) or GFPlow (n=3) adults sorted for GFP labeling at larval stages and probed with
radiolabeled gfp sequence. The probe recognizes restriction fragments which extend from the BamHI site 5’ to gfp (red) to the nearest BamH1 site in 3’ flanking genomic sequence (blue). Independent integrations produce restriction fragments of distinct sizes because the nearest BamH1 in flanking DNA will vary in distance from the transgene integration site. The lower band (arrow, 2.0 kb) corresponds to the predicted molecular weight of the BamH1 fragment for the insertion mapping to the odz4 locus. Individuals from both expression classes were identified that contain only this insertion (GFPhigh lanes 1 and 3, GFPlow
lanes 1 and 2). The arrowhead indicates a second insertion in two individuals that was unlinked to the
M. Goll et al. 3 SI
FIGURE S2.—Transactivation of UAS:mCherry in GFPhigh and GFPlow transgenic lines. Corresponding
images of GFP (left) and mCherry (right) fluorescence in GFPhigh and GFPlow transgenic larvae from lines c251,
M. Goll et al.
4 SI
FIGURE S3.—Widespread methylation of the UAS in sperm from GFPoff adults. DNA from sperm
isolated from GFPoff adults was subjected to bisulfitesequencing. The methylation status of each CG
site is reported as a percentage of total sites tested for 8 clones from one individual and ten clones from a second GFPoff individual. Methylated sites are indicated black; bars indicate pairs of CpG
M. Goll et al. 5 SI
FIGURE S4.—Methylation of the 14X UAS is decreased in larvae showing reactivation of gfp. (A-B) c269
GFPoff, UAS:mCherry adults heterozygous for the dnmt1S872 mutation were intercrossed and genomic DNA
was isolated from pooled progeny that showed either mCherry and GFP labeling in the brain (GFP+) or
only mCherry labeling (GFP-). DNA was digested with SacI and one half of each sample was further
digested with HpaII. (A) Ethidium bromide staining of GFP+ and GFP- DNA samples run on an agarose gel.
Increased smearing is observed in HpaII digested DNA from GFP+ larvae (lane 2) compared to GFP
-siblings (lane 4), indicating a genome wide decrease in methylation. (B) Southern blot of (A) using a DNA fragment encompassing the 14X UAS as probe. SacI digestion isolates an 840 bp fragment corresponding to the 14X UAS regulating gfp expression. Increased HpaII digestion in larvae showing reactivation of gfp
compared to their GFP- siblings (lane 4) indicates that a reduction in methylation at the 14X UAS
M. Goll et al.
6 SI
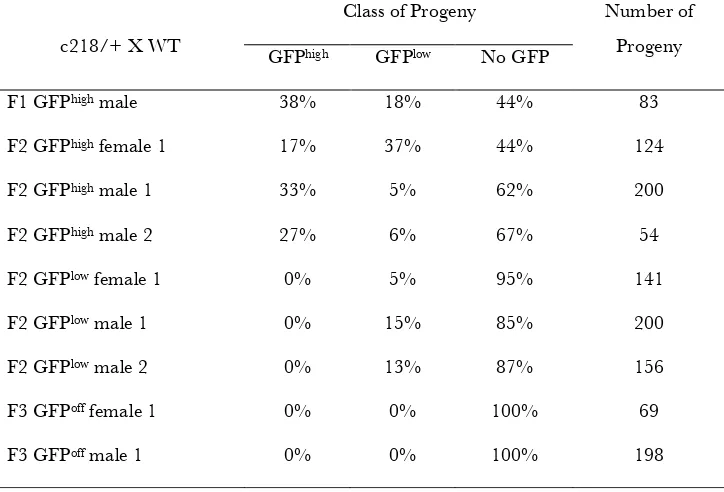
TABLE S1
Inheritance of c218 epialleles
Class of Progeny c218/+ X WT
GFPhigh GFPlow No GFP
Number of Progeny
F1 GFPhigh male 38% 18% 44% 83
F2 GFPhigh female 1 17% 37% 44% 124
F2 GFPhigh male 1 33% 5% 62% 200
F2 GFPhigh male 2 27% 6% 67% 54
F2 GFPlow female 1 0% 5% 95% 141
F2 GFPlow male 1 0% 15% 85% 200
F2 GFPlow male 2 0% 13% 87% 156
F3 GFPoff female 1 0% 0% 100% 69
M. Goll et al. 7 SI
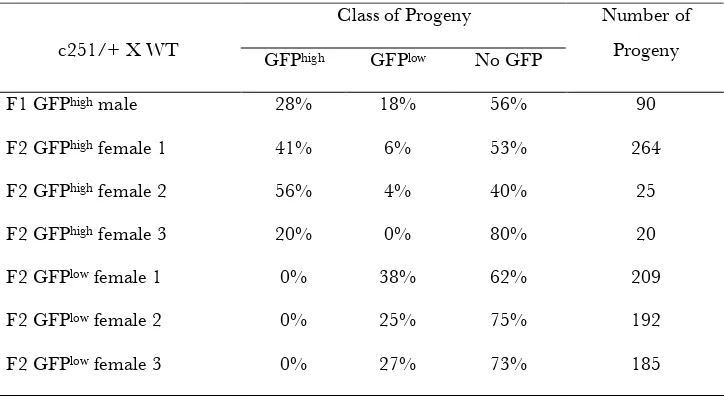
TABLE S2
Inheritance of c251 epialleles
Class of Progeny
c251/+ X WT GFPhigh GFPlow No GFP
Number of Progeny
F1 GFPhigh male 28% 18% 56% 90
F2 GFPhigh female 1 41% 6% 53% 264
F2 GFPhigh female 2 56% 4% 40% 25
F2 GFPhigh female 3 20% 0% 80% 20
F2 GFPlow female 1 0% 38% 62% 209
F2 GFPlow female 2 0% 25% 75% 192