Mutations That Suppress the Deletion
of
an Upstream Activating
Sequence in Yeast: Involvement
of a Protein Kinase and Histone H3
in
Repressing Transcription
in
Vivo
Gregory Prelich' and Fred
WinstonDepartment of Genetics, Haroard Medical School, Boston, Massachusetts 021 15 Manuscript received April 25, 1993
Accepted for publication July 14, 1993
ABSTRACT
Regulated transcription of most protein-encoding genes in Saccharomyces cerevisiae requires an upstream activating sequence (UAS); in the absence of UAS elements, little or no transcription occurs. In certain mutant strains, however, promoters that have been deleted for their UAS can direct significant levels of transcription, indicating that the remaining promoter elements (the basal pro- moter) are capable of directing higher levels of transcription, but they are normally repressed in wild- type strains. T o analyze this repression, we have selected for mutations that cause increased transcrip tion of the SUC2 gene in the absence of its UAS. In addition to some previously studied genes, this selection has identified five genes that we have designated BURl, BURP, BUR3, BUR5 and BUR4 (for
Bypass UAS Requirement). T h e bur mutations cause pleiotropic phenotypes, indicating that they affect transcription of many genes. Furthermore, some bur mutations suppress the requirement for the SNF5 trans-activator at both SUCZ and T y . Additional analysis has demonstrated that BURl is identical to SGVl, which encodes a CDC28-related protein kinase. This result indicates that protein phosphorylation is important for repression of the SUCZ basal promoter as well as other aspects of transcription in vivo. Finally, BUR5 is identical to HHTl, encoding histone H3, further implicating chromatin structure as important for expression of SUC2.
T
HE regulation of transcription initiation by RNA polymerase I1 in eukaryotic cells is an extremely complex process, requiring the concerted action of a large number of proteins. Most of the proteins studiedso far can be divided into three general classes. First, RNA polymerase I1 and the general transcription factors assemble over the T A T A box to form a prein- itiation complex that is required for basal transcrip- tion at all pol II-dependent transcription units (see CONAWAY and CONAWAY 1991 for review). Second, regulatory transcription factors are required for con- trolling the levels of transcription in response to either intracellular or extracellular signals, usually for a lim- ited set of coordinately regulated genes (see MITCHELL and TJIAN 1989 for review). Finally, the core histones and other components or regulators of chromatin also play a vital role in the process of transcription initia- tion, as many studies have shown that nucleosomes can have a general repressive effect on transcription initiation both in vivo and in vitro (see GRUNSTEIN
1990; FELSENFELD 1992 for reviews).
T o identify proteins that are important for tran- scription initiation and to investigate their function in vivo, we have used genetic analysis in the yeast Saccha- romyces cerevisiae. Previously, o u r lab has identified a
College of Medicine, 1300 Morris Park Avenue, Bronx, New York 10461. Genetics 135: 665-676 (November, 1993)
' Present address: Department of Molecular Genetics, Albert Einstein
large number of genes by selection for mutations that suppress transcriptional defects caused by T y or 6
insertion mutations at HIS4 or LYS2 (WINSTON et al.
1984; WINSTON et al. 1987; CLARK-ADAMS et al. 1988; FASSLER and WINSTON 1988). Since these mutations (named spt for suppressor of T y ) affect transcription initiation and cause pleiotropic phenotypes, the SPT proteins were expected to have general and important roles in transcription. This prediction has been con- firmed in some cases: SPTIS encodes the TATA binding protein TBPITFIID (EISENMANN, DOLLARD and WINSTON 1989), and SPTIIIHTAI and SPTl2/
HTBI encode histones H2A and H2B (CLARK-ADAMS
et al. 1988). Although the biochemical functions of the other SPT proteins are not known, mutations in many SPT genes cause similar phenotypes as mutations in either SPT15 (WINSTON et al. 1987) or S P T l l /
HTAI-SPTI2IHTBl (CLARK-ADAMS and WINSTON
1987; MALONE et al. 1991; SWANSON and WINSTON 1992). Thus, many SPT genes can be grouped into either of two distinct phenotypic classes: one that is functionally related t o TBP, and another that is func- tionally related to histones.
666 G. Prelich and F. Winston
and WINSTON 1992; C. DOLLARD, J. HIRSCHHORN and F. WINSTON, unpublished data). Upstream activating sequences are promoter elements in yeast that are functionally equivalent to mammalian transcriptional enhancers, in that they can activate transcription at variable distances from the TATA box and in either orientation (GUARENTE 1988). T h e SUC2 UAS has been shown by deletion analysis to extend from around -650 to -418, relative to the first AUG in
the SUC2 coding region (SAROKIN and CARLSON
1984). In addition, a DNA fragment that contains the
SUC2 UAS confers glucose repression upon a heter- ologous promoter (SUC2 transcription is regulated by glucose repression) and responds to trans-acting mu- tations that affect the wild-type SUC2 promoter (SA- ROKIN and CARLSON 1985). In the absence of its UAS, SUC2 transcription is virtually undetectable, resulting in the inability of strains containing a suc2AUAS allele to grow on media containing sucrose as the carbon source. However, mutations in most members of the histone class of SPT genes suppress the Suc- pheno- type of a S U C ~ A U A S , indicating that chromatin plays an important role in transcription at this promoter, and that the SUC2 UAS may function by overcoming repression by nucleosomes (HIRSCHHORN et al. 1992). T h e suppression of a suc2AUAS by certain spt mu- tations is similar to the effects observed upon deplet- ing a cell of histone H4 (HAN and GRUNSTEIN 1988). Depletion of histone H4 in vivo suppresses deletions of UAS elements at P H 0 5 , CYCl and G A L l , resulting in elevated transcription from the mutant promoters under both repressing and derepressing conditions. (A suc2AUAS was not examined in that study.) These results indicate that mutations that affect chromatin structure can allow transcription from multiple pro- moters in the absence of a UAS, perhaps by alleviating repression of the assembly or function of the transcrip- tion preinitiation complex. This model is supported by biochemical studies, which demonstrate that chro- matin also represses basal transcription in vitro (KNE- ZETIC and LUSE 1986; LAYBOURN and KADONAGA 1991; LORCH, LAPoINTEand KORNBERG 1987,1992), and that one function of UAS-binding proteins is to counteract chromatin repression (WORKMAN and
ROEDER 1987; CROSTON et al. 1991; WORKMAN, TAY- LOR and KINGSTON 1991 ; WORKMAN and KINGSTON
Although mutations in the histone group of SPT
genes can suppress a S U C ~ A U A S , the spt mutations were originally identified through other selections and screens, and therefore they may not identify the full spectrum of mutations capable of suppressing a
suc2AUAS. We therefore decided to select specifically for suppressors of a suc2AUAS mutation, with the intention of identifying other genes that affect tran- scription from basal promoters. Clearly, one class of 1992).
mutations expected to be identified by such a selection would be mutations in genes encoding either compo- nents of chromatin or regulators of chromatin func- tion; however, other classes of interesting mutations were also possible.
In this paper, we describe the identification and analysis of suppressors of a suc2AUAS. Using this selection, we have identified many new alleles of pre- viously identified SPT genes, as expected, based on previous characterization of mutations in those genes. In addition, we have identified recessive mutations in five other genes that can suppress a suc2AUAS. One of these genes encodes histone H3, and another one encodes a CDC28-related protein kinase, indicating that chromatin structure and protein phosphorylation are required for repression of the SUC2 basal pro- moter. Since these mutations are pleiotropic and af- fect transcription from other promoters besides SUC2,
they may identify elements of a general transcriptional repression mechanism.
MATERIALS AND METHODS
Yeast strains: S. cerevisiae strains used in this study are listed in Table 1. All strains with the prefix FY or GY are isogenic to FY2, a GAL2+ ura3-52 derivative of S288C. DBY2448 was obtained from M. CARLSON. 0653 was de- rived from LM67-17C (obtained from G. JOHNSTON) by crossing in the suc2AUAS(-1900/-390) and lys2-1286 mu- tations. The suc2AUAS(-l900/-390) allele was integrated into the genome at the SUCZ locus by two-step gene replace- ment using plasmid pLS29A(-1900/-390) (SAROKIN and CARISON 1984). The his4-9126 (CHALEFF and FINK 1980; FARABAUCH and FINK 1980) and lys2-1286 (SIMCHEN et al. 1984) alleles are solo 6 insertions that cause His- and Lys- phenotypes, respectively. To create the burlA1::TRPl al- lele, a 0.2-kb BglII fragment from BUR1 was replaced by a 1.2-kb BamHI-BglII TRPl fragment. The snflAl0 and snf5- 5::URA3 alleles were described previously (CELENZA and CARLSON 1989; ABRAMS, NEICEBORN and CARLSON 1986).
Media and genetic methods: Rich media (YPD), synthetic complete dropout media (for example, SC-Ura), minimal media (SD) and sporulation media were made as described (ROSE, WINSTON and HIETER 1990). YPSuc plates contained YEP
+
2% sucrose+
1 pg/ml antimycin A (Sigma), YPGal plates contained YEP+
2% galactose + 1 pg/ml antimycin A. YPRaf plates contained YEP+
2% raffinose + 1 pg/ml antimycin A. SC-inositol plates were made as described (SHERMAN, FINK and LAWRENCE 1978). Standard genetic methods for mating, sporulation and tetrad analysis (MOR- TIMER and HAWTHORNE 1969; ROSE, WINSTON and HEITER 1990) were used. Yeast cells were transformed by the lith- ium acetate procedure (ITO et al. 1983).Isolation of bur mutants: To select for Suc+ revertants
of a suc2AUAS strain (bur mutants), strains FY 198 and FY 12 1 (Table 1) were streaked for single colonies on YPD plates, and individual colonies were resuspended in 100 pl
of sterile H20 and spread onto YPSuc plates. To increase the mutation frequency, cells were mutagenized with 300
Suppressors of a UAS Deletion
TABLE 1
S. cerevisiae strains
Strain Genotype
FY 1 FY 2 FY 78 FYI21 FY 129 FY 130 FY143 FY 198 FY357 FY874 FY875 DBY2448 LM67-17C 0653 CY60 CY80 CY8 1
CY82 CY83 CY84 CY85 GY9 1
CY93 CY94 CY95 CY96 CY97 CY99 C Y 100 CY101 CY102 CY103 CY110 CY1 14 GYll9 CY141 CY142 G Y 144 C Y 146 C Y 148 C Y 152 C Y 153 C Y 154 CY155 CY156 C Y 158 C Y 159 GY162 CY167 C Y 169 G Y 177 CY201 GY203 CY227 CY233 CY234 CY237 CY238
MATa ura3-52 MATa GAL2+ ura3-52 MATa his3A200
MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 aded spt6-140 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 spt6-140 MATa his4-91213 lys2-1286 ura3-52 aded
MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 aded
MATa lys2-1286 suc2AUAS(-l900/-390) ura3-52 ade8 leu2A1 (htal-htb1)A::LEUZ MATa his4-9126 lys2-1286 ura3-52 leu2A1 s n f l A l 0
MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 (htal-htbl)A::LEU2 trplA63 MATa suc2A9 his4-539am ura3-52
MATa trpl met4 arof ura3 leu2-3 cdc67-1
MATa lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2 t r p l aro7 cdc67-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 aded bur2-2 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 spt5-194 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 ade8 spt5-194 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 spt16-197 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 spt16-197
MATa his4-9126 lys2-1286 suc2AUAS(-l900/-390) ura3-52 leu2A1 trplA63 sptl0::TRPl MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 trpl A63 sptl0::TRPl MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 bud-1
MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 bur4-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 burl-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 bur4-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 ade8 bur6-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 b u d - 1 MATa his4-9126 lys.2-1286 suc2AUAS(-1900/-390) ura3-52 aded burl-2 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 burl-2 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trpl A63 burl-2 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 aded bur2-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trpl A63 bur2-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 bur5-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 ade8 burl-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 ade8 bur5-1 MATa his4-9126 lys2-1286 ura3-52 leu2A1 snflAl0 burl-1
MATa his4-9126 lys2-1286 ura3-52 aded snflAlO burl-2 MATa his4-9126 lys2-1286 ura3-52 leu2AI s n f l A l 0 bur2-1 MATa his4-9126 lys2-1286 ura3-52 trplA63 snflAl0 bur.?-2 MATa his4-9126 lys2-1286 ura3-52 leu2Al snflAl0 burl-1 MATa his4-9126 lys2-1286 ura3-52 aded snf5-5::URA3 burl-2 MATa his4-9126 lys2-1286 ura3-52 leu2A1 snf5-5::URA3 burl-2 MATa his4-9126 lys2-1286 ura3-52 leu2A1 snf5-5::URA3 bur2-1 MATa his4-9126 lys2-1286 ura3-52 aded snf5-5::URAj bur2-1 MATa his4-9126 lys2-1286 ura3-52 leu2A1 snf5-5::URA3 bur2-2 MATa his4-9126 lys2-1286 ura3-52 leu2Al snf5-5::URA3 burl-1 MATa his4-9126 lys2-1286 ura3-52 leu2A1 snf5-5::URA3 bur4-1
MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 bur6-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 leu2A1 burl-1 MATa his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 aded burl-2 MATO his4-9126 lys2-1286 suc2AUAS(-1900/-390) ura3-52 trplA63 bur2-2 MATa his4-9126 lys2-1286 ura3-52 leu2A1 snf5-5::URAj
MATa his4-9126 lys2-1286 ura3-52 trplA63 snf5-5::URA3 bur5-1 MATa his4-9126 lys2-1286 ura3-52 leu2A1 s n f l A l 0 bur3-1 MATa his4-9126 lys2-1286 ura3-52 ade8 snf5-5::URA3 bur3-1 MATa his4-9126 lys2-1286 ura3-52 ade8 snf5-5::URA3 bur3-1 MATa his4-9126 lys2-1286 ura3-52 snflAlO bur5-1
668 G. Prelich and F. Winston
purification. Since the mutagenesis increased the reversion frequency more than 2O-fold, it is likely that these colonies represent independent reversion events.
Dominance and complementation tests: T o test for dom- inance, the Suc+ revertants were replica mated to suc2AUAS (Suc-) tester lawns, selected for diploids by complementation of auxotrophic phenotypes, and the diploids were then replica plated to YPSuc plates. T o test whether any of the mutations were in known SPT genes, the 259 recessive Suc+ mutants were crossed by tester lawns containing suc2AUAS in combination with mutations in SPT genes known to suppress this deletion: HTAl-HTBl, SPT5, SPT6, SPTlO or SPT16. Diploids were selected by complementation of aux- otrophic markers and replica plated to test for growth of the diploids on YPSuc plates. One strain, initially designated bur4, was later shown to contain a mutation in SPT21 (NATSOULIS et al. 199 1) by both complementation and link- age tests. The seven recessive bur mutants that did not contain mutations in SPT genes were crossed into both mating types and then complementation tests were per- formed by pairwise mating in all possible combinations.
Invertase assays: Invertase assays were performed as described previously (HIRSCHHORN et al. 1992).
RNA isolation and Northern analysis: Cells were grown in YPD liquid media to a concentration of 5 x lo6 to 1 X
l o 7 cells per ml, and RNA was isolated as described previ- ously (CARLSON and BOTSTEIN 1982). In experiments where SUC2 RNA levels were examined, cells were washed in H 2 0
and derepressed for 165 min in YEP
+
0.05% glucose before RNA was isolated. RNA was separated by electrophoresis in a 1 % formaldehyde agarose gel. Blotting and hybridiza- tion to DNA probes was performed as described previously (SWANSON, MALONE and WINSTON 1991). RNA was cross- linked to GeneScreen (New England Nuclear) by exposure to UV radiation in a Spectrolinker XL1500 (Spectronics Corp.). The probes, plasmids B161 (Ty; WINSTON et al.1987), and pYST138 (TUB2; SOM et al. 1988) were nick translated using a kit from Boehringer Mannheim.
Primer extension analysis: Twenty pg of RNA prepared from derepressed cultures and 1 pmol of "P 5'-end labeled oligonucleotide primers were combined in 100 pl of H z 0 and precipitated on ice for 30 min by addition of 100 pl of 4 M ammonium acetate and 500 pl ethanol. The RNA/oligo pellet was washed with 70% ethanol, dried and resuspended in 9.5 pl of H 2 0 . The sample was heated to 90" for 5 min and then cooled on ice. Three pl of 5X R T buffer (250 mM Tris, pH 8.0, 25 mM MgC12, 250 mM KCI) was added, the samples were heated for 15 min at 65 , and then frozen on powdered dry ice. The tubes were moved to room temper- ature and 1 pl of 0.1 M DTT, 1 pl of a solution containing 10 mM each dNTP, and 3 units of AMV reverse transcrip- tase (Boehringer Mannheim Biochemicals) were added and the tube was incubated for 45 min at 50". Five pl of 1 mg/ ml RNAase111 were added and the reactions were incubated for 15 min at 37". The reactions were diluted to 100 p1
with H20, extracted once with an equal volume of phenol/ chloroform/isoamyl alcohol (25:24: l), and once with chlo- roform. DNA was precipitated on ice with 100 pl 4 M ammonium acetate and 500-pl ethanol for 30 min, washed with 70% ethanol, dried and resuspended in 2-pl dye form- amide
+
2 pl TE, pH 8.0. The samples were heated at 90" for 3 min and subjected to electrophoresis through an 8% urea acrylamide sequencing gel. Two oligonucleotides were used as probes: suc2-3 (5' GTG AAG TGG ACC AAA GGT CTA TCG C 3') and U6.48-72 (5' GCA GGG GAA CTG CTG ATC ATC T C T 3'). Extension of suc2-3 pro- duces a 14 1 nucleotide product that corresponds to the 1.9- kb SUC2 start site (CARLSON et al. 1983), and extension ofU6.48-72 produces a 72 nucleotide U6-specific product (BROW and GUTHRIE 1988).
Plasmids: pGP59 contains the original BURl clone that was isolated from a YCp50-based yeast genomic DNA li- brary (ROSE et al. 1987). A 4.5-kb EcoRI fragment of pGP59 was subcloned into the EcoRI site of YIp5, creating pGP89. pGPlO3 contains a 2.2-kb ClaI fragment of BURl in pRS316. A 0.2-kb BglII fragment of pGP103, internal to the BURl open reading frame, was deleted and replaced with a 1.2-kb T R P l BamHI-BglII fragment from YRp7, creating pGP105. This plasmid contains the burl null allele burlA1::TRPl. pGP73 and pGP74 are the two original clones from the YCp50 library (ROSE et al. 1987) that complemented the bur5-1 mutation. pGP74 contains H H T l - H H F l , and pGP73 contains HHT2-HHF2. A 5.4-kb BamHI- Hind111 fragment of pMS191 (SMITH and MURRAY 1983), which contains H H T l - H H F l , was subcloned into the BamHI-Hind111 sites of pRS305, creating pGP156, which was used to show that bur5-1 is linked to H H T l - H H F l . For subcloning analysis of the BUR5 complementing activity, the following plasmids were constructed: pGP147 contains a 6.0-kb SphI fragment of pGP74 in the SphI site of YCp50; pGP150 contains a 3.0-kb PvuII-KpnI fragment of pGP147 in the KpnI-SmaI sites of pRS416; pGPl 53 contains a 1.6 kb SphI-ClaI fragment of pGP147 in the SphI-ClaI sites of YCp50; and pGPl54 contains a 1.2-kb EagI-KpnI fragment of pGP14'1 in the Eagl-KpnI sites of pRS416.
Sequencing the burs-1 mutation: T o sequence BURS+ and bur5-I, the wild-type and mutant genes were first am- plified by the polymerase chain reaction (PCR). To do this, an FY 12 1 (BUR5+) or GY56 (bur5-1) yeast colony was added to a 10-pl PCR reaction that contained 10 mM Tris pH 8.3, 50 mM KCI, 2.5 mM MgC12, 170 pg/ml BSA, 200 PM each dNTP, 2.5 units Taq polymerase, and 300 ng of each primer. Reaction conditions were 94" for 3 min, 35 cycles of (94" for 15 sec, 55" for 15 sec, 72" for 60 sec), followed by 70" for 5 min in a Perkin Elmer 9600 cycler. The 570- bp amplified fragments were purified by electroelution and directly sequenced using the Circumvent Version 2.0 kit
(New England Biolabs). We identified two differences be- tween our sequence of wild-type H H T l and the H H T l Genbank sequence, a C to T change at nt 535, and a G to A change at nt 478; these are each silent changes that are in both BUR5+ and bur5-1.
RESULTS
Isolation and genetic analysis of mutations that suppress suc2AUAS Selection for mutations that can suppress a deletion of t h e SUC2 UAS used strains FY121 and FY198, which contain the
Suppressors of a UAS Deletion 669
TABLE 2
Classification of suc2AUAS suppressors
TABLE 3
Phenotypes of bur mutations
Phenotypes
Class of mutation isolates
No. of
Dominant mutations Previously identified genes
HTAl-HTBl SPTlO SPT6 SPTl6 SPT21 BUR genes
BUR 1 BUR2 BUR3 BUR5 BUR6
10
144 7 0 35 2 1
-Genetic characterization of Suc+ revertants of suc2AUAS. 269
revertants of suc2AUAS were isolated and analyzed as described in the text. The ability of diploid strains to grow on YPSuc plates was used to determine both dominance and complementation as sum- marized here. The mutation originally designated b u d 1 was shown to be a mutation in SPT21.
starting strains for the mutant hunt also contained the his4-9126 and lys2-1286 insertion mutations. These insertion mutations cause His- and Lys- phenotypes that can be suppressed by most spt mutations, and therefore allow easy determination of the Spt pheno- type of any mutants isolated. T o select Suc+ rever- tants, FY121 and FY198 colonies were spread onto YPSuc plates, mutagenized with ultraviolet light, and 269 independent
Sue+
colonies were chosen for ge- netic analysis.T o test for dominance, each revertant strain was crossed by a suc2AUAS strain of the opposite mating type and diploid colonies were selected. Analysis of the SUC phenotype of these diploids showed that 259 mutations are recessive and 10 mutations are domi- nant to wild type. Although the dominant mutations may be very interesting, the remainder of this report will concentrate on the analysis of the recessive mu- tations. T o test whether any recessive mutations might be in known SPT genes, the 259 strains containing recessive mutations were crossed by five strains that contained suc2AUAS in combination with mutations in HTAl-HTBl, SPT5, SPT6, SPTlO or SPTl6, and scored for possible complementation of the Suc+ phe- notype. T h e results of this analysis (Table
2)
demon- strated that the majority of the revertants fail to complement mutations in previously identified SPT genes, with most of these mutations being in the HTAl-HTBl complementation groups. A later set of tests showed that one mutant, initially designated bur4-1, contained a mutation in SPT21 (NATSOULIS et al. 1991). Seven of the recessive mutations, however, were not in known SPT genes. We have designated the genes containing these mutations BUR, for Bypass UAS Requirement.Genotype SPt Ts Gal Ino
BUR+ burl-1 burl-2 bur2-1 bur2-2 bur3-1 bur5-1 bur6-1
+
+
+
+
+
+
+
+
+
+
-
-
-
-
-
-
-I++
+
-I++
+
+
+
Phenotypes of the seven recessive bur mutants. Strains containing each of the seven recessive bur mutations were tested for unselected phenotypes, including suppression of b insertion mutations at HIS4 and LYS2 (Spt phenotype), growth on YPD at 37" (Ts phenotype), growth on YPgalactose plates (Gal phenotype), and growth on plates that lack inositol (Ino phenotype). Wild-type growth is indicated by
+,
strong mutant phenotypes are indicated by -, and weak mutant phenotypes are indicated by-/+.
For the Spt phenotype, the -/+designation indicates that the bur mutation suppresses his4-9128 but not lys2-1286.
To determine the number of genes that are repre- sented by the seven bur mutations, all seven mutations were crossed into both mating types, mated with each other in all pairwise combinations, and the resulting diploids were scored for complementation of the SUC+ phenotype. This analysis revealed that the seven re- cessive mutations comprise five complementation groups: there were two alleles of burl, two alleles of bur2, and one allele each of bur3, bur5 and bur6. Crosses of bur suc2AUAS by BUR+ SUC2+ yielded parental ditype: nonparental ditype: tetratype tetrads in approximate 1 : 1:4 ratios, indicating that each of the BUR genes is unlinked to SUC2. A combination of linkage analysis, genetic mapping, and complementa- tion tests with the cloned BUR genes (see below) indicate that the five bur complementation groups represent five different genes.
bur mutations cause pleiotropic phenotypes: Since we anticipated that bur mutations might cause general effects on transcription, the bur mutants were tested for other phenotypes. As summarized in Table 3, all of the bur strains displayed various combinations of Spt-, Ts-, Gal- and Ino- phenotypes, showing that the bur mutations are not specific for SUC2, nor are they specific for affecting promoters lacking a UAS. For each mutant, analysis of at least 15 tetrads indi- cated that all phenotypes are caused by a mutation in a single nuclear gene. Examples of some of these phenotypes are shown in Figure 1.
bur mutations increase transcription at suc2AUAS:
670 G . Prelich a n d F. Winston
YPD 30' YPsucrose
YPD 37' -Lysine -Inositol
TABLE 4
Suppression of suc2AUAS by bur mutations
Invertase units
Genotype Repressed Derepressed
s u c 2 + < I 121 f 1 1
suc2AUAS BUR+ 2 2 0.2 2 f 0.3
suc2AUAS burl-I 1 6 2 2 2 0 2 1
suc2AUAS bur2-1 1 1 f 2 1 2 2 2
suc2AUAS bur3-I 6 f 1 8 f 1 suc2AUAS bur5-1 5 + 1 1 0 2 1
suc2AUAS bur6-I 8 2 1 1 1 f 1
S U C ~ A U A S b u r l - 2 9 + 3 4 + 1
SUCPAUAS bur2-2 1 4 2 1 1 8 + 2
The bur mutations cause increased invertaseactivity in suc2AUAS strains. Strains containing suc2AUAS and the seven bur mutations were grown under both repressing (2% glucose) and derepressing (0.05% glucose) conditions for SUC2 expression. Cells were har- vested and assayed for invertase activity as described previously (HIRSCHHORN et al. 1992). Invertase unitsare micromolesof glucose released per minute per 100 mg of cells. At least two different bur mutant strains were used for each allele, and the invertase activity reported is the average and standard error for at least three assays.
strated that invertase levels are increased up to 10- fold in bur suc2AUAS strains when compared with
BUR+ suc2AUAS strains. Furthermore, a comparison of invertase levels under repressing and derepressing conditions indicated that suc2AUAS expression is rel- atively independent of glucose repression in bur mu- tants. This result was not very surprising, since glucose repression of SUC2 requires the SUC2 UAS (SAROKIN
and CARLSON 1985), and suppression of suc2AUAS by
spt5 and sptb/ssn20 mutations also shows little effect of glucose repression (NEIGEBORN, CELENZA and CARLSON 1987; SWANSON and WINSTON 1992)
T o test whether the increased invertase activity caused by the bur mutations affects transcription of
S U C ~ A U A S , primer extension analysis was performed
FIGURE 1 .-Phenotypes of bur mutants. Patches of the bur mutants and two control strains were grown on YPD plates at 30" and then were replica plated to test for growth on the indicated plates. Photographs were taken after 2 days of growth. The control strain labeled WT is wild type for all phenotypes tested, while the control strain labeled BUR+ is one of the starting strains for the Bur- selection, and therefore contains the his4-9126 lys2-1286 and suc2AUAS alleles. The bur mutant strains (indicated by their allele num- bers in the squares) also contain the his4-9126 lys2- 1286 and suc2AUAS alleles. The strains shown are: (WT) FYI. (BUR+) FY121. ( b u r l - I ) GY114, ( b u r l - 2 ) GYIOI, (bur2-I) GY103, (bur2-2) GY177, (bur3-I) GY91. ( b u d - 1 ) GY93, (bur5-I) GYIIO, (bur6-I) GY 162. Subsequent work showed that bur4-1 is an allele of SPT21 (see MATERIALS and METHODS AND
RESULTS).
+
9 sucZAUAS u .
r
-.
"SUCZa e
I
-
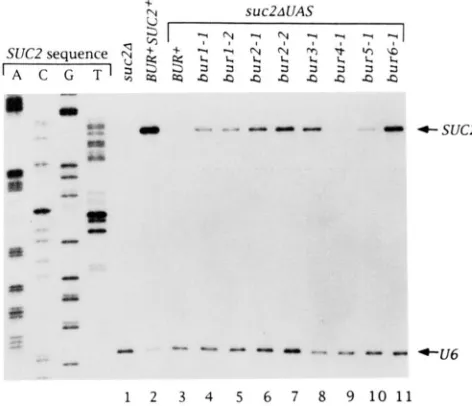
*U6 1 2 3 4 5 6 7 8 9 1 0 1 1FIGURE 2.-The bur mutations suppress suc2AUAS at the tran- scriptional level. Strains containing bur mutations in combination with suc2AUAS were grown under derepressing conditions for SUC2 transcription, total R N A was prepared, and 20 r g of R N A from each sample was used for primer extension analysis. Two primers were used for each reaction: suc2-3, which is specific for SUC2 RNA, and U6.48-72, which is specific for the polII1-dependent U6 RNA. The positions of the wild-type SUC2 and U6 initiation sites are indicated to the right of the figure. A SUC2 sequencing ladder using the suc2-3 primer was used to determine the initiation site for SUC2 and the size for the U6 transcript. Note that strain DBY2448 (lane 1) contains a deletion of the SUC2 coding region. to show the specificity of the suc2-3 primer, while the strains used in lanes 3-1 1 contain a deletion of only the SUC2 UAS. The strains used are: (1) DBY2448, (2) FY143. (3) FY198, (4) GY167, ( 5 )
GY169, (6) GY102, (7) GY60, (8) CY89, (9) GY94. (10) GY119, (1 1) GY96.
on RNA prepared from derepressed bur suc2AUAS
Suppressors of a UAS Deletion 67 1
TABLE 5
Analysis of bur snf double mutants
Growth on sucrose
snfl snfs
BUR+
-
-
burl
-
+
bur2
-
+
bur3
-
bur5
-
+
bur6
-
-
-
Some bur mutations can suppress the requirement for SNF5 for growth on sucrose. Double mutant bur snflAl0 and bur snf5-5 strains were constructed, and growth of these strains was tested on YPsucrose and YPraffinose plates. Strong suppression, assessed by growth on both types of plates, is indicated by
+.
Strains marked by-
are unable to grow on either YPsucrose or YPraftinose.only a weak effect in this assay). In addition, the SUC2 transcripts in bur suc2AUAS strains initiate at the normal SUC2 1.9-kb mRNA start site. These results suggest that the BUR genes negatively regulate tran- scription from the SUC2 basal promoter. T h e low level of the unregulated 1.8-kb SUC2 mRNAs (CARL- SON and BOTSTEIN 1982) was not affected by the bur mutations (data not shown). In a SUC2+ background the bur mutations caused a low level of SUC2 transcrip tion under repressing conditions, and little or no difference under derepressing conditions (data not shown).
Certain bur mutations suppress transcriptional defects caused by a snf5 mutation: In addition to the UAS requirement, SUC2 transcription is dependent on several trans-acting factors encoded by the SNF and SWZ genes (CARLSON, OSMOND and BOTSTEIN
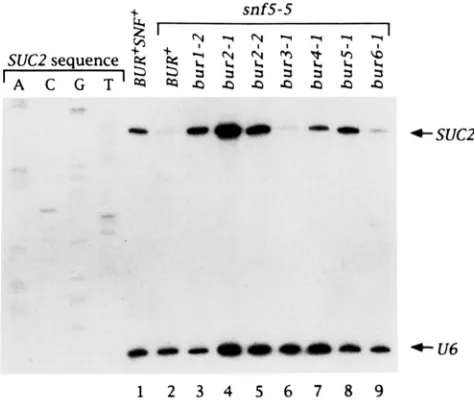
198 1 ; NEICEBORN and CARLSON 1984; VALLIER and CARLSON 1991 ; PETERSON and HERSKOWITZ 1992). There are two classes of SNFISWZ genes required for SUC2 transcription. One contains SNFl and SNF4, and the other contains SNF2/SWZ2, SNF5, SNF6, SWZl and SWZ3. T o test whether bur mutations sup- press the requirement for the SNF gene products, bur snflA and bur snf5A double mutant strains were con- structed and analyzed for growth on YPSuc (Table 5). Based on this analysis, we conclude that snf5 is suppressed by burl, bur2, bur4/spt21 (weakly) and bur5, but not by bur3 or bur6. In contrast, snfl is not suppressed by any of the bur mutations. T o test whether the suppression of snf5 is due to transcrip- tional effects, primer extension analysis was per- formed. These results (Figure 3) correlate with the growth on sucrose: for burl, bur2, bur4/spt21 and bur5 mutants, suppression of snf5 corresponds to an increase in SUC2 mRNA levels, while for bur3 and bur6 there is no increase.
T h e SNF5 protein is required for transcription of many diversely regulated yeast genes besides SUC2, including T y elements, PH05, HO and IN01 (NEIGE-
-
-
0-
-
-
C S U C 2"-0000" +U6
1 2 3 4 5 6 7 8 9
FIGURE 3.-Some bur mutations can suppress the requirement
for SNF5 at SUC2. Strains containing the indicated bursnf5-5 double mutant combinations were grown under derepressing conditions
for SUC2 transcription, R N A was prepared from those strains, and 20 pg of total R N A was used for primer extension analysis using primers suc2-3 and U6.48-72. The positions of the SUC2 and U6 initiation sites are shown. The strains used are: (1) FY143, (2) GY201,(3)GY152,(4)GY155,(5)GY157,(6)GY234,(7)GY158,
(8) GY203, (9) GY238.
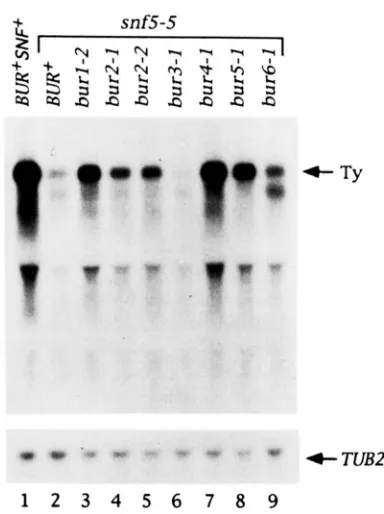
BORN and CARLSON 1984; HAPPEL, SWANSON and WINSTON 1991 ; HIRSCHHORN et al. 1992; PETERSON and HERSKOWITZ 1992). Since burl, bur2, bur4 and bur5 mutations suppress the snf5 defect for SUC2 transcription, we determined whether bur mutations also suppress the snf5 defect for T y transcription. Northern blot analysis (Figure 4) shows that burl, bur2, bur4/spt21 and bur5 mutations suppress the requirement for SNF5 at T y . In this case, bur6 shows weak suppression, while bur3 still shows no suppres- sion of snf5. Thus, the bur mutations that suppress snf5 can do so at multiple SNF5-dependent pro- moters.
672 G . Prelich and F. Winston
t'
P
o
t."
1 2 3 4 5 6 7 0 9
FIGURE 4."Some bur mutations can suppress the requirement for SNF5 for Ty transcription. Strains containing the bur snf5-5
double mutant combinations indicated at the top of the figure were grown in YPD media, RNA was prepared from those cells, and 10 r g of total RNA was subjected to electrophoresis through a 1% formaldehyde agarose gel. The R N A was transferred to Gene- Screen and first hybridized to a probe specific for TUB2 RNA to show that equivalent amounts of RNA were loaded in each lane. The TUB2 probe was stripped off the filter, and the filter was then reprobed with an internal T y l fragment. Some cross hybridization with rRNA can be seen below the Ty-specific band. The positions of full length T y and TUB2 RNA are indicated by arrows on the right. The strains used were (1) FY143, (2) GY201, (3) GY152, (4)
GY 155, (5) GY 157, (6) GY234, (7) GY158, (8) GY203, (9) GY238.
cating that the insert directed integration to the BURl
locus.
T o determine the map position of BUR1, two types of analyses were performed. First, the pGP59 insert was hybridized to a set of overlapping ordered yeast genomic DNA clones, which indicated that BURl
maps distal to SUP16 on the right arm of chromosome 16 (L. RILES and M. OLSON, unpublished data). Sec- ond, this map position was confirmed by meiotic map- ping (Table 6), which demonstrated that burl is linked to cdc67 and aro7, in the order aro7-cdc67-burl. This order was determined by examining recombinants between burl and cdc67. In those 48 recombinant tetrads, aro7 had also recombined with burl 41 times, while aro7 and cdc67 had only recombined in 16 of those tetrads.
To determine the phenotype caused by a burl null mutation, the burlA1::TRPl allele was integrated into a diploid strain and tetrads were dissected. Viability segregated 2:2 in 18 of 20 tetrads and 1 :3 in the other two, and all viable spores were Trp- Bur+. These results demonstrate that BURl is essential for viability. Sequence analysis of 1.9 kb of BURl revealed ex-
TABLE 6
Mapping of burl by tetrad analysis
Segregating markers PD NPD TT cM
aro7-cdc67 43 1 25 22.5 aro7-burl 16 4 51 52.8 cdc67-burl 18 1 49 40.4
Genetic mapping of burl. Tetrads were dissected from a cross of 0 6 5 3 (aro7 cdc67-I lys2-1286) X GY99 (burl-2 lys2-1286). aro7 was scored on SC-tyr-phe dropout plates, cdc67-I was scored for temperature sensitive growth on YPD at 37". and burl-2 was scored on SC-lys dropout plates. The burl-2 mutation does not cause a Ts- phenotype; therefore scoring cdc67 for Ts- was unambiguous. PD, parental ditype; NPD, nonparental ditype; TT, tetratype; cM, centimorgans.
tensive homology to the CDC28 family of protein kinases, and showed that BURl is identical to a previ- ously identified gene, SGVl (IRIE et al. 1991). Our
BUR1 sequence corresponds to bases -36 1 to
+
1542 of the published SGVl sequence (data not shown).SGVl was originally identified by mutations that sup- pressed the g p ~ l " ~ ' ~ ' allele, in an attempt to identify genes that were required for recovery from a-factor mating arrest. In that study, SGVl was shown to be essential for viability, displayed 42% DNA sequence identity to CDC28, and hybridized to chromosome 16, all consistent with our results. T h e identity of BURl
and SGVl suggests that this protein plays a pleiotropic role in gene expression.
BUR5 encodes histone H3: Since the bur5-1 muta- tion suppresses snf5 defects and causes Spt- pheno- types, we began a molecular analysis of BURS. The
BUR5 gene was cloned by transforming a YCp50- based yeast genomic DNA library into a bur5-1 uru3- 52 strain and screening for complementation of the
bur5-1 Spt- phenotype. T w o plasmids (pGP73 and pGP74) were obtained, and surprisingly the inserts of these two plasmids were shown to have completely different restriction maps. Hybridization of these two plasmids to filters containing an ordered array of X
clones of yeast genomic DNA (L. RILES and M. OLSON, unpublished data), combined with DNA sequence analysis, demonstrated that each plasmid contains one of the two histone H3-H4 loci: pGP74 contains H H T I - H H F I , while pGP73 contains HHT2-HHF2.
Several steps were taken to determine whether
Suppressors of a UAS Deletion 673
pCP147
pCPl5 3 pGP154
pCPl50
PVUII ClaI KpnI SmaI SphI
I ,I I
4
c
3
+
PPAl HHTl HHFl
-
0.5 kbH H T l - H H F l . T o confirm this possibility, a LEU2 H H T l - H H F l plasmid (pGP156) that had been line- arized with SmaI in the H H T l - H H F l intergenic region was integrated into a leu2 BURS+ strain. Two different Leu+ integrants were each crossed by a leu2 bur5-1
strain and tetrads were dissected. All viable spores from 40 tetrads were either Leu+ Bur+ or Leu- Bur-, demonstrating that BUR5 is tightly linked to H H T l - H H F l . Next, to determine whether the bur5-1 muta- tion was in H H T l or H H F l , subcloning analysis of the original pGP74 complementing plasmid was per- formed. T h e results (Figure 5) indicated that the bur5- 1 mutation is in H H T l , since a fragment that contains histone H 3 coding sequences, but not the histone H4 coding region, was able to fully complement the b u r 5 1 mutation. Finally, to determine the site of the mu- tation in bur5-1, oligonucleotide primers specific for
H H T l were used to amplify the BURS+ and bur5-I
coding regions. Direct sequencing of these PCR prod- ucts indicated that there is a single G to A missense mutation in bur5-I, causing a T h r to Ile substitution at amino acid 119 of histone H3.
DISCUSSION
In this work we have selected mutations that cause increased transcription in the absence of an upstream activating sequence. As expected, we have recovered many new alleles of previously identified SPT genes, but we have also identified a,set of genes (BUR genes) that have multiple effects on transcription. In addition to suppressing SUCPAUAS, these mutations result in pleiotropic phenotypes, and some bur mutations sup- press the requirement for SNF5 at two different pro- moters. Since the bur mutations that we have analyzed are recessive, result in increased transcription in both
suc2AUAS and sn.5 strains, and have widespread ef- fects, the BUR genes appear to encode general repres- sors of transcription. This idea is supported by the identification of BUR5 as one of the two genes encod- ing histone H3, since nucleosomes are repressors of transcription initiation both in vitro and in vivo (GRUN- STEIN 1990; FELSENFELD 1992).
comalementatlrm
+
FIGURE 5.”Subcloning analysis of the BUR5 complementing activity. Plasmids containing the four indicated subclones of the original pGP74BUR5+ clone were transformed into CY56 (bur5-1
+
his4-9126 lys2-1286 suc2AUAS u r d - 5 2 ) and the transformants were scored for Bur and Spt phe--
notypes on SC-Ura sucrose, SC-Ura-His and SC- Ura-Lys plates. Plasmids that complemented the+
bur5-I mutation are indicated by a (+) at the rightof the figure. The coding regions for P P A l , H H T l and HHFl are indicated by the open arrows.
T h e ability of some, but not all bur mutations to suppress the requirement for SNF5 function at SUC2
and T y suggests that the bur mutations affect tran- scription through two different mechanisms. Since
BUR5 encodes histone H3, and bur5, burl and bur2
mutations suppress a snf5 mutation, BUR1 and BUR2 may also repress transcription through chromatin ef- fects. This idea is supported by recent data that im- plicates SNF5 in activation via alterations in chroma- tin structure (HIRSCHHORN et al. 1992). In contrast, since bur3 and bur6 mutations do not suppress snf5
defects, BUR3 and BUR6 may repress suc2AUAS via a chromatin-independent mechanism. For example, they may repress the general transcription factors directly, analogous to the N C l , NC2 and DR1 pro- teins that have been identified as repressors of TBP activity in vitro (MEISTERERNST and ROEDER 199 1 ;
MEISTERERNST et al. 199 1 ; INOSTROZA et al. 1992). Of course, since we have only a single allele each of bur3
and bur6, the inability of these mutations to suppress
snf5 may be an allele-specific effect.
Although the suc2AUAS allele used in this study removes more than 1.5 kb of the SUCZ UAS region, 350 bp of the SUC2 promoter still remain intact. It is not yet known whether the increased transcription observed at suc2AUAS in bur strains is still dependent on the T A T A box, or whether any other elements within these 350 base pairs of the promoter are also required.
T h e bur mutations described here are part of a larger group of mutations that suppress UAS dele- tions. Previous studies have shown that mutations in
SPT5 (SWANSON and WINSTON 1992), SPT6 (NEIGE-
BORN, CELENZA and CARLSON 1987; CLARK-ADAMS and WINSTON 1987), SPTI6 (MALONE et al. 1991),
SPTlO, HTAl-HTBl (C. DOLLARD, J. HIRSCHHORN
674 G . Prelich and F. Winston
by the bur selection indicates that this selection is still not saturated. This raises the question as to why mutations in so many genes confer this phenotype. Since the histone mutations establish a clear involve- ment of chromatin, it is possible that many genes are required for nucleosome function, either by affecting the expression, assembly, positioning or modification of histones. As described above, chromatin-independ- ent pathways that involve the general transcription factors may also be involved. It is also tempting to speculate that the BUR/SPT/SZN genes in yeast may be functionally analogous to the polycomb group of genes in Drosophila, which are repressors of homeotic gene transcription. Both sets of genes identify a large number of repressors that are thought to function primarily through chromatin effects, and display in- teractions with a large group of transcriptional acti- vators that in yeast includes SNF2/SWZ2, and in Dro- sophila includes brahma, a SNF2 homologue (see WIN- STON and CARLSON 1992; PARO 1990 for reviews). Clearly, continued studies of the few proteins in the BUR/SPT/SIN group with known biochemical activi- ties, such as BURl, will be important to understand how they affect transcription.
BURlISGVl has now been identified by two unre- lated selections, first as a suppressor of the gpalva150
mutation, in an attempt to identify genes involved in recovery from mating factor arrest, and here as a mutation that causes increased transcription in the absence of the SUC2 UAS. If BURl/SGVl functions by affecting transcription directly, then the pleiotropic effects may result either because BURl/SGVl has many substrates that each affect transcription of spe- cific genes, or it may phosphorylate one key substrate that has general effects on transcription. Obvious potential candidates for BURl /SGV1 substrates that have widespread effects on transcription include his- tones and the largest subunit of RNA polymerase 11, which is encoded by RPBl (NONET and YOUNG 1989). Both RPBl and histones are phosphorylated in vivo,
and mutation or altered expression of the genes that encode H2A, H2B, H3, H4 and RPBl each cause similar phenotypes as burl mutations. Furthermore, RPBl is a substrate for other CDC28-related protein kinases both in vivo and in vitro (LEE and GREENLEAF
1989; CISEK and CORDEN 1989; FEAVER et al. 1991; Lu et al. 1992).
The bur5-1 allele appears to be a gain-of-function mutation in H H T l . This hypothesis is supported by several results. First, a deletion of the H H T l - H H F l
locus does not cause an Spt- phenotype (CLARK-AD- AMS et al. 1988), yet the buri-1 mutation does, indi- cating that bur5-1 is not a loss-of-function allele. Sec- ond, haploid bur5-1 strains have mutant phenotypes even though there is a second H3-encoding gene present. However, bur5-1 is recessive in diploids, in-
dicating that the relative level of mutant us. wild-type histone H3 is critical in determining the Bur pheno- type. Finally, the bur5-l mutation causes a Thr to Ile change at amino acid 1 19, in a loop between two a
helices near the carboxyl terminus of histone H3 (ARENTS et al. 199 1). The importance of this region was confirmed by an independent genetic study which revealed that SIN2 is identical to HHTIIBURS, and that mutations in H H T l or HHTZ that confer a Sin- phenotype cause amino acid substitutions in the same region of histone H3, including one mutation in
HHT2 that causes the identical amino acid change as
bur5-1 (W. KRUGER, C. PETERSON, A. SIL, C. COBURN, E. MOUDRIANAKIS and I. HERSKOWITZ, unpublished data). Mutations in SIN2 were identified as suppres- sors of mutations in SWZl, SWZ2/SNF2 and SWZ3
(STERNBERC et al. 1987), which encode proteins that have been proposed to be components of a multipro- tein complex with SNF5 and SNF6 (LAURENT, TREI- TEL and CARLSON 1991; PETERSON and HERSKOWITZ
1992; LAURENT and CARLSON 1992). Further detailed analysis of the location of this loop within the octamer structure should reveal whether Thr 1 19 contacts other histones, DNA or other cellular proteins.
In conclusion, our studies have identified recessive mutations in five genes that suppress a deletion of the
SUC2 UAS. The pleiotropic phenotypes caused by these mutations suggest that they have general and important roles in transcription initiation. The iden- tity of one of these genes as encoding histone H3 further strengthens the evidence that chromatin struc- ture plays a central role in gene expression. The identification of BURl/SGVl can lead to direct bio- chemical tests of the role of this essential kinase in transcription initiation. Finally, further biochemical and genetic studies of BURllSGVI and BUR5IHHTI
should help to determine the function of the other
BUR genes.
We thank M. CARLSON, G. JOHNSTON and M. M. SMITH for plasmids and yeast strains, L. RILES for assistance with the physical mapping studies, I . HERSKOWITZ, E. MOUDRIANAKIS, W. KRUGER, C. PETERSON, A. SIL and C. COBURN for communicating unpub- lished results, and A. BORTVIN, L. GANSHEROFF, J. HIRSCHHORN and L. Wu for helpful comments on the manuscript. This work was supported by grant GM13019 from the National Institutes of Health to G.P., and grant GM32967 from the National Institutes of Health to F.W.
LITERATURE CITED
ABRAMS, E., L. NEIGEBORN and M. CARLSON, 1986 Molecular analysis of S N F 2 and SNFS, genes required for expression of glucose-repressible genes in Saccharomyces c e m i s i u e . Mol. Cell. Biol. 6: 3643-3651.
ARENTS, G., R. W. BURLINGAME, B.-C. WANG, W. E. LOVE and E.
BROW, D. A., and C. GUTHRIE 1988 Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature 334:
CARLSON, M. and D. BOTSTEIN, 1982 Two differentially regulated mRNAs with different 5’ ends encode secreted and intracel- lular forms of yeast invertase. Cell 28: 145-154.
CARLSON, M., B. C. OSMOND and D. BOTSTEIN, 1981 Mutants of yeast defective in sucrose utilization. Genetics 98: 25-40. CARLSON, M., R. TAUSSIC, S. K u s ~ u and D. BOTSTEIN, 1983 The
secreted form of invertase in Saccharomyces cerevisiae is synthe- sized from mRNA encoding a signal sequence. Mol. Cell. Biol.
3: 439-447.
CELENZA, J. L. and M. CARLSON, 1989 Mutational analysis of the Saccharomyces cerevisiae SNFl protein kinase and evidence for functional interaction with the SNF4 protein. Mol. Cell. Biol.
CHALEFF, D. T., and G. R. FINK, 1980 Genetic events associated with an insertion mutation in yeast. Cell 21: 227-237. CISEK, L. J.. and J. L. CORDEN, 1989 Phosphorylation of RNA
polymerase by the murine homologue of the cell-cycle control protein cdc2. Nature 3 3 9 679-684.
CLARK-ADAMS, C. D., and F. WINSTON, 1987 The SPT6 gene is essential for growth and is required for delta-mediated tran- scription in Saccharomyces cerevisiae. Mol. Cell. Biol. 7: 679- 686.
CLARK-ADAMS, C. D., D. NORRIS, M. A. OSLEY, J. S. FASSLER and F. WINSTON, 1988 Changes in histone gene dosage alter transcription in yeast. Genes Dev. 2: 150-159.
CONAWAY, J. W., and R. C. CONAWAY, 1991 Initiation of eukar- yotic messenger RNA synthesis. J. Biol. Chem. 2 6 6 17721-
17724.
CROSTON, G. E., L. A. KERRICAN, L. M. LIRA, D. R. MARSHAK and J. T. KADONACA, 1991 Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase I1 transcription. Science 251: 643-649.
DAVIS, J. L., R. KUNISAWA and J. THORNER, 1992 A presumptive helicase (MOTZ gene product) affects gene expression and is required for viability in the yeast Saccharomyces cereuisiae. Mol. Cell. Biol. 12: 1879-1892.
EISENMANN, D. M., C. DOLLARD and F. WINSTON, 1989 S P T I 5 , the gene encoding the yeast TATA binding factor TFIID, is required for normal transcription initiation in vivo. Cell 58:
FARABAUCH, P. J., and G. R. FINK, 1980 Insertion of the eukary- otic transposable element TyZ creates a 5-base pair duplication. Nature 2 8 6 352-356.
FASSLER, J. S., and F. WINSTON, 1988 Isolation and analysis of a novel class of suppressor of T y insertion mutations in Saccha- romyces cerevisiae. Genetics 118: 203-21 2.
FEAVER, W. J., 0. GILEADI, Y. LI and R. D. KORNBERG, 199 1 CTD kinase associated with yeast RNA polymerase I1 initiation factor b. Cell 67: 1223-1230.
FELSENFELD, G., 1992 Chromatin as an essential part of the tran- scriptional mechanism. Nature 3 5 5 2 19-224.
GRUNSTEIN, M., 1990 Histone function in transcription. Ann. Rev. Cell Biol. 6: 643-678.
GUARENTE, L., 1988 UASs and enhancers: common mechanism of transcriptional activation in yeast and mammals. Cell 52:
HAN, M. and M. GRUNSTEIN, 1988 Nucleosome loss activates yeast downstream promoters in vivo. Cell 5 5 1137-1 145.
HAPPEL, A. M., M. S. SWANSON and F. WINSTON, 1991 TheSNF2, SNF5 and SNF6 genes are required for T y transcription in Saccharomyces cerevisiae. Genetics 128: 69-77.
HIRSCHHORN, J. N., S. A. BROWN, C. D. CLARK and F. WINSTON, 1992 Evidence that SNF2/SWI2 and SNF5 activate transcrip tion in yeast by altering chromatin structure. Genes Dev. 6:
2288-2298. 213-218.
9 5034-5044.
1183-1191.
303-305.
INOSTROZA, J. A., F. H. MERMELSTEIN, I. HA, W. S. LANE and D. REINBERG, 1992 D r l , a TATA-binding protein-associated phosphoprotein and inhibitor of class I1 gene transcription. Cell 7 0 477-489.
IRIE, K., S. NOMOTO, I. MIYAJIMA and K. MATSUMOTO, 1991
SGVZ encodes a CDC28/cdc2-related kinase required for a Ga subunit-mediated adaptive response to pheromone in S. cerevis- iae. Cell 65: 785-795.
ITO, H., K. FUKUDA, K. MURATA and A. KIMURA, 1983 Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153: 163-168.
JIANG, Y. W., and D. J. STILLMAN, 1992 Involvement of the SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol. Cell. Biol. 12: 4503-4514. KNEZETIC, J. A,, and D. S. LUSE, 1986 The presence of nucleo-
somes on a DNA template prevents initiation by RNA polym- erase I1 in vitro. Cell 45: 95-104.
LAURENT, B. C., and M. CARLSON, 1992 Yeast SNF2/SWI2, SNF5, and SNFG proteins function coordinately with the gene- specific transcriptional activators GAL4 and Bicoid. Genes Dev.
LAURENT, B. C., M. A. TREITEL and M. CARLSON, 199 1 Functional interdependence of the yeast SNF2, SNF5, and SNFG proteins in transcriptional activation. Proc. Natl. Acad. Sci. USA 88: 2687-2691.
LAYBOURN, P. J., and J. T. KADONACA, 1991 Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase 11. Science 2 5 4 238-245.
LEE, J. M., and A. L. GREENLEAF, 1989 A protein kinase that phosphorylates the C-terminal repeat domain of the largest subunit of RNA polymerase 11. Proc. Natl. Acad. Sci. USA 8 6
LORCH, Y., J. W. LAPOINTE and R. D. KORNBERC, 1987 Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 4 9
LORCH, Y. J. W., LAPOINTE and R. KORNBERG, 1992 Initiation on chromatin templates in a yeast RNA polymerase I1 transcrip- tion system. Genes Dev. 6: 2282-2287.
Lu, H., L. ZAWEL, L. FISHER, J.-M. ECLY and D. REINBERG, 1992 Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase 11. Nature 358: 641-645.
MALONE, E. A., C. D. CLARK, A. CHIANC and F. WINSTON, 1991 Mutations in SPTZ6/CDC68 suppress cis- and trans- acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 5710-5717.
MEISTERERNST, M., and R. G. ROEDER, 1991 Family of proteins that interact with TFIID and regulate promoter activity. Cell 67: 557-567.
MEISTERERNST, M., A. L. ROY, H. M. LIEU and R. G. ROEDER, 1991 Activation of class I1 gene transcription by regulatory factors is potentiated by a novel activity. Cell 6 6 981-993. MITCHELL, P. J., and R. TJIAN, 1989 Transcriptional regulation
in mammalian cells by sequence-specific DNA binding proteins. Science 245: 371-378.
MORTIMER, R. K. and D. C. HAWTHORNE, 1969 Yeast genetics, pp. 385-460 in The Yeasts, Vol. 1, edited by A. H. ROSE and J.
S. HARRISON. Academic Press, New York.
NATSOULIS, G., C. DOLLARD, F. WINSTON and J. D. BOEKE, 1991 The products of the SPTZO and SPT2Z genes of Saccha- romyces cereuisiae increase the amplitude of transcriptional reg- ulation at a large number of unlinked loci. New Biol. 3: 1249- 1259.
NEIGEBORN, L. and M. CARLSON, 1984 Genes affecting the regu- lation of SUC2 gene expression by glucose repression in Saccha- romyces cerevisiae. Genetics 108: 845-858.
NEICEBORN, L., J. L. CELENZA and M. CARLSON, 1987 SSN20 is
6 1707-1715.
3624-3628.
676 G . Prelich and F. Winston
an essential gene with mutant alleles that suppress defects in SUCP transcription in Saccharomyces cereuisiae. Mol. Cell. Biol.
NONET, M. L. and R. A. YOUNG, 1989 Intragenic and extragenic suppressors of mutations in the heptapeptide repeat domain of Saccharomyces cereuisiae RNA polymerase 11. Genetics 123:
PARO, R. 1990 Imprinting a determined state into the chromatin of Drosofhila. Trends Genet. 6 4 16-42 1 .
PETERSON, C. L., and I. HERSKOWITZ, 1992 Characterization of the yeast SWIl, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell 68: 573-583.
ROSE, M. D., F. WINSTON and P. HIETER, 1990 Methods in Yeast Genetics: A Laboratoy Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
ROSE, M. D., P. NOVICK, J. H. THOMAS, D. BOTSTEIN and G. R. FINK, 1987 A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60:
SAROKIN, L., and M. CARLSON, 1984 Upstream region required for regulated expression of the glucose-repressible SUCZ gene of Saccharomyces cereuisiae. Mol. Cell. Biol. 4: 2750-2757.
SAROKIN, L. and M. CARLSON, 1985 Upstream region of the SUCZ gene confers regulated expression to a heterologous gene in Saccharomyces cereuisiae. Mol. Cell. Biol. 5: 2521-2526.
SHERMAN, F., G . R. FINK and C. W. LAWRENCE, 1978 Methods in Yeast Genetics, rev. ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
SIMCHEN, G., F. WINSTON, C. A. STYLES and G. R. FINK, 1984 Ty- mediated gene expression of the LYSZ and HIS4 genes of Saccharomyces cereuisiae is controlled by the same SPT genes. Proc. Natl. Acad. Sci. USA 81: 2431-2434.
SMITH, M. M., and K. K. MURRAY, 1983 Yeast H3 and H4 histone messenger RNAs are transcribed from two non-allelic gene sets. J. Mol. Biol. 1 6 9 641-661.
SOM, T., K. A. ARMSTRONG, F. C. VOLKERT and J. R. BROACH, 7: 672-678.
715-724.
237-243.
1988 Autoregulation of 2 micron circle gene expression pro- vides a model for maintenance of stable plasmid copy levels. Cell 52: 27-37.
STERNBERG, P. W., M. J. STERN, 1. CLARK and I. HERSKOWITZ,
1987 Activation of the yeast HO gene by release from multi- ple negative controls. Cell 48: 567-577.
SWANSON, M. S., E. A. MALONE and F. WINSTON, 1991 SPTS, an essential gene important for normal transcription in Saccharo- myces cereuisiae, encodes an acidic nuclear protein with a car- boxy-terminal repeat. Mol. Cell. Biol. 11: 3009-3019.
SWANSON, M. S., and F. WINSTON, 1992 SPT4, SPT5, and SPT6 interactions: effects on transcription and viability in Saccharo- myces cerevisiae. Genetics 132: 325-336.
VALLIER, L. G., and M. CARLSON, 1991 New SNF genes, GAL11 and G R R l affect SUCZ expression in Saccharomyces cerevisiae. Genetics 129: 675-684.
WINSTON, F., and M. CARLSON, 1992 Yeast SNF/SWI transcrip tional activators and the SPT/SIN chromatin connection. Trends Genet. 8: 387-391.
WINSTON, F., D. T . CHALEFF, B. VALENT and G. R. FINK,
1984 Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107: 179-197.
WINSTON, F., C. DOLLARD, E. A. MALONE, J. CLARE,^. G. KAPAKOS, P. FARABAUGH and P. L. MINEHART, 1987 Three genes are required for trans-activation of T y transcription in yeast. Ge- netics 115: 649-656.
WORKMAN, J. L. and R. E. KINGSTON, 1992 Nucleosome core displacement in vitro via a metastable transcription factor- nucleosome complex. Science 258: 1780-1 784.
WORKMAN, J. L., and R. G. ROEDER, 1987 Binding of transcrip- tion factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase 11. Cell 51: 613-622.
WORKMAN, J. L., I . C. TAYLOR and R. E. KINGSTON, 1991
Activation domains of stably bound GAL4 derivatives alleviate repression of promoters by nucleosomes. Cell 64: 533-544.