Copyright 0 1984 by the Genetics Society of America
T I M E OF RECOMBINATION IN T H E DROSOPHILA
MELANOGASTER OOCYTE. 111. SELECTION AND
CHARACTERIZATION
OF TEMPERATURE-SENSITIVE AND
-INSENSITIVE, RECOMBINATION-DEFICIENT ALLELES I N
DROSOPHILA
R. F. GRELL
Biology Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 Manuscript received October 24, 1983
Revised copy accepted June 5 , 1984
ABSTRACT
The procedure for the selection of a temperature-sensitive recombination mutant in Drosophila is described. Use of this procedure has led to the recov- ery of three alleles at a new recombination locus called rec-Z, located within the region of chromosome 3 circumscribed by Deficiency(3R)~bd'~~. One allele, rec-Z26, is temperature sensitive, and the other two alleles, rec-Z6 and rec-1I6, are temperature insensitive. Gene dosage studies reveal rec-lZ6 to be a leaky mutant with greater recombination activity in two doses than in one. The other two alleles show no dose response, implying that they may be null mutants. The temperature response curves of rec-1" as a homozygote and in heteroal- lelic combination with rec-Z " suggest that the sharp decrease in recombination between 28" and 31" indicates temperature denaturation of an enzyme or other protein specified by the mutant and associated with the recombination process. The ability of small changes in temperature to reverse or abolish polarity in recombination along the X chromosome arm in rec-ZZ6/rec-Zl6 fe- males brings into question the use of the "polarity" criterion to partition mu- tants into two functional types, i.e., precondition mutants that display polarity and exchange mutants that do not. Evidence that rec-1 may be part of a complex locus residing in a chromosome segment harboring a variety of re- combination-related genes is presented.
EMPERATURE-sensitive mutants are valuable tools for localizing the time
T
of gene expression. They have been used to determine the time of action of a particular gene, even when the specific nature of the gene product is unknown (EDGAR and EPSTEIN 1965). By applying the restrictive temperature at sequential time points during development, in temperature shift experi- ments, the period during which wild-type activity is blocked identifies the active phase of the gene product. T h e alteration in the biological activity of the protein at the restrictive temperature has been shown to be the consequence in most cases of a single amino acid substitution in a polypeptideUOCKUSCH
1966).In earlier studies with Drosophila, oocytes were exposed to elevated tem- perature at sequential time points during their development to mark the sen-
426 R. F . GRELL
sitive period for the induction and enhancement of recombination. T h e du- ration of sensitivity has been shown to be coextensive with the S-phase during premeiotic interphase ( G R E L L , 197 1 , 1973, 1978c; GRELL and DAY 1974). Availability of a temperature-sensitive (ts), recombination (rec) mutant could provide an independent indicator of the time of action of a gene product associated with the recombination process. A search, initiated in 1970, suc- ceeded in isolating a ts rec mutant 5 yr later. Subsequent studies demonstrated that the response of the mutant to the restrictive temperature coincided pre- cisely with the heat-sensitive period for enhancement of recombination in the normal genome (GRELL 1978a). T h e protocol for isolation of the mutants and the characterization of the three alleles recovered are described in this report.
MATERIALS AND METHODS
Strategyfor selection of a ts rec mutant: Isolation of ts rec mutant poses several problems. First, a rec mutant in Drosophila has no visible phenotype and recognition requires a genetic test. Second, most mutants are recessive so that approximately four generations are needed to recover the mutant as a homozygote before testing for expression. Following large numbers of treated chro- mosomes through five generations in order to score for the presence of a rec mutant is a forlniddbk and impracticable task.
An alternative approach has been adopted that permits mutant expression at an earlier gener- ation. This can be accomplished if the targeted region is made hemizygous through the use of a deficiency for that region in the homologous chromosome. T h e limitation of this method is the restriction of mutant detection to the small fraction of the genome delimited by the deficiency. Probability of success is increased if the region targeted for mutant induction is one known to carry o r suspected of carrying a rec gene.
I n the present study, the region chosen was defined by a deficiency in the right arm of chro- mosome 3, DJ3R)sbdlo5 (sbdlo5), which uncovers 2 4 bands. T h e region includes the locus of c(3)G
whose mutations abolish the synaptonemal complex and in so doing act indirectly to eliminate meiotic recombination. We desired a ts rec mutant that acts directly upon exchange and not upon synapsis as C(3)G does. Choice of the sbdlo5 deficiency was based on the following rationale. Homozygous mutants of c(3)C act as aniorphs. On this basis a heterozygous mutant and a heter- ozygous deficiency for the locus should be formally equivalent. Genetic results d o not support this conclusion. T h e genotype +/c(3)C shows enhanced recombination, whereas +/sbd IO5 significantly
reduces recombination (HINTON 1966). O n e explanation for the reduction could be the existence of a second rec locus within the 2 4 bands uncovered by the deficiency.
T h e protocol that was followed to isolate a ts rec mutant is diagrammed in Figure 1. Males homozygous for the eye mutant brown (bw) located at 104.5 near the tip of the right arm of Chromosome 2 and for the mutant b i t h ~ r a x ’ ~ “ ( b ~ ’ ~ ‘ ) at 58.8 on chromosome 3 were mutagenized within ethyl methanesulfonate (EMS) according to the procedure of LEWIS and BACHER (1968). T h e bx locus lies close to the distal boundary of sbdlo5, but not within it, and, therefore, serves to mark the inutagenized third chromosome. Treated males were mated to females carrying the eye mutant cinnabar (cn) (located near the centromere of 2R at 57.5) in the free second chromosome and sbdlo5 (located between 88F9-89.41 and 89B10-11 on BRIDGES’ (1941) revised salivary map of chromosonie 3) in the free third chromosome. Both chromosomes were balanced over the translocation T(2;?)Xa,cn2 which carries the conspicuous, dominant mutant upXo.
G2 virgin females were selected which carried the treated second and third chromosomes of paternal origin and the free second and third chromosomes of maternal origin so that cn and bw
are present in trans configuration on chromosome 2. T h e females were individually mated in vials to males homozygous for cn and bw on chromosonie 2 and for crossveinless-c (m-c,54.1) and stubloid* (sbd2,58.2) which lie just to the left of and within sbdlo5, respectively. Following eclosion of the Gs progeny, the population of each vial was inspected for recombinants between cn and bw,
-0
A TS REG MUTANT IN D. MELANOCASTER
-0
H
r-4 w
2 w U
427
-
m
In m
4.28 R. F. GRELL
(+ +/cn bw) eyes in contrast to the noncrossover “bw” (+ bw/cn bw) and “cn” (cn +/cn bw) classes and indicate that a mutant affecting recombination has not been induced in the targeted region. Vials yielding predominantly or solely parental classes indicate that a mutant has probably been induced.
Stocks of putative rec mutants were constructed by selecting from vials showing no o r few recombinants, those bw-eyed males that carried the treated third chromosome over the tester third chromosome (*sbd bxs4“/cv-c sbd, where
*
indicates the treated third chromosome) and were“sbd+” in contrast to ~ b d ’ ~ ~ / c v - c sbd‘ males which were “sbd.” T h e G S “bw;sbd+” males were mated to females carrying bw homozygously and the multiply inverted third chromosome balancer,
In(3LR)TM3,Sb bxs4‘Ser, heterozygously, so as to produce in G4, males and females carrying the putative rec mutant balanced over T M 3 , bxS4‘. They appeared “bx’”” in phenotype and provided a balanced stock in Gs. At the same time G3 “bw;sbd+” males were mated in duplicate sets to females of the GI genotype to test for temperature sensitivity at 21
Genetic studies: Complementation tests for allelism with c(3)G were carried out for each rec
mutant by crossing b ~ ~ ~ ‘ / T M 3 , b x ~ ~ ‘ virgin females to males carrying an X chromosome marked with y2 sc v f.y+ and third chromosomes homozygous for c(3)G”. Crossing over was measured among the male progeny of FI y2 sc v f.y+/+; * ~ X ~ ~ ’ / C ( ~ ) G ’ ~ females. Absence of crossing over indicated allelism with c(3)GI7 and not the desired new rec locus.
Mutants shown by complementation tests to represent a new locus were studied for their recombination properties. Two stocks were prepared for each mutant. O n e stock carried X chro- mosomes homozygous for y2 cv v f and a rec mutant balanced over T M 3 , bx34‘ or over T(2;3)Xa.
T h e second stock carried X chromosomes homozygous for the markers y2 sc car and, sometimes t o the right of the centromere, y+ (a duplication of the yellow locus) in combination with *bxs4‘/ TM3, Sb bxs4‘ Ser or * b ~ ~ ~ ‘ / T ( 2 ; 3 ) X a , c n ~ . T h e X chromosome mutants in the two stocks demarcated five regions from the distal tip to the centromere. In other crosses females homozygous for y2 CO v f and bearing a rec mutant in chromosome 3 were crossed t o males with an unmarked X chromosome and the same rec mutant in chromosome 3 so as t o provide three regions for study covering -90% of the chromosome. In all cases the F1 females, heterozygous for the Xchromo- some mutants, were mated to males carrying an attached-X and -Y chromosome, (XU, y B) and a free Y. Recombination was measured among the male progeny, and nondisjunction was calculated from exceptional progeny of both sexes.
Allele tests for rec mutants shown to complement c(3)GI7 were performed by inter se crosses, utilizing one rec stock carrying marked X chromosomes. Allelism was indicated when the cross gave significantly lower recombination values than the heterozygote of either mutant with wild ‘ Y F
Temperature-sensitive tests for the ts mutant in homozygous, heterozygous, hemizygous and het- eroallelic combinations were carried out by maintaining females at the desired temperature throughout development, eclosion, mating and egg laying until the full complement of eggs had been laid; or, in the case of the restrictive temperature, by making use of the ”pupal system” (see GRELL 1973 for procedure) and treating on days 6 and 7. These methods ensured that all eggs utilized had undergone recombination at the prescribed temperature.
(kindly supplied by R. NOTHINGER) were used in an attempt to cytologically separate the locus of rec-1 from c(3)G. T h e deficiencies are Dj(3R)sbdZ6, which is deleted from 89B9-10 to 89C7-D1, and D j ( 3 R ) ~ b d ~ ~ , deleted from 89B4 to 89B10. T h e mutants c(3)GoR1, c(3)G0R12-179 and rec-I6 were tested with each deficiency.
Electron microscopy: Ovaries were prepared for electron microscopy according to the method described in GRELL and GENEROSO (1980) and examined for the presence or absence of the synaptonemal complex.
Further descriptions of the point mutations and rearrangements employed in this study can be found in the book by LINDSLEY and GRELL (1968).
and 28”.
Deficiency tests: T w o deficiencies that overlap Dj(3R)sbd
RESULTS
A TS REC MUTANT IN D . MELANOGASTER
TABLE 1
Isolation of mutants affecting recombination
429
No. chromo- Candidates rec-Z 43)G
Run no. Date some tested tested mutants mutants
1 l a 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Totals 611970 711972 811972 811972 911972 1111972 1111972 1211 972 611973 611973 711973 711973 811973 511974 1211 973 1211 973 411974 511974 511974 511974 511974 511974 611974 811974 811974 911974 911974 911974 111975 111975 1000 408 119 500 483 47 1 72 1 773 754 797 8 5 1 369 943 257 566 35 1 518 757 772 450 562 6 3 1 549 55 420 346 63 1 614 352 320 16,340 2 5 5 2 5 1 3 5 7 4 2 16 - -
-
-
3 3 2 3 1 2 2 1 2 5 2-
-
-
-83 3
1
-
1 1
15
-
indicates that none were found.430 R . F. GREI-1.
r . . ? .
I.'ICC'RE ~ . - ~ ~ t l ~ t ~ ~ l o ~ l ~ ~ t ~ ~ ~ t ~ c ~ o ~ ~ ~ ~ ~ ~ ~ s c ~ s in rrc-1 niutants and ;Inonioloiis structltres in cytoplasm of d3)G nilitant. A . rf3)(;''R'2'72*: 13, (:, and I). Nuclei of rec-16, rer-/I6, rrc-lz6 prcmwvtes, rrspec- tivclv. Arrow indicates syn;ipronctnal coniplex.
revealed that the synaptonemal coniplex was absent. Accumulation of cyto- plasmic structures, possibly identifiable as lamellae, in abnormal amounts were observed for most mutants (Figure 2A).
I he remaining three mutants coniplemented fully with c(3)G 17. Electron niicroscopic study of their pro-oocyte nuclei disclosed synaptonemal complexes in each case (Figure 2 R , C, and I)). 'These mutants were provisionally desig- nated rec", rec'" and ret" (corresponding to the run in which they arose) pending allelism tests to determine whet her more than one new locus was represented.
The three new rec mutants: The heterozygote and homozygote of each mu- tation were studied for the effect on crossing over, nondisjunction and fecun- dity. Examination of the three heterozygotes at 25" on a 7-ti;iv satnple ('fable 2) shows that crossing over and nondisjunction are not significantly different
A TS REC MUTANT IN D. MELANOGASTER 431
TABLE 2
Recombination, nondisjunction and fecundity in 7-day sample ofprogeny f r E y2 cv v f/+ or y2 sc car.y+ females heterozygous f o r rec6, recI6 or mated to XY, y B/Y
males and grown at 25"
Crossing over among 6 progeny (76)
Region" Nondis-
Total Total junction No. d
Genotype of mother 1 2 3 4 5 y'-f y'y' (X) progeny Progeny/?
y 2 cv v ff+; rec6/+ 10.4 22.1 21.3 53.8 0 2743 343
y 2 cv v fly' sc c a r . 9.5 23.5 21.3 7.4 5.3 54.3 67.0 0.1 1680 289
y 2 cv v fly' sc car. 9.4 22.1 23.3 7.1 4.6 54.8 66.5 0.1 2271 260
y 2 cv v f/+; rec16/+ 11.0 22.4 22.1 55.6 0.1 1560 129
y 2 cv v ff+; recZ6/+ 10.6 19.5 22.1 52.2 0 888 218
y 2 cv u fly' S E c a r . 12.1 20.9 22.2 6.8 2.6 55.3 64.7 0 1257 314
y 2 cv v f / y 2 s c car. 11.8 22.0 20.8 5.9 4.6 54.7 65.2 0 1358 280
y + ; rec6/+
y + ; rec6 /+
y+; recZ6/+
y + ; recZ6/+
y 2 cv v fly' sc c a r . 11.4 20.0 21.8 6.8 3.9 53.2 63.8 0 1344
y+; +f+b
a Region 1 (y2-cv); 2 (cv-U); 3 (U;$; 4 (f-car); 5 (car-y+).
'
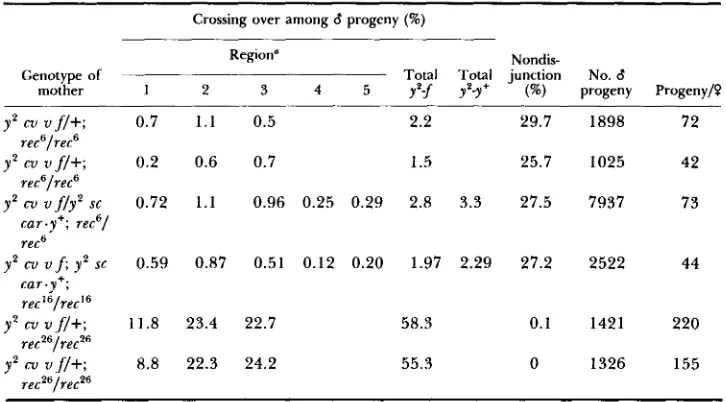
From GRELL (1978b).from normal and indicates that the mutations are recessive. By contrast, cross- ing over in the homozygotes of rec6 and rec16 is greatly reduced from -56 to -2% (Table 3). Nondisjunction in the homozygotes shows the usual reciprocal relationship to crossing over; it is increased from -0.1 to -27%. Fecundity is markedly reduced in both homozygotes. Increased nondisjunction and de- creased fecundity are expected consequences of reduced recombination. As elucidated by the distributive pairing model of meiosis (GRELL 1962a,b, 1976), reductions in recombination lead to high frequencies of noncrossover univalent chromosomes, following the exchange process. Noncrossover univalents will, during the distributive phase of meiosis, pair and segregate from nonhomolo- gous univalents, such as an X from a 2, a 2 from a 3 or an X from a 4 , and in this way undergo distributive nondisjunction with their homologue. When X chromosomes nondisjoin, the resulting progeny will be inviable one-half of the time; when a major autosome nondisjoins the progeny will be inviable all of the time. In this way a reduction in recombination increases nondisjunction and reduces fertility. On the other hand, the homozygote of recZ6 at the permissive temperature shows no reduction in recombination and no increase in nondisjunction (Table 3), although fecundity is slightly reduced.
Temperature-sensitivity of rec2': Selection of recZ6 as a recombination mutant was based on its behavior at an elevated temperature as a hemizygote. T o reexamine the recombinational properties of rec", the homozygote was treated at 3 1 O , the maximal temperature compatible with fertility. Synchronous pop-
432 R . F. GRELL
TABLE 3
Recombination, nondisjunction and fecundity in 7-day sample of progeny
fz
y2 cv vf/+ or y2 sc car.y+females homozygous for rec6, recl6 or mated to XY, y B/Y
males and grown at 25"
Crossing over among 6 progeny (W)
Region" Nondis-
Genotype of Total Total junction No. d
mother 1 2 3 4 5 y'f yp-y+ ( W ) progeny Progeny/?
y2 cu v f l + ; 0.7 1 . 1 0 .5 2.2 29.7 1898 72
y2 cv v f/+; 0.2 0.6 0.7 1.5 25.7 1025 42
rec6/rec6
rec6/rec6
car.y+; rec6/ rec6
car.y+; rec
'
'/ret'
recZ6/recz6
recZ6/recz6
y2 cv v f / y 2 s c 0.72 1 . 1 0.96 0.25 0.29 2.8 3.3 27.5 7937 73
y 2 cv v f ; y 2 sc 0.59 0.87 0.51 0.12 0.20 1.97 2.29 27.2 2522 44
y 2 cv v f/+; 11.8 23.4 22.7 58.3 0.1 1421 220
y 2 cv v f l + ; 8.8 22.3 24.2 55.3 0 1326 155
a Regions as in T a b l e 2.
the pupal system (GRELL 1973). Heat treatment was applied on day 6, 7 or on both days 6 and
7,
based on earlier studies which defined the period between 126 and 162 h post-egg laying as heat sensitive for the induction and enhance- ment of recombination (GRELL 1971, 1973; GRELL and DAY 1974). Synchro- nous samples of heat-treated and untreated eggs were secured by collecting the first 12-hr brood from the newly eclosed F1 females.As seen in Table 4, the restrictive temperature, when applied on day 6, significantly reduced recombination from the control value of 50.6 to 43.3%. T h e reduction is greater for day 7 (24.3%), and it is maximal when applied on both days (10.5%). Clearly, recZ6 is a temperature-sensitive recombination mutant. Recombination in the 12-hr control brood of rec26/rec26 at 25" is significantly less (Table 4, line 4) than that observed for the 7-day brood maintained at 25" (Table 3, lines 5 and 6). Reductions localize to regions 1 and 2.
A T S REC MUTANT IN D. MELANOGASTER 433
TABLE 4
Recombination, nondisjunction and fecundity in a 12-hr sample of progenyJom heat- treated and untreated y2 cv v €/+ females homozygous for recZ6 mated to XY, y B/Y
males
31" treatment Genotype of mother (day)
y 2 CO v f/+; recZ6/recz6 Experiment 1 6
Experiment 3 6 and 7
Experiment 4 None Experiment 2 7
Crossing over among d progeny
Region"
1 2 3 Total
5.9 16.0 21.5 43.3* 4.9 9.7 9.7 24.3" 2.6 5.2 2.6 10.5'
7.2 18.7 24.7 50.6
Nondis- junction
(%)
No d
progeny Progeny/?
0.4 545 10
4.9 144 3
9.2 76 1
0.6 656 12
a Regions as in Table 2.
'
Significant at 10% level.Significant at < I % level.
TABLE 5
Allelism tests for rec6 and rec'6 in 7-day samples at 25"
Crossing over among d progeny (%)
Region"
Total Total Genotype of mother 1 2 3 4 5 y'-f y*-y+
y 2 cu v f/+; rec6/recI6 0.9 1 . 1 0.7 2.7
y 2 cv v f/+; rec6/recI6 1.4 1.4 1.0 3.8
y 2 cu v f/+; rec6/rec'6 0.9 1.3 0.8 2.9
y 2 cu v f / y ' s c car.y+; 0.6 1.0 0.9 0.3 0.2 2.5 3.0
rec6/recI6
Nondis- junction No. d
(%) progeny Progeny/?
32 1805 146 30 1620 145 30 2217 126 29 4120 75
* Regions as in Table 2.
heterozygote, rec6/recI6, is markedly increased over each homozygote, suggest- ing complementation for this property. On the basis of these tests, allelism is established between rec6 and recI6, permitting the assignment of both mutants to a new locus designated recombination-I (rec-I) with the designations rec-l6 and rec-1 l 6 for the alleles. This locus lies within the confines of sbdlo5; genet- ically, it is located to a map position between 57.0 and 58.2.
Allelism tests for rec26 are less simple since the homozygote of retz6 shows normal recombination at 25" (Table 3, lines 5 and 6). In this circumstance, marked deviations from normal are not expected for rec26/rec-l or rec26/rec-
434 R. F. GRELL
2
s
2
-
iE
a z
U2 2
2
gus
._
E
b :
s
2
-
-
-
Tm _ ;
" 2
72%+ =
.E rl
Z d z o .Ei E
k?o
&?;t
A TS REC MUTANT IN D. MELANOGASTER 435
TABLE 7
Effect of dose of rec-1 alleles on recombination, nondisjunction and fecundity at 25"
~
~ _ _ _ _ _ _ _ ~~ ~~ ______ -
Crossing over among 6 progeny
Region" Nondis-
junction No. 6
Genotype of mother 1 2 3 Total ( W ) progeny Progeny/P
y 2 cv v f/+; rec-16/sbd'05 1.3 0.8 0.7 2.7 22.9 1676 66 y2 cu v f/+; rec-16/rec-16 0.7 1 . 1 0.9 2.6 27.7 10860 70
y 2 cv v f/+; r e ~ - l ' ~ / s b d ' ' ' ~ 1 . 1 0.8 0. 8 2.7 19.0 1315 44 y2 cv v f/+; rec-1'6/rec-1'6 0 .6 0.9 0.5 2.0 27.2 2522 38
y 2 cv v f/+; r e ~ - l ~ ~ / s b d ' ~ ~ 13.2 16.9 18.0 48.1 1 . 1 4498 168
y 2 cv v f/+; rec-lZ6/rec-lz6 10.4 22.9 23.4 56.7 0.1 2747 189 Regions as in Table 2.
8 and 9), similar treatment of recZ6/rec-l6 and recZ6/rec-l (Table 6, lines 10-
12) causes drastic reductions resembling that observed for rec26/rec26 under identical conditions (Table 4, experiment 3). T h e contrasting behavior at the restrictive temperatures of rec26/+ and rec26/rec-l ti or rec26/rec-l " serves to
identify recZ6 as a rec-1 allele with the designation rec-1 26.
Dosage: One method of characterizing different alleles of a gene is through gene dosage. A leaky mutant or hypomorph acts to reduce the amount of a gene product, causing mutant expression to be more extreme with one dose than two; a null mutant or amorph produces no functional product and is expected to show unaltered expression, regardless of dose. T h e effect of one vs. two doses of each rec-1 allele on recombination, nondisjunction and fecun- dity was studied at the permissive temperature (25 ") by comparing homozygote and hemizygote expression through use of sbdlo5. T h e results are given in Table
7.
Recombination of rec-16 and rec-116 is not altered by dose (Table
7,
lines 1-4),
suggesting that both are null mutants. This possibility is strengthened by the observation that recombination shows the same low level in the homozy- gotes of rec-1 ti and rec-1 as well as in their hemizygotes. To account for the residual recombination it is postulated that it might reflect a minor, independ- ent recombination pathway. If so, the responsible gene is probably not located within the region of the deficiency since there is no dosage response. Nondis- junction, showing the expected reciprocal relation to recombination, is high in the four situations. Fecundity, which appears to be typical for each allele regardless of dose, is again lower in the rec-1 ' t i hemizygote than in the rec-1 tihemizygote, as previously observed for their homozygotes. If these are null mutants with respect to recombination, the difference in fertility suggests a complex locus.
436
100
-i
R. F. GRELL
1 ' ! I
17 25 2 8 31
TEMPERATURE ("C)
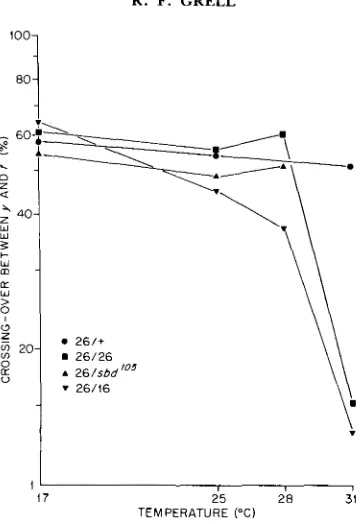
FIGURE 3.-Recombinational response of rec-1 26 genotypes to increasing temperatures in the range between 17" and 31".
to 85% of normal. Nondisjunction shows a slight increase with one dose and fecundity is unaltered.
A surprising consequence of hemizygosity is an increase in recombination in region 1 for each allele. Values for rec-1' and rec-1 are approximately dou- bled; rec-1
''
shows a significant increase which is accompanied by decreases in regions 2 and 3. This result provides additional evidence that the effects of rec-1 and sbd l o 5 on recombination are not equivalent and implies the existence of other gene(s) affecting recombination within the region defined by the deficiency.Putative denaturatzon of the rec-1" product by heat: T h e extreme sensitivity of rec-1
''
to a small temperature increase prompted an examination of thermal response over a wider temperature range. The study utilized four different rec-1''
genotypes, namely, the heterozygote with wild-type, the homozygote, the hemizygote and the heteroallelic combination with rec-1".
Response was measured in the X chromosome at 17", 2 5 " , 28" and 31" (Figure 3).A TS REG MUTANT IN D . MELANOGASTER 437
TABLE 8
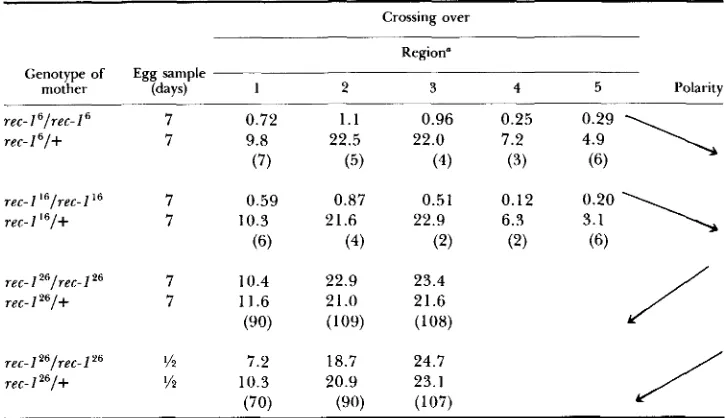
Polarity associated with the three rec-1 homozygotes at 25'
~
Crossing over
Region" Genotype of Egg sample
mother (days) 1 2 3 4 5 Polarity
8:i9
\
9::O
\
rec-1 'lrec- I
'
7 0.72 1 . 1 0.96 0.25rec- 1 'I+ 7 9.8 22.5 22.0 7.2
(7) ( 5 ) (4) (3) (6)
rec-1 "/rec-I" 7 0.59 0.87 0.51 0.12
rec- I "1-1- 7 10.3 21.6 22.9 6.3
(6) (4) (2) (2) (6)
rec-1
"/ret-
I 26 7 10.4 22.9 23.4rec-1 2b/+ 7 11.6 21.0 21.6
(90) (109) (108)
rec-IZb/rec-I2' Yz 7.2 18.7 24.7
rec-1 "/+ v2 10.3 20.9 23.1
(70) (90) (107)
/
Regions as in Table 2. Numbers in parentheses are % normal.
recombination value at 17" and the lowest at 31". Its slope between 17" and 28" is steeper than that seen for the other genotypes; between 28" and 31 O it decreases sharply and parallels that of the homozygote. T h e sharp decline seen for the homozygote and the heteroalleles within the narrow temperature range of 2"-3" is typical of a denaturation curve. T h e results are most simply interpreted as temperature denaturation of an enzyme or other protein pro- duced by the rec-1'' allele and associated with the recombination process. One dose of r e c - l + is sufficient to maintain normal recombination at the restrictive temperature as evidenced by the rec-1 26/+ response curve.
The matter of polarity: T h e ability to uniformly or nonuniformly reduce re- combination along a chromosome arm has been used to partition meiotic mu- tants into two functional types (CARPENTER and SANDLER 1974). All but one occur in the latter category and are postulated to be "precondition" mutants which are concerned with the establishment of sites or "nodes" where exchange may occur; mei-9 which effects a uniform reduction is postulated to be involved directly with exchange. When the reduction is nonuniform, the direction of polarity has been found to be distally directed with the greatest reduction at the chromosome tip.
438 R. F. GRELL
TABLE 9
Polarity modijicatwns imposed by hemizygosity at 25"
Crossing over
Region" Genotype of Egg sample
mother (days) 1 2 3 Polarity
rec-1 6/sbd 7 1.3
rec-1 '/+ 7 9.8
(13)
rec-1 '6/sbd ' 0 5 7 1.1
(1 1)
rec- 1 '6/+ 7 10.3
rec- 1 26/sbd
'
O5 7 13.2rec-1 26/+ 7 11.6
(1 14)
0.8 22.5
(4) (3)
0.8 21.6
(4) (3)
Regions as in Table 2. Numbers in parentheses are % normal.
I 26, although allelic to rec-1 ti and rec-1 16, shows the converse polarity. If both
of the latter prove to be null mutants, the residual recombination associated with them would, in fact, be the expression of a different gene with a different polarity.
T h e modifications induced in each of the rec-1 alleles by hemizygosity are seen in Table 9. In the cases of rec-1 and rec-1 1 6 , polarity remains proximally
directed but its expression is greatly enhanced by the near doubling of recom- bination frequency in region 1. In the case of rec-Iz6 recombination is also increased in region 1 but is accompanied by reductions in regions 2 and 3. This leads to a reversal of polarity so that the three alleles now exhibit the same proximal direction.
T h e greatest modifications are seen with the response of the rec-1 26/rec-I l 6
combination to shifts in temperature (Table 10). At 17" polarity is proximally directed; at 25" it is weakly distally directed in both 12-hr and 7-day egg samples. At 3 1 " polarity is abolished. This is seen whether the control utilized is a 31 ", 12-hr sample of rec-Iz6/+, a 25" 12-hr sample of rec-126/rec-1'6, a
25" 12-hr sample of rec-Iz6/+ or a 25" 12-hr sample of
+/+.
A TS REC MUTANT IN D. M E L A N O G A S T E R
TABLE 10
Polarity modijications of rec-1 "jrec- 1 I 6 by temperature
439
Crossing over
Egg sam- Region"
Genotype of ple Tempera-
mother (days) ture 1 2 3 Polarity
rec- I 26 jrec- I l 6
rec- 1 26 /+
rec-126/rec-1'6 rec- 1 26 /+
rec-126/rec-1'6 rec- 1 26 /+
rec-126/rec-I'6b rec- 1 26/+
rec-126/+
rec- I 26 jrec-I '6
+/+'
17" 17.1 17" 13.7 (125) 25" 9.0 25" 11.6 (78) 25" 7.0 25" 10.3 (68)
31 O 1.2
31" 10.6 (1 1)
25' 10.3
(12) 25" 7.0
(17) 25" 11.1
21.6 20.5
( 105) 16.7 21.0 (80) 15.8 20.9 (76) 2.4 16.3 (15)
(1 1)
(15) 20.9 15.8 21.3 25.1 24.5
( 102) 19.4 21.6 (90) 19.2 23.1 (83) 2.9 23.8 (12) (13) (15) 23.1 19'.2 23.1
Regions as in Table 2. Numbers in parentheses are % normal.
'
From GRELL (1 978a).' From GRELL (1978b).
DISCUSSION
T h e search for a t s rec mutant was initiated so that it might be used to define the time that the restrictive temperature acts to block recombination during oocyte development. Recovery of rec-Z26 provided the means to answer this question. Heat treatments of synchronous oocyte populations at sequential developmental time periods revealed that in rec-lZ6/rec-l l 6 females developing
at 25" response to the restrictive temperature (31 ") in the first oocytes formed is limited to a 36-hr period between 126 and 162 hr after egg deposition
(GRELL 1978a). This time span corresponds precisely to the sensitive period
for the induction and enhancement of recombination by heat in the normal genome for the same oocyte sample (GRELL 1973; GRELL and DAY 1974). Cytological and electron microscopic autoradiographic studies show that this oocyte sample enters premeiotic interphase at -129 hr (BUCHER 1957; GRELL
440 R . F. GRELL
complexes have formed by 132 hr (GRELL and GENEROSO 1982) and that the S-phase continues for -30 hr (GRELL 1973; GRELL and DAY 1974; DAY and
GRELL 1976; GRELL and GENEROSO 1982). Thus, DNA replication, synapsis
and the temperature-sensitive period for recombination are coextensive during premeiotic interphase.
A subsequent electron microscopic study of synaptonemal complexes in rec- 1 26 at the permissive and restrictive temperature was initiated to determine
whether at the restrictive temperature the level of pairing is altered or the synaptonemal complex is eliminated. Measurements of complex lengths in the two situations showed no significant difference. Likewise, complex distribution and fine structure displayed no detectable difference between treated and untreated samples. Based on these observations, it was provisionally concluded that at the restrictive temperature the exchange process itself is altered (GRELL
and GENEROSO 1980).
T h e present study has provided some additional information concerning the rec-1 gene. Specifically, use of its ts allele has furnished insight into its function. First, extension of the temperature range has shown that expression of rec-1 26
is fairly constant between 17 O and 28 O . Activity of the rec-1 2fi gene product is
acutely reduced at some temperature greater than 28", presumably through denaturation of a protein required for recombination. The loss of recombi- nation activity within a narrow temperature range is reminiscent of the behav- ior of the ts recA200 mutant of E. coli. In this case recombination activity is reduced more than one order of magnitude by a temperature increase of 2"
(KOBAYASHI and IKEDA 1978). Second, gene dosage tests have revealed that
rec-12fi is a leaky mutant with sufficiently high activity in one dose at the permissive temperature to attain 85% of normal expression. Failure of rec-1 and rec-lI6 to respond to dose as well as the virtually identical recombination
values of each as homozygotes suggests that they are null alleles whose inactiv- ity exposes an alternative recombination pathway controlled by one or more genes located outside the region delineated by sbdIo5.
Third, possible evidence for functional complexity is suggested by the expression of the putative null alleles, rec-1' and rec-1 l f i . If the product of the rec-1 gene is a monofunctional polypeptide, showing pleiotropic effects on recombination and fecundity, these properties might be expected to be simi- larly affected by null mutants, or in the case of slightly leaky mutants, similarity of expression for one parameter might be expected to extend to the other. Although recombination values are virtually identical, a marked difference is noted in fecundity; the homozygote and hemizygote of rec-16 produce close to twice as many progeny per female as do the homozygote and heterozygote of rec-1 1 6 . T h e possibility of a mutation affecting fertility elsewhere in the genome
A TS REC MUTANT IN D . MELANOCASTER 44 1
Analysis of recombination as a two-step process was first made by BRIDGES
(1 91 5). T h e establishment of sites where exchange might occur constituted the first step; exchange, based on a fixed probability per site, the second. As long as the second event was assumed to have constant value, it was possible to determine whether alterations in recombination were the consequence of shifts in the distribution of sites, as revealed by alterations in interference (expressed as coincidence values). If coincidence was unaltered, the change in recombi- nation was attributed to altered probability of exchange which still remained constant for the genome and so maintained the same interference values.
When a number of meiotic mutants were isolated, BRIDGES’ concept was
utilized to categorize their defects (LINDSLEY et al. 1968; SANDLER et al. 1968). A difficulty arose in estimating the coefficient of coincidence with mutants that severely reduced exchange, since double exchanges were eliminated or greatly reduced. A substitute method was adopted based on uniformity (= exchange mutant) or nonuniformity (= precondition mutant) in reduction along a chro- mosome arm (CARPENTER and SANDLER 1974). In the case of one mutant, abo,
a contradiction existed since reductions were nonuniform but coincidence was unchanged. To accommodate this mutant as well as others showing polarized reductions, a formulation was proposed in which the probability of exchange along the chromosome arm also became a variable. A drawback with this formulation was an insufficiency of information required to derive values for all of the variables. It is apparent, nevertheless, that as long as recombination frequencies are a product of two variables, probability of site establishment and probability of exchange per site, polarized reductions may result from a defect in either variable and do not necessarily indicate a defect in a precon- dition.
T h e assumption that the probability of exchange is inconstant finds support from experiments that compare the effects of modifiers on coincidence and recombination. If the probability of exchange is a constant, coincidence be- comes a function solely of the manner by which preconditions are established. When different modifiers alter coincidence values in dissimilar ways, the dis- similarities are expected to be reflected in their recombination patterns. A study of two modifiers, heat and interchromosomal effects (GRELL 1978b), revealed little similarity in coincidence patterns but strikingly similar recom- bination patterns. One way to account for these results is to invoke variable recombination rates.
T h e present experiments provoke additional questions concerning the valid- ity of categorizing mutants as precondition or exchange on the basis of uni- formity or nonuniformity in recombination reductions. Small changes in tem- perature acting on the same genotype (Table 10) are capable of not only reversing polarity but of abolishing it. In view of this result, polarity does not appear to be a sufficiently fundamental property upon which to base a func- tional dichotomy.
T h e region of the third chromosome circumscribed by sbd lo5 apparently
442 R . F. GRELL
bination process, namely, c(?)G and rec-I, the former indirectly through pair- ing, lie within the region. There is reason to suspect other rec genes are located here. First, isolation of whatever factor(s) is responsible for the decrease in recombination caused by sbd"'/l+ (the reason for targeting this region) was not realized with the recovery of rec-1 alleles, all of which give completely normal recombination as heterozygotes with wild type. Second, evidence for a regional modifier of recombination, located within the region defined by the deficiency, which operates to increase recombination comes from gene dosage studies which show recombination in region 1 to be either significantly in- creased or doubled when each allele is made heterozygous for the deficiency. A reduction in dose for a null or leaky allele is not expected to enhance recombination; nor is the reduction in dose of a normal component of the recombination system expected to do so unless the modifier is charged with the function of constraining the level of exchange and requires a double dose to do so. Finally, GREEN (1 976) has described a mutator gene, designated mu,
which lies within the region defined by sbdlo5 and localizes to map position
57
on the third chromosome. In addition to mutator activity, mu reduces recom- bination frequency both as a homozygote and a heterozygote. GREEN suggests that mu affects a DNA replication or DNA repair function.
In summary, this segment appears to harbor a number of recombination- related genes which makes it a desirable choice for further exploration.
I wish to thank E. H. GRELL for his great help in devising a method for isolating a temperature- sensitive recombination mutant. I am grateful to R. J. PRESTON, E. H. GRELL and F. W. LARIMER for their critical review of the manuscript, to R. NOTHINGER for generously supplying deficiency stocks and to E. E. GENEROSO for technical assistance. This research was sponsored by the Office of Health and Environmental Research, United States Department of Energy, under contract DE- AC05-840R2 1400 with the Martin Marietta Energy Systems, Inc.
LITERATURE CITED
BRIDGES, C. B., 1915 BRIDGES, P. N., 1941
BUCHER, N., 1957
A linkage variation in Drosophila. J. Exp. Zool. 1 9 1-21.
A revision of the salivary gland 3R-chromosome map of Drosophila mela- nogaster. J. Hered. 32: 299-300.
Experimentelle Untersuchungen uber die Beziehungen zwischen Keimzellen und soniatischen Zellen in Ovar von Drosophila melanogaster. Rev. Suisse Zool. 64: 91-188.
O n recombination-defective meiotic mutants in
Drosophila melanogaster. Genetics 7 6 453-475.
Synaptonemal complexes during premeiotic DNA synthesis in oocytes of Drosophila melanogaster. Genetics 83: 67-79.
Conditional lethal mutations in bacteriophage T4. In:
Genetics Today. Proceedings of the X I International Congress of Genetics, Edited by S. J. GEERTS. Pergamon Press, New York.
Mutable and mutator loci. pp. 929-946. In: Genetics and Biology of Dro- sophila, Vol. l b , Edited by M. ASHBURNER and E. NOVITSKI. Academic Press, New York.
A new hypothesis on the nature and sequence of meiotic events in the female of Drosophila melanogaste. Proc. Natl. Acad. Sci. USA 4 8 165-172.
A new model for secondary nondisjunction: the role of distributive pairing. Genetics 47: 1737-1754.
CARPENTER, A. T. C. and L. SANDLER, 1974
DAY, J. W. and R. J. GRELL, 1976
EDGAR, R. S. and R. H. EPSTEIN, 1965
GREEN, M. M., 1976
GRELL, R. F., 1962a
A TS REC MUTANT IN D. MELANOGASTER 44 3 Heat-induced exchange in the fourth chromosome of diploid females of
Recombination and DNA replication in the Drosophila melanogaster oocyte.
Distributive Pairing. pp. 435-486. In: Genetics and Biology of Drosophila, Vol. l a , Edited by M. ASHBURNER and E. NOVITSKI. Academic Press, New York.
Time of recombination in the Drosophila melanogaster oocyte: evidence from a temperature-sensitive recombination-deficient mutant. Proc. Natl. Acad. Sci. USA 75: 3351- 3354.
GRELL, R. F., 197813 A comparison of heat and interchromosomal effects on recombination and interference in Drosophila melanogaster. Genetics 89: 65-77.
GRELL, R. F., 1978c High frequency recombination in centromeric and histone regions of Dro- sophila genomes. Nature 272: 78-80.
Intergenic recombination, DNA replication and synaptonemal complex formation in the Drosophila oocyte. pp. 327-349. In: Mechanisms in Recombination,
Edited by R.F. GRELL. Plenum Press, New York.
Time of recombination in the Drosophila melanogaster
oocyte. 11. Electron microscopic and genetic studies of a temperature-sensitive recombination mutant. Chromosoma (Berl.) 81: 339-348.
A temporal study at the ultrastructural level of the developing pro-oocyte of Drosophila melanogaster. Chromosoma (Berl.) 87: 49-75.
Enhancement of recombination associated with c(3)G mutant of Drosophila melanoguster. Genetics 53: 157-164.
Relations between temperature sensitivity, amino acid replacements, and
O n the role of recA gene product in genetic recombination: an analysis by in vitro packaging of recombinant DNA molecules formed in the absence of protein synthesis. Mol. Gen. Genet. 166: 25-29.
Method of feeding ethyl methanesulfonate (EMS) t o D. males. Drosophila Inform. Serv. 47: 133-1 34.
Genetic variations of Drosophila melanogaster. Carnegie Inst. Wash. Publ. 627.
Genetic control of recombi- nation in Drosophila. pp. 253-276. In: Replication and Recombination of Genetic Material, Edited by W. J. PEACOCK and R. D. BROCK. Australian Academy of Science, Canberra.
In vivo interactions of genes and proteins in DNA replication and recombination of phage T 4 . Cold Spring Harbor Symp. Quant. Biol. 43: 501-515.
Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics 6 0 525-558.
GRELL, R. F., 1971
Drosophila melanogaster. Genetics 69: 523-527.
Genetics 73: 87-108. GRELL, R. F., 1973
GRELL, R. F., 1976
GRELL, R. F., 1978a
GRELL, R. F. and J. W. DAY, 1974
GRELI., R. F. and E. E. GENEROSO, 1980
GRELL, R. F. and E. E. GENEROSO, 1982
HINTON, C. W., 1966
JOCKUSCH, H., 1966
KOBAYASHI, I. and H. IKEDA, 1978
quaternary structure of mutant proteins. Biochem. Biophys. Res. Commun. 2 4 577-583.
LEWIS, E. B. and F. BACHER, 1968
LINDSLEY, D. L. and E. H . GRELL, 1968
LINDSLEY, D. L., L. SANDLER, B. NICOLETTI and G. TRIPPA, 1968
MOSIC, G., A. LUDER, G. GARCIA, R. DANNENBERC and S. BOCK, 1979
SANDLER, L., D. L. LINDSLEY, B. NICOLETTI and G. TRIPPA, 1968