SLEEP, Vol. 29, No. 7, 2006 INTRODUCTION
PHYSIOLOGICALLY, THE ONSET OF SLEEP UNDER NOR-MAL CONDITIONS IN NORNOR-MAL ADULTS IS THROUGH
NON-RAPID EYE MOVEMENT (NREM) SLEEP.1 Thus, a
pre-dictable pattern of sleep has been described, with sleep onset oc-curring with NREM sleep, and rapid eye movement (REM) sleep occurring approximately 90 minutes thereafter. NREM and REM sleep then alternate in a cyclic fashion every 90 minutes through the night. This was initially described by Dement and Kleitman and remains essentially unchanged. This fundamental principle of normal human sleep reflects a highly reliable finding and is im-portant when considering normal versus “pathologic” sleep.2
The occurrence of sleep-onset REM was first described by
Vogel in 1960 and subsequently linked to narcolepsy by Re-chtschaffen et al in 1963.3,4 During the 1960s, on overnight sleep
studies as well as single daytime nap recordings, sleep-onset REM was found to be exclusively associated with narcolepsy.4-7
In addition, with the First International Symposium on Narco-lepsy, held in 1975, a clear consensus on the definition of narco-lepsy was established to include REM abnormalities.8 This led to
the idea that polysomnographically identified sleep-onset REM periods (SOREMPs) could be used to diagnose narcolepsy. The development of the Multiple Sleep Latency Test (MSLT)9 in 1976
provided an opportunity to analyze multiple sleep-onset episodes and, therefore, the potential for multiple sleep-onset REM epi-sodes, in a single test.10 Early studies confirmed that the presence
of multiple SOREMPs (i.e., ≥ 2) on the MSLT were found to oc-cur exclusively with narcolepsy11 and were considered “highly
diagnostic” of narcolepsy.10,12 In addition, many reports have
con-firmed that multiple SOREMPs are very rare in normal subjects tested in standard conditions.13-15
However, in the 1980s, these reports were brought into ques-tion by studies done in healthy young adults, as well as in patients with frequent periodic limb movements and sleep apnea, among whom multiple SOREMPs were found to be relatively com-mon.16-19 Bishop et al found the frequency of multiple SOREMPs
to be 17% in a study done in healthy young adults.16 In a series of
187 patients with sleep apnea, 25% were found to have multiple SOREMPs.18 Recently, 4.7% of 1145 patients with obstructive
sleep apnea were found to have multiple SOREMPs.19 Multiple
SOREMPs have also been seen among patients with Prader-Willi syndrome,20 Kleine-Levin syndrome,21 Parkinson disease,22 and
periodic limb movements of sleep.17 Thus, recent data suggest
that multiple SOREMPs are not pathognomonic for narcolepsy. Despite this increasingly large body of work, the prevalence
The Prevalence of Multiple Sleep-Onset REM Periods in a Population-Based Sample
Meeta Singh, MD; Christopher L. Drake, PhD; Thomas Roth, PhDHenry Ford Sleep Disorders Center, Detroit, MI
Prevalence of SOREMPs—Singh et al Disclosure Statement
This was not an industry supported study. Dr. Drake has participated in speaking engagements supported by Sepracor, Cephalon, and Takeda. Dr. Roth has received research support from Aventis, Cephalon, GlaxoSmith-Kline, Neurocrine, Pfizer, Sanofi, Sepracor, Somaxon, Syrex, and Takeda; has participated in speaking engagements supported by Sanofi; and is a consultant for Accadia, Acoglix, Arena, AstraZeneca, Aventis, Cephalon, Eli Lilly, GlaxoSmithKline, Hypnion, King, Ludbeck, McNeil, Merck, Neurocrine, Organon, Orginer, Pfizer, Roche, Sanofi, Sepracor, Somaxon, Syrex, Take-da, Transoral, Vivometric, and Wyeth. Dr. Singh has indicated no financial conflict of interest.
Submitted for publication October 11, 2005 Accepted for publication February 2, 2006
Address correspondence to: Meeta Singh MD, Sleep disorders Center, Hen-ry Ford Hospital, 2799 W Grand BLD, Sleep Clinic, CFP 3, Detroit, MI 48202; Tel: (313) 916-5154; Fax: (313) 916-5150; E-mail: msingh2@hfhs.org
Study Objective: The presence of 2 or more sleep-onset rapid eye move-ment periods (SOREMPs) on a Multiple Sleep Latency Test (MSLT) has been used as 1 of the criteria for the diagnosis of narcolepsy and is thought to be specific to this disorder. However, previous studies have shown the prevalence of SOREMPS in healthy volunteers and apneic patients to be higher than expected. The present study determined the prevalence of 2 or more SOREMPs in a representative sample of the population from southeast Michigan and investigated potential associations with other sleep-related variables.
Design: Cross-sectional laboratory-based analysis.
Settings: Sleep disorders clinic.
Participants: Population-based sample.
Interventions: N/A.
Measurements: A population-based sample of 333 subjects was as-sessed by nocturnal polysomnography and daytime MSLT (5 naps), and an additional 206 subjectively sleepy people were also assessed (total = 539). Sample demographics were comparable to the 2000 census. Epworth Sleepiness Scale scores were also determined. Groups were formed based on a median split of each sleep variable (Epworth
Sleepi-ness Scale, MSLT, total sleep time from nocturnal polysomnography) for comparisons of SOREMPs in each group.
Results: The prevalence of 2 or more SOREMPs was 3.9%. Only mean sleep latency on the MSLT was a discriminator for the presence of 2 or more SOREMPs (short latency = 6.3%, long latency = 1.9%, p < .05). Among the subjects who had an MSLT of 5 minutes or less (an indicator of a pathologic level of sleepiness), 9.5% had 2 or more SOREMPS.
Conclusions: The overall prevalence of 2 or more SOREMPs in our sample is 3.9%. Interestingly, of the variables assessed (MSLT, Epworth Sleepiness Scale, and total sleep time from nocturnal polysomnography), objective sleepiness, as determined by the MSLT, was the only measure significantly associated with 2 or more SOREMPs. Therefore, subpopula-tions with excessive sleepiness (eg, shift workers, young adults, patients with apnea) are likely to have a greater prevalence of SOREMPs.
Keywords: Multiple SOREMPs, population based sample, sleepiness
Citation: Singh M; Drake CL; Roth T. The prevalence of multiple sleep-onset rem periods in a population-based sample. SLEEP 2006;29(7): 890-895.
SLEEP, Vol. 29, No. 7, 2006
of multiple SOREMPs in the general population is still unknown, making the diagnostic value of “multiple SOREMPs” a question-able issue. Specifically, the prevalence of any given variquestion-able in the general population affects the specificity and positive predictive value of a test that uses the variable to diagnose a specific disease. Studies done to date have looked for SOREMPs in different pa-tient populations.17-22 Although, the subjects of the study done by
Bishop et al consisted of healthy young adults with no sleep-wake complaints, these subjects were also a sample of convenience and, hence, may not be representative of the general population. Our study was, therefore, done with the objective of assessing the prevalence of multiple SOREMPs in a population-based sample. We also investigated the associations of sleep-related variables with multiple SOREMPs.
METHODS Subjects
The current study was performed as a part of a larger study of excessive daytime sleepiness. Participants were drawn from the general population of the tricounty Detroit area. The tricounty area includes 84% of the population of southeastern Michigan and is similar to the United States as a whole, with the exception of a different racial/ethnic distribution (Table 1). Our sample is therefore representative of southeastern Michigan. The research design was composed of 2 components: (1) a random digit dial, computer-assisted, telephone survey and (2) a laboratory-based evaluation. This is depicted in Figure 1.
For eligibility, the calling address had to be a residence and the participant an adult between the ages of 18 and 65 years. A ran-dom-probability selection procedure was used to determine the sex of the target adult. If 2 or 3 adults within a target sex were present in a household, a random-probability selection procedure (oldest/second, oldest/youngest) was used to determine the target respondent. If 4 or more adults with the target sex were present in the household, last-birthday method was used to determine the target respondent. In order to maintain an unbiased sample, only individuals who could not answer the questionnaire due to sensory or mental impairment were excluded from the sample.
From 4682 eligible participants, 3283 individuals completed the telephone survey (response rate calculated by the number of in-terviews that were conducted relative to the number of eligible participants was 70.1%). The demographic details of the sample including race, age, and socioeconomic status are shown in Table 1 and are nearly identical to the 2000 census data for the area.23
Of the 3283 subjects, 333 were randomly selected for a labora-tory-based evaluation. Further, as this study was a part of a larger study on daytime sleepiness, we also selected 206 high scorers (sleepy subjects) on the Daytime Sleepiness Scale24 to be brought
into the laboratory.
The institutional review board approved all procedures, and informed consent was obtained from all participants. Individuals were paid for study participation.
Procedures
Participants completed a 20-minute telephone interview, which included questions related to sleep and health habits, along with general information regarding medical and psychiatric status and use of medications. They also reported on subjective sleepiness by answering questions on the Daytime Sleepiness Scale.24
Sub-jects who were chosen to come into the laboratory, completed a 2-week sleep diary describing their sleep-wake patterns, and came into the laboratory at the end of the 2 weeks. They were in-structed to continue all medications, including REM-suppressant
Prevalence of SOREMPs—Singh et al
Table 1—Sociodemographic Characteristics of the Study Sample and Comparative Data From the 2000 United States Census
Characteristic Sample Tri county US Census (N=3283) (N=4,043,467) (N=281,421,906) Sex Men 50.3 48.5 49.1 Women 49.7 51.5 50.9 Annual income, in thousands of ($)a < 10 6.4 8.5 9.5 10 < 15 4.4 5.1 6.3 15 < 25 11.1 11.0 12.8 25 < 35 11.3 11.1 12.8 35 < 50 14.5 15.0 16.5 50 < 75 22.5 20.2 19.5 > 75 29.8 29.1 22.4 Race/Ethnicity (%) Caucasian 68.9 68.9 75.1 African American 24.9 25.0 12.3 Asian/Pacific 1.9 2.5 3.7 Islander Native American 0.9 0.3 0.9 Other/refused 3.4 3.3 7.9 Age, (years) 18-24b 10.9 18.7 21.0 25-34 21.4 22.5 21.4 35-44 24.9 25.0 24.3 45-54 24.9 21.0 20.2 55-64 17.8 12.7 13.0
Data for the socioeconomic status are presented in percentage of “households.”
a5% of individuals refused to answer any 1 of the questions
bUnited States Census and Tricounty census data from this category
included individuals ages 15-17 years, which accounted for the in-creased percentages in these categories.
Of the eligible respondents n=3,283
Completed the telephone survey
Randomly selected sample n=333
Daytime Sleepiness Scale high scorers: ≥10
n = 206 Mean latency* = 10.3 Prevalence of multiple SOREMP =
3.8% Mean latency*= 11.1
Prevalence of multiple SOREMP = 3.9%
n = 539 Prevalence of multiple
SOREMP = 3.9%
Figure 1—Flow chart summary of the study sampling methods and sleep-onset rapid eye movement periods (SOREMPs)/ sleepiness prevalence. *Mean latency of a Multiple Sleep Latency Test.
SLEEP, Vol. 29, No. 7, 2006
medications. All subjects received an 8.5-hour polysomnogram prior to the MSLT procedure. The nocturnal recordings included 4 electroencephalographic channels (C2, 3 and O1 and o2), 2 chan-nels for electrooculography (bilateral horizontal), chin electro-myogram, a nasal/oral thermistor, an electrocardiogram-record-ing channel, and an anterior tibialis electromyogram channel. All recordings were made using standard sleep laboratory procedures and were scored according to standard criteria.24 Nocturnal
poly-somnographic variables included total sleep time (TST); sleep latency; REM latency; percentages of stage 1, 2, 3/4, and REM sleep; and the respiratory event index. Following the nocturnal recording, subjects were administered a 5-nap clinical MSLT. In accordance with the standard procedures, sleep latency and the presence or absence of REM sleep was determined.25 In addition,
on the morning of the MSLT, subjects filled out various sleep- and health-related questionnaires, including the Epworth Sleepiness Scale.26
Analysis
For the first part of the analysis, we calculated the mean sleep latency (MSLT) as well as the prevalence of multiple SOREMPs in both groups of subjects n = 333 (random sample) and n = 206 (subjectively sleepy). Toward our second objective, we inves-tigated the potential associations of multiple SOREMPs with various other sleep-related variables. Specifically, we looked at sleepiness, both objective sleepiness as measured by a mean la-tency on the MSLT and self-reported sleepiness as measured by the Epworth Sleepiness Scale. We also looked at various poly-somnographic variables, including TST; sleep efficiency; stage1 latency; REM latency; percentages of stage 1, 2, 3, 4, and REM sleep; and the respiratory event index. For this part of the analysis, groups were formed based on a median split of the data within each variable and then compared for the prevalence of multiple SOREMPs using the χ2 test. Respiratory event index scores were
used to divide the sample into 2 groups based on a cutoff of >5
events or more. These groups were similarly compared, using the χ2 test. Variables explored are listed in Table 2 along with the
means and their standard deviations. Self-reported TST was also analyzed to look for an association with multiple SOREMPs. TST was calculated based on the 2-week sleep diary data.
We also evaluated the use of REM-suppressant drugs and their association with multiple SOREMPs. For this part of the analysis, the following drugs were included as potential REM suppressants: tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, serotonergic or noradrenergic reup-take inhibitors, β-antagonists, α2-agonists, anticholinergic agents,
carbamazepine, and barbiturates.
Using a mean latency of 5 minutes or less—an accepted cut-off for pathologic sleepiness11—we compared individuals with a
mean latency of 5 minutes or less on the MSLT to those with a mean latency of longer than 5 minutes to determine the associa-tion with multiple SOREMPs. In addiassocia-tion, since a mean latency of 8 minutes or less is also used clinically to determine daytime sleepiness,we performed a similar analysis using a cutoff of 8 minutes or less.
Finally, to investigate the relationship between sleepiness and number of SOREMPs on an MSLT, we looked at the mean sleep latency in each of the 5 SOREMP-frequency groups (having 0, 1, 2, 3, and 4 or 5 SOREMPs). In order to clarify if the shorter latency seen with a higher number of SOREMPs is a result of true sleepiness or due to a higher REM drive that is reflected by a more rapid (REM) sleep onset, we looked at the sleep latency of NREM and REM naps. Because our hypothesis was that the latency on NREM versus REM naps would not differ (supporting the notion of true sleepiness), we also performed a t-test to look at the difference between NREM and REM sleep latencies among individuals with 1 SOREMP and those with multiple SOREMPs.
RESULTS
Since the results (ie, the prevalence of multiple SOREMPs and the mean MSLT latency) did not statistically differ for the 2 groups (Daytime Sleepiness Scale high scorers and randomly selected subjects), we combined our groups for a final number of subjects of 539. (Figure 1)
The prevalence of multiple SOREMPs in our population-based sample (n = 539) was 3.9%. The actual number (and prevalence) of subjects with 0, 1, 2, 3, 4, and 5 SOREMPs is as follows: 491 (86.7%), 53 (9.4%), 14 (2.5%), 6 (1.1%), 1 (0.2%), and 1 (0.2%) subjects, respectively. The age and sex distribution of the subjects who had 0, 1, or 2 or more SOREMPs on their MSLT is presented in Table 3 and did not differ significantly.
Of all the variables tested, only objective sleepiness, as mea-sured by a mean latency on the MSLT, was associated with a
Prevalence of SOREMPs—Singh et al
Table 2—Sleep-Related Polysomnography Variables for the Entire Sample and Each of the SOREMP Groups
PSG variable Entire 0 SOREMP 1 SOREMP ≥ 2
Sample SOREMPS Mean latency 10.85±4.58 11.14±4.5 9.6±4.3 7.3±3.6 (MSLT) ESS, score 8.3±4.19 9.8±4.8 9.3±4.7 10.9±4.8 TST(PSG), 427.48±64.24 425.0±66.0 439.5±54.94 442.3±51.8 min SE (PSG), 83.84±12.43 83.3±12.7 86.0±10.9 86.5±9.4 % Latency to sleep stage, min Stage 1 15.95±23.49 16.7±25.0 10.9±10.0 11.6±10.5 REM 108.57±64.96 111.1±67.0 90.86±48.8 96.9±61.15 Sleep stage, % 1 10.79±8.71 10.8±8.9 9.9±6.7 11.3±9.3 2 57.39±22.72 57.3±24.4 57.3±8.9 57.2±9.2 3 and 4 14.33±9.97 14.5±9.5 13.1±10.75 13.4±12.7 REM 18.42±7.08 18.3±7.0 19.4±7.8 17.9±6.9 Data are presented as mean ± SD unless otherwise indicated. SOREMP refers to sleep-onset rapid eye movement periods; PSG, polysomnog-raphy; MSLT, Multiple Sleep Latency Test; TST, total sleep time; ESS, Epworth Sleepiness Scale; REM, rapid eye movement.
Table 3—Mean Age and Sex Distribution for Each SOREMP Group
Variable Number of SOREMPS
0 1 ≥ 2
n = 464 n = 53 n = 22
Sex, % men 47.7 49.2 68.2
Mean age, y, ± SD 42.0±12.4 38.1±14.4 38.1±14.8 No statistical differences were present between groups (p > .05); SOREMPs refers to sleep-onset rapid eye movement periods on the Multiple Sleep Latency Test.
SLEEP, Vol. 29, No. 7, 2006
higher prevalence of multiple SOREMPS. Specifically, subjects with a shorter mean latency on the MSLT had a higher frequency of multiple SOREMPs (Figure 2). For the remaining sleep-related variables tested, none of the groups differed statistically in their association with the frequency of multiple SOREMPs. A break-down of the mean and standard deviations of these variables in the 3 groups (0, 1, and 2 or more SOREMPs), is presented in Table 2.
In addition, the 3 groups, based on the presence of 0, 1, and 2 or more SOREMPs, did not differ statistically on self-reported TST, as measured by a 2-week diary. Specifically, groups having 0, 1, and 2 or more SOREMPs had a mean TST of 7.3 ± 3.0, 7.0 ± 1.5, and 7.4 ± 1.5 hours, respectively (p > .05).
Forty-eight subjects (8.6%) were on REM-suppressant medica-tions. Groups with 1 or 2 or more SOREMPs did not differ in their association with REM-suppressant medications (0 SOREMPs– 9.2%, 1 SOREMP–10.6%, ≥ 2 SOREMPs–10.0% [p > .05]). Of the individuals who had a mean latency of less than 5 mutes (n = 63), 9.5% had multiple SOREMPs, versus 3.2% of in-dividuals (n=476) who had a mean latency of 5 minutes or longer (p < .005). Similarly, 7.8% of individuals who had a mean latency of 8 minutes or shorter (n = 178) had multiple SOREMPs, versus 2.1% of those who had a mean latency of longer than 8 minutes (n = 387) (p < .005).
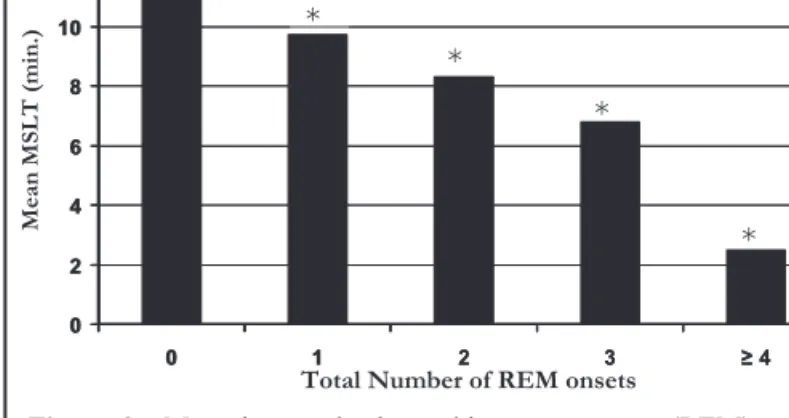
Among the SOREMP-frequency groups, mean latency on the MSLT and number of SOREMPS had an inverse relationship; as the number of SOREMPs increased, mean latency decreased, re-vealing a linear trend (p < .05) (Figure 3).
Finally, our hypothesis that NREM latency and REM latency would not differ was rejected when looking at individuals who had only 1 SOREMP (p < .004) (Table 4). Specifically, in individ-uals who had only 1 SOREMP, the sleep latency on the REM naps was significantly shorter, as compared with that on the NREM naps (p < .05) (Table 4). However, in individuals who had mul-tiple SOREMPs, the mean latency in NREM and REM naps did not differ.
DISCUSSION
The presence of multiple SOREMPs on an MSLT was once considered to be diagnostic for narcolepsy, provided that other
causes of SOREMPs have been excluded, including drug with-drawal, REM sleep deprivation, obstructive sleep apnea, alcohol-ism, major depression, and sleep-wake schedule abnormalities.27
Though various studies have evaluated the diagnostic value of using the MSLT for narcolepsy,28,29, it is difficult to interpret the
specificity and sensitivity of this criterion without determining the prevalence of multiple SOREMPs in the general population. Our study was aimed at calculating the prevalence of multiple SOREMPs in a population-based sample of southeastern Michi-gan and was found to be 3.9%. This is, in fact, much higher than the prevalence of narcolepsy in the general population (0.05%).30
In clinical situations, therefore, the sole reliance on the pres-ence of multiple SOREMPs on the MSLT for the diagnosis of narcolepsy, in the absence of auxiliary symptoms of the disease (cataplexy, sleep paralysis, hypnagogic hallucinations), should continue to be discouraged. The prevalence of 3.9% was also found to be much lower than the 17% previously found among healthy young adults. This previous study done by Bishop et al was done on a sample of convenience.16 Young adults are known
to be sleepier, 31 which may explain the higher prevalence of
mul-tiple SOREMPs in the Bishop et al study. Indeed, this association of sleepiness and multiple SOREMPs was a robust finding in our analysis. The prevalence of 3.9% in our population-based sample is comparable with the 4.7% found among apneic patients,19 who
are known to be objectively sleepy.
The second part of our study investigated which sleep-related variables were associated with multiple SOREMPs. Of all the variables tested, only objective sleepiness, as measured by a low mean latency, was associated with multiple SOREMPs. Again, SOREMPs were not related to nocturnal sleep but, rather, to ex-cessive daytime sleepiness. Thus, the SOREMPs seen in apneic patients19 may not be due to sleep fragmentation as much as to
excessive daytime sleepiness.
Self-reported sleepiness did not have a significant relationship with multiple SOREMPs, which is not surprising given that self-reported sleepiness does not accurately correlate with objective sleepiness.32
The presence of multiple SOREMPs in a patient with narco-lepsy has been thought to reflect a higher REM pressure. How-ever, our results would imply that any condition associated with sleepiness would be associated with multiple SOREMPs. There-fore, shift-workers, apneic patients, sleep-deprived individuals, etc. would have an elevated prevalence of multiple SOREMPs,
Prevalence of SOREMPs—Singh et al 0 1 2 3 4 5 6 7 Sleepy Sleepy Subjective Sleepiness(ESS) * Alert Objective Sleepiness (MSLT) % S O R E M P ≥ 2 * P < .001 Alert
Figure 2—Prevalence of multiple sleep-onset rapid eye movement periods (SOREMP) in sleepy versus alert subjects. On the Multiple Sleep Latency Test (MSLT), sleepy: n = 279, mean latency = 7.0 ± 2.4 min; alert: n = 275, mean latency = 14.6 ± 2.6 min. On the Epworth Sleepiness Scale (ESS), sleepy: n = 277, mean score 11.3 ± 2.9, alert: n = 230, mean score 4.6 ± 1.8; *Alert versus sleepy groups on the MSLT. * * * 0 2 4 6 8 10 12 0 1 2 3 ≥ 4 ). ni m( TL S M n ae M
Total Number of REM onsets
* 0 2 4 6 8 10 12 0 1 2 3 ≥ 4
Figure 3—Mean latency in the rapid eye movement (REM)-onset frequency groups. For the groups having 0, 1, 2, 3, 4, and 5 sleep-onset REM periods, n = 491, 53, 14, 6, 1, and 1, respectively. MSLT refers to Multiple Sleep Latency Test. *p < .05 vs 0 Group
SLEEP, Vol. 29, No. 7, 2006
albeit lower than the number seen in narcolepsy. In our review of the sleep-deprivation literature that used MSLT to document day-time effects, we did not find an increase in multiple SOREMPs. One explanation could be that the MSLT administered in these studies is not a clinical MSLT but a research MSLT, in which sub-jects are awakened after 3 epochs of sleep onset, and the presence of SOREMPs is not fully explored. In fact, this also corresponds with our finding that prior-night TST on PSG and sleep diary TST did not significantly predict the presence of multiple SOREMPs. The number of SOREMPs is linearly related to how objective-ly sleepy an individual is. This result can impobjective-ly 2 things. Either sleepier individuals are more likely to have more SOREMPs or the mean latency to REM sleep is shorter than that to NREM sleep. To clarify this result, we looked at the sleep latency on REM versus NREM naps within each SOREMP-frequency group. We found that, among individuals who had multiple SOREMPs, sleep laten-cy did not significantly differ between NREM versus REM naps. However, within individuals with a single SOREMP, the REM nap had a significantly shorter latency versus the NREM naps. This is an interesting finding. In the past, the presence of multiple SOREMPs has frequently been attributed to a high REM pressure, which leads to REM intrusions into wakefulness and NREM sleep stages.33-36 This higher REM pressure has been described as REM
sleepiness and was found to be associated with a shorter latency on REM naps versus NREM naps among 12 untreated patients with narcolepsy.33 Thus, the idea that REM sleepiness versus NREM
sleepiness might be 2 separate processes was advanced. However, in the largest study done to date on this topic,37 in 103
narcolep-tics, sleep latency did not differ on REM naps versus NREM naps. Consistent with this finding, in our study, individuals with mul-tiple SOREMPs did not differ on REM versus NREM latencies. Thus, a shorter mean latency on the MSLT and its association with multiple SOREMPs seem to imply true sleepiness rather than higher REM pressure. To be more specific, this implies that sleepier individuals have more SOREMPs and not the other way around, ie, the presence of more SOREMPs results in a shorter mean latency on the MSLT, secondary to a higher REM pressure intruding into wakefulness. In contrast, REM pressure appears to be higher in individuals with only 1 SOREMP. This may imply that REM pressure gets masked by the higher degree of sleepiness in sleepy individuals who have multiple SOREMPs.
In summary, “2 or more SOREMPs” have been thought to be pathognomonic of narcolepsy. However, our finding that
higher SOREMP-frequency groups have lower mean sleep la-tency would imply that, as sleepiness increases, the number of SOREMPs would increase even in the absence of narcolepsy and thus “2 or more SOREMPs” does not appear to have any specific pathognomonic significance.
ACKNOWLEDGEMENTS
Support for this study was provided by MH grants MH59338 and MH068372 to Drs Roth and Drake.
REFERENCES
1. Dement W, Kleitman N: Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming. Elec-troencephalogr Clin Neurophysiol 1957;9: 673-90.
2. Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger MH, Roth T, Dement, WC, eds. Principles and Practice of Sleep Medicine, 4th ed. Philadelphia: WB Saunders; 2005.
3. Vogel G: Studies in the psychopathology of dreams, III: The dream of narcolepsy. Arch Gen Psychiatry 1960;3:421-8.
4. Rechtschaffen A, Wolpert EA, Dement WC, Mitchell SA, Fisher C. Nocturnal sleep of narcoleptics. Electroencephalogr Clin Neuro-physiol 1963;15:599-609.
5. Takahashi Y, Jimbo M. Polygraphic study of the narcoleptic syn-drome, with special reference to hypnagogic hallucinations and cataplexy. Folia Psychiatr Neurol Jpn 1963; Suppl 7:343-7. 6. Dement W, Rechtschaffen A, Gulevich G. The nature of the
narco-leptic sleep attack. Neurology 1966;16:18-33.
7. Dement WC, Daytime sleepiness and sleep attacks. In: Guil-leminault C, Dement WC, Passouant P, eds. Narcolepsy. New York: Spectrum; 1975: 17-42.
8. Guilleminault C, Dement WC, Passouant P. Narcolepsy. In: Weitzman ED, ed. Advances in Sleep Research, vol 3. New York: Spectrum Publications; 1976.
9. Carskadon MA, Dement WC. Sleep tendency: an objective measure of sleep loss. Sleep Res 1977:6:200.
10. Mitler MM, van den Hoed J, Carskadon MA, et al. REM sleep epi-sodes during the multiple sleep latency test in narcoleptic patients. Electroencephalogr Clin Neurophysiol 1979;46:479-81.
11. Richardson GS, Carskadon MA, Flagg W, van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency test in narcoleptic and control subjects. Electroen-cephalogr Clin Neurophysiol 1978;45: 621-7.
12. Mitler M. The multiple sleep latency test as an evaluation for exces-sive somnolence. In: Guilleminault C, ed Disorders of Sleeping and Waking: Indications and Techniques. Menlo Park: Addison Wesley; 1982:145-153.
13. Carskadon MA, The second Decade. In: Guilleminualt C, ed. Dis-orders of Sleeping and Waking: Indications and Techniques. Menlo Park: Addison-Wesley; 1982:145-153.
14. Roth T , Hartse KM, Zorich F, Conway W. Multiple naps and the evaluation of daytime sleepiness in patients with upper airway sleep apnea. Sleep 1980:3:425-39.
15. Carskadon MA, Orav EJ, Dement WC. Evolution of sleep and day-time sleepiness in adolescents. In: Guilleminault C, Lugaresi E. eds. Sleep/Wake Disorders: Natural History, Epidemiology, and Long-Term Evolution. New York: Raven Press; 1976:499-517.
16. Bishop C, Rosenthal L, Helmus T, Roehrs T, Roth T. The frequency of multiple sleep onset REM periods among subjects with no exces-sive daytime sleepiness. Sleep 1996:19:727-30.
17. Moscovitch A, Partinen M, Guilleminualt C. The positive diagnosis of narcolepsy and narcolepsy’s borderland. Neurology 1993;43:55-60.
18. Biniaurishvilli RG, Fry JM, DiPhillipo MA, Goldberg R, MSLT REM sleep episodes, excessive daytime sleepiness and sleep
struc-Table 4—REM and NREM latency in individuals who had 1 SOREMP and multiple SOREMPs on the Multiple Sleep Latency Test
Number of SOREMPS Nap Type Time, (min)
1 (n = 53) REM nap 5.67 ± 4.8a
NREM nap 8.19 ± 4.4
≥ 2 REM nap 5.80 ± 3.0
NREM nap 6.70 ± 4.0
Data are presented as mean ± SD unless and are based on the results of t-tests.
ap< .05 versus non-rapid eye movement (NREM) naps; REM refers
to rapid eye movement; SOREMP, sleep-onset REM periods on the Multiple Sleep Latency Test (MSLT). Naps with 0 SOREMPs are excluded by definition, as there are no REM onsets to compare MSLT values.
SLEEP, Vol. 29, No. 7, 2006
ture in obstructive sleep apnea. Sleep Res 1994:23:131.
19. Chervin RD, Aldrich MS. Sleep onset REM periods during multiple sleep latency tests in patients evaluated for sleep apnea. Am J Respir Crit Care Med. 2000;161:426-31.
20. Helbing-Zwanenburg B, Kampheisen HAC, Mourtazaev MS. The origin of excessive daytime sleepiness in the Prader-Willi syndrome. J Intellect Disabil Res 1993;37:533-41.
21. Reynolds CF, Kupfer DJ, Christiansen CL, et al. Multiple sleep latency test findings in Kleine-Levin Syndrome. J Nerv Ment Dis 1984;172:41-4.
22. Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK daytime sleepiness in Parkinson’s disease. J Sleep Res 2000;9:63-9. 23. United States Census Bureau. (US Government). 2000 Census of
Population and Housing. 2005.
24. Johnson EO. Breslau N. Roth T. Roehrs T. Rosenthal L. Psycho-metric evaluation of daytime sleepiness and nocturnal sleep on-set scales in a representative community sample. Biol Psychiatry 1999;45:764-70.
25. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring Systems for Sleep Stages of Human Sub-jects. Washington: U.S. Government Printing Office; 1968. 26. Johns MW. A new method for measuring daytime sleepiness: the
Epworth sleepiness scale. Sleep 1991;14:540-5.
27. Sleep Disorders Classification Committee, Roffwarg HP, Chair-man. Diagnostic classification of sleep and arousal disorders. Sleep 1979;2:1-139.
28. Amira SA, Johnson TS, Logowitz NB. Diagnosis of narcolepsy us-ing the multiple sleep latency test: analysis of current laboratory criteria. Sleep 1985;8:325-31.
29. Aldrich MS, Chervin RD, Malow BA. Value of the Multiple Sleep Latency test (MSLT) for the diagnosis of narcolepsy. Sleep 1997;20:620-9.
30. Hublin C, Kaprio J, Partinen M. The prevalence of narcolepsy: an epidemiological study of the Finnish twin cohort. Ann Neurol 1994;35:709-16.
31. Levine B, Roehrs TA, Zorick F, Roth T. Daytime sleepiness in young adults. Sleep 1998;11:39-46.
32. Drake CL, Roehrs TA, Burduvali E, Bonahoon A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology 2001;38:979-87.
33. Broughton RJ. Aguirre M. Differences between REM and NREM sleepiness measured by event-related potentials (P300, CNV), MSLT and subjective estimate in narcolepsy- cataplexy. Electroen-cephalogr Clin Neurophysiol 1987;67:317-26.
34. Aguirre M, Broughton RJ. Objective and subjective measurements of (REM and NREM) sleepiness in narcolepsy-cataplexy. Sleep Res 1984;13:128.
35. Broughton RJ, Agguirre M. Evidence of qualitatively different types of excessive daytime sleepiness. In: Koella WP, Ruther E, Schulz H. eds Sleep ’84. Stuttgart: Fischer Verlag: 1985:86-7.
36. Broughton RJ, Valley V, Aguirre M, Roberts J, Suwalski W, Dun-ham W. Excessive daytime sleepiness and the pathophysiology of Narcolepsy- cataplexy: a laboratory prespective. Sleep 9(1): 205-215
37. Zorick F. Roehrs T, Wittig R. Lamphere J. Sicklesteel J. Roth T. Sleep-wake abnormalities in narcolepsy. Sleep 1986;9:189-93.