FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis and
regulate gastrulation during sea urchin development
Eric Röttinger, Alexandra Saudemont, Véronique Duboc, Lydia Besnardeau, David McClay and Thierry Lepage
There was an error published in Development135, 353-365.
The title of this article was not corrected in the on-line version before publication. The correct title appears above. The printed version of the article is correct.
INTRODUCTION
The sea urchin embryo, which is transparent, simple and readily accessible to experimental perturbations, offers a unique opportunity to study the regulation of morphogenesis in an organism closely related to vertebrates (Ettensohnn and Winklbauer, 2004; Hardin, 1996; McClay et al., 1992; McClay et al., 2004). During early development of the sea urchin embryo, a well-defined series of morphogenetic events transforms the hollow epithelial ball of the blastula into a three-layered bilaterally organized pluteus larva with its characteristic organization of mesodermally derived spicules. Spicules are constructed by a population of mesodermal cells called the primary mesenchyme cells (PMCs). At the blastula stage, PMCs precursors delaminate into the blastocoel and then undergo a dramatic sequence of morphogenetic behaviors (Decker and Lennarz, 1988). Immediately following ingression, they stop dividing and begin to migrate along the extracellular matrix that lines the blastocoel wall. During migration, the PMCs constantly extend and retract long and thin filopodia that survey the inner surface of the ectoderm (Malinda et al., 1995; Miller et al., 1995). Then, before the onset of gastrulation, they gradually adopt a stereotyped pattern consisting of two ventrolateral clusters joined by a ring of cells surrounding the archenteron. PMCs within the clusters
subsequently fuse through their filopodial processes, forming a syncytium that begins to accumulate calcium and magnesium carbonates, leading to formation of a small tri-radiate crystalline structure: the spicule rudiment, deposited within an organic matrix. Each rudiment then increases in size and complexity (Decker and Lennarz, 1988) and branches in a specific pattern to form the extraordinary polycrystalline skeleton that gives the pluteus larva its easel-like shape.
It has been shown experimentally that the ectoderm plays a crucial role in guiding PMC migration as well as in regulating the skeletogenic gene expression program (Armstrong et al., 1993a; Ettensohn, 1990b; Ettensohn and Malinda, 1993; Ettensohn and McClay, 1986; Katow et al., 2000; Malinda and Ettensohn, 1994; McClay et al., 1992; Peterson and McClay, 2003; Wilt, 1997). For instance, PMCs taken from embryos treated with the ventralizing agent NiCl2 form a normal skeleton when transplanted into untreated host embryos, whereas normal PMCs recombined with a nickel-treated ectoderm form multiple spicule rudiments (Hardin et al., 1992). The molecular nature of the spatial cues provided by the ectoderm to the underlying PMCs remained elusive for a long time, but one signal has recently been identified as VEGF (Duloquin et al., 2007). VEGF ligands emitted from two small regions of the ectoderm are specifically required for the oriented migration and differentiation of the PMCs, which specifically express VEGFR.
While the formation of spicules from PMCs is a process unique to echinoderms, the invagination of the archenteron and delamination of the secondary mesenchyme cells (SMCs) are clearly comparable to those driving vertebrate gastrulation. In the sea
FGF signals guide migration of mesenchymal cells, control
skeletal morphogenesis of the skeleton and regulate
gastrulation during sea urchin development
Eric Röttinger1, Alexandra Saudemont1, Véronique Duboc1, Lydia Besnardeau1, David McClay2and Thierry Lepage1,*
The sea urchin embryo is emerging as an attractive model to study morphogenetic processes such as directed migration of mesenchyme cells and cell sheet invagination, but surprisingly, few of the genes regulating these processes have yet been characterized. We present evidence that FGFA, the first FGF family member characterized in the sea urchin, regulates directed migration of mesenchyme cells, morphogenesis of the skeleton and gastrulation during early development. We found that at blastula stages, FGFA and a novel putative FGF receptor are expressed in a pattern that prefigures morphogenesis of the
skeletogenic mesoderm and that suggests that FGFA is one of the elusive signals that guide migration of primary mesenchyme cells (PMCs). We first show that fgfA expression is correlated with abnormal migration and patterning of the PMCs following treatments that perturb specification of the ectoderm along the oral-aboral and animal-vegetal axes. Specification of the ectoderm initiated by Nodal is required to restrict fgfA to the lateral ectoderm, and in the absence of Nodal, fgfA is expressed ectopically throughout most of the ectoderm. Inhibition of either FGFA, FGFR1 or FGFR2 function severely affects morphogenesis of the skeleton.
Furthermore, inhibition of FGFA and FGFR1 signaling dramatically delays invagination of the archenteron, prevents regionalization of the gut and abrogates formation of the stomodeum. We identified several genes acting downstream of fgfA in these processes, including the transcription factors pea3and pax2/5/8 and the signaling molecule sproutyin the lateral ectoderm and SM30and SM50in the primary mesenchyme cells. This study identifies the FGF signaling pathway as an essential regulator of gastrulation and directed cell migration in the sea urchin embryo and as a key player in the gene regulatory network directing morphogenesis of the skeleton.
KEY WORDS: Fgf, Sea urchin, Gastrulation, Skeletogenesis, Cell migration, PMCs, FGF, Sprouty, FGFR1, FGFR2, Pea3, Pax Development 135, 353-365 (2008) doi:10.1242/dev.014282
1UMR 7009 CNRS, Université Pierre et Marie Curie (Paris 6) Observatoire
Océanologique, 06230 Villefranche sur mer, France. 2Department of Biology, French
Family Science Center, Duke University Durham, NC 27708, USA.
*Author for correspondence (e-mail: lepage@obs-vlfr.fr)
Accepted 30 October 2007
D
E
V
E
LO
P
M
E
N
urchin, internalization of the endodermal precursors occurs by buckling of the vegetal plate rather than by ingression or involution (McClay et al., 1992). Formation of the blastopore is thought to involve the coordinated constriction of the apical surface of cells at the center of the vegetal plate. Although recent studies have demonstrated the crucial role played by a subpopulation of SMCs and by non-canonical Frizzled 5/8 and Rho signaling in triggering primary invagination of the gut, archenteron morphogenesis remains poorly understood at the molecular level (Beane et al., 2006; Croce et al., 2006; Kominami and Takata, 2004).
In this study we describe a molecular pathway that is initiated before gastrulation within the ectoderm of the blastula and involves the sequential and spatially restricted expression of FGFA and of transcription factors in the Ets and Pax families in discrete regions of the ectoderm to which migrating mesenchymal cells are attracted. We report that this pathway is required not only for oriented migration of the PMCs, which is required for construction of the skeleton, but also that it regulates invagination of the archenteron. Therefore, this study has identified a regulatory module essential for morphogenesis in the sea urchin embryo and the FGF pathway as a key player of the gene regulatory network directing patterning of the PMCs and gastrulation.
MATERIALS AND METHODS
Cloning of fgfA and FGFR2
A partial cDNA corresponding to the 3⬘UTR region of the fgfA transcript was first isolated in the course of an in situ hybridization screen. A full-length cDNA was subsequently isolated from a pluteus cDNA library. A partial clone encoding FGFR2 was identified in a ParacentrotusEST database and extended by 5⬘RACE using mesenchyme blastula-stage cDNA.
Accession numbers for novel sequences used in this study are: fgfA, EF157978; sprouty, EF157979; FGFR2, EF439654; pea3, EF439653.
Constructs, RNA and morpholino injections, treatments
The coding sequence of fgfA was amplified by PCR using the Pfx DNA polymerase and inserted at the BamHI-XhoI sites of pCS2+ (Turner and Weintraub, 1994). To construct the dominant-negative FGFR1, a portion of the cDNA coding for the signal peptide, extracellular and transmembrane domains but truncated of the intracellular kinase domain was amplified by PCR and cloned into pCS2. Capped RNA was injected at the following concentrations: fgfA:600 g/ml; dn-FGFR1, 780 g/ml.
The exon2-intron2 boundary located within the FGF core domain was selected for morpholino targeting. A genomic DNA fragment containing intron 2 was first amplified using PCR primers within exon2 and exon3 and its sequence was determined. The in-vivo specificity and efficiency of the splice-blocking morpholino oligonucleotide was monitored via semi-quantitative RT-PCR. RNA was extracted at the early gastrula stage from batches of 150 embryos injected with increasing doses of the morpholino and reverse transcribed. PCR primers located within exon1 and exon3 were used to amplify the mRNA products generated in the presence of the splice-blocking oligonucleotide. The PCR products were gel eluted, cloned and deletion of exon 2 was confirmed by sequencing. The FGFA-splice-Mo was effective when injected at relatively low doses (0.5 mM), and it did not cause any toxicity even when injected at high concentrations (up to 2 mM). Similarly, the FGFR2-Mo could be injected at doses up to 1.5 mM without causing toxicity. By contrast, when injected at doses above 1 mM, the FGFA-ATG and the FGFR1 morpholinos started to be toxic. Therefore, these morpholinos were used below 1 mM (0.6-0.9 mM).
Sequence for morpholino oligonucleotides are: FGFAsplice: 5⬘ACA CATTTTGGATACTTACAGCTCC; FGFAATG: 5⬘ACTTTCATCC A TTTTCGCTTTCATG; FGFA 5⬘UTR: 5⬘ATGGATGCCGCGTCGT ACACACGAG; FGFR1Mo1: 5⬘CATCATGCCGTGGCTGCCT T -GAGCA; FGFR1-Mo2: 5⬘-AGTTCCAGCAAAAGATGACGAAAAG; Mo1: 5⬘-TAAAGCATCGGATCGCCATTTCCAT; and FGFR2-Mo2: 5⬘-TTCCGTTTAATTTTCTCCAAATCAC.
All the injections were repeated many times with different batches of embryos and for each experiment, 100-150 embryos were analysed. Only representative phenotypes present in at least 80% of the injected embryos are presented.
Immunocytochemistry and U0126 treatment
Detection of phosphorylated ERK and treatments with U0126 were performed as described previously (Röttinger et al., 2004). Treatments with human bFGF (a kind gift from Hitoyoshi Yasuo) were performed at 50 ng/ml starting at early blastula stage. LiCl was used at 30 mM and NiCl2at 0.5 mM.
Sequence analysis and phylogenetic analysis
To predict the signal sequence of FGFA, we used the SignalP software available at http://www.cbs.dtu.dk/services/SignalP/, and to predict the secondary structure we used the Porter software available at http://distill.ucd.ie/porter/. For phylogenetic analysis, the amino acid sequences of the core domain of FGF ligands and of the tyrosine kinase domains of FGFRs were collected from GenBank using the SMART software and aligned with ClustalW with default parameters (http://www.ebi.ac.uk/clustalw/). The tree was calculated using the maximum likelihood method with PhyML using the WAG substitution model (http://atgc.lirmm.fr/phyml/) (Guindon et al., 2005). A consensus tree with 50% cut off value was derived from bootstrap analysis (500 iterations) using Mega 3.1 (http://www.megasoftware.net/). Numbers above branches represent posterior probabilities, calculated from this consensus.
RESULTS
Identification of the sea urchin FGFA ligand and of a novel putative FGF receptor expressed during early development
A cDNA encoding a protein with homology to FGF peptides was first isolated in the course of an in situ hybridization screen as a gene displaying restricted expression within the ectoderm. The predicted sea urchin protein contains a hydrophobic sequence at the N-terminus characteristic of signal peptides for secretion and a core FGF domain located in the C-terminal portion (see Fig. S1A in the supplementary material). The latter contains the invariant residues characteristic of the FGF core domain as well as several conserved residues implicated in binding of FGFs to Heparin and heparin sulphate proteoglycan (HSPG) (Bellosta et al., 2001; Ornitz, 2000; Ornitz and Itoh, 2001) (see Fig. S1B in the supplementary material). The predicted protein sequence shows similarities with vertebrate FGF9, FGF16 and FGF20, as well as with FGF8, FGF17 and FGF18. However, phylogenetic analysis gave ambiguous results, and therefore, in the absence of strong evidence for grouping this sequence with other known FGF subfamilies, we decided to name this FGFA (see Fig. S1C in the supplementary material).
A sea urchin FGF receptor named FGFR1 has been characterized previously in Strongylocentrotus purpuratus(McCoon et al., 1996; McCoon et al., 1998), but its function has not been studied. The expression pattern of FGFR1 has been re-examined recently in Paracentrotus lividus, revealing a highly dynamic expression of FGFR1 in various territories, including mesodermal and endodermal precursors (Lapraz et al., 2006). By screening a P.lividusEST database for sequences related to FGFR1, we identified a second putative FGFR receptor, which we named FGFR2 (Lapraz et al., 2006). FGFR2 has the typical architecture of FGFRs, with 3Ig domains in the extracellular ligand-binding domain, a Fibronectin III (FN III) domain, a transmembrane region and a tyrosine kinase domain (see Fig. S1E in the supplementary material). The organization of these domains is slightly different from that of FGFR1 (in which the FN III domain is upstream of the Ig domains rather than downstream). Also, FGFR2 apparently lacks the acidic box usually
D
E
V
E
LO
P
M
E
N
found between Ig domains 1 and 2, required for interaction between FGFR and N-CAM and/or cadherins (Sanchez-Heras et al., 2006). The kinase domain of FGFR2 contains all the structural motifs required for coordinating Mg2+ATP, including the GTGSFGKV, VAIK and DFG motifs, as well as the HRDLXXXN motif that constitutes the catalytic loop of the kinase. Blast analysis indicated that the amino acid sequence of FGFR2 is mostly related to FGFRs from insects and cephalochordates (see Fig. S1F in the supplementary material). However, phylogenetic analysis using a large set of sea urchin RTKs and FGFRs from various species indicated that the tyrosine kinase domain of FGFR2 is divergent compared with that of other FGFRs (see Fig. S1G in the supplementary material). Taken together, these results suggest that this receptor kinase encodes a putative FGF receptor with a divergent kinase domain.
fgfA and FGFR2 are co-expressed at the onset of morphogenesis
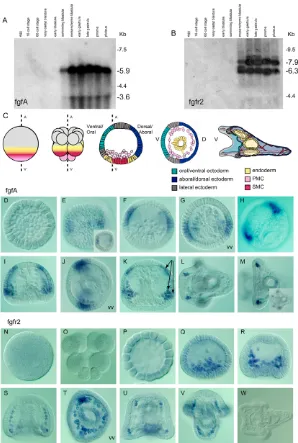
Northern blot analysis revealed the presence of two zygotic fgfA transcripts that started to accumulate in the embryo at the swimming blastula stage (Fig. 1A). The abundance of fgfA transcripts increased
dramatically after ingression of the primary mesenchyme cells and remained elevated from the onset of gastrulation up to the pluteus stage.
The temporal expression of FGFR2 is largely parallel to that of fgfA. Two zygotic fgfr2transcripts started to accumulate in the embryo at the mesenchyme blastula stage (Fig. 1B). Their abundance peaked during gastrulation, both transcripts being still expressed at a high level at the prism and pluteus stages.
fgfA expression prefigures formation of the bilateral clusters of PMCs and foreshadows the branching pattern of the skeleton
[image:4.612.50.348.295.738.2]Expression of fgfA was first detected at the hatching blastula stage in an equatorial belt of ectodermal cells surrounding the embryo (Fig. 1E). Immediately after ingression of the primary mesenchyme cells, while there is yet no morphological sign of bilateral symmetry, the expression of fgfA was downregulated in part of this equatorial belt and progressively restricted to two broad domains of the ectoderm (Fig. 1F,G). Double in situ hybridization with probes for fgfA and nodal, which is expressed on the presumptive ventral side,
Fig. 1. Temporal and spatial expression of fgfA and FGFR2 in sea urchin.(A,B) Northern blot analysis of fgfA and FGFR2 expression. (C) Scheme of early development of the sea urchin embryo describing morphogenesis of the skeleton. Spatial distribution of fgfA (D-M) andfgfr2(N-W) transcripts during normal development analysed by in situ hybridization. Insets in E and M show the same embryo in a different focal plane. (N) Egg, (O) 16-cell stage, (P) 120-cell stage, (D,E) swimming blastula, (F,G,Q) mesenchyme blastula, (I,J,R,S) early gastrula, (K,T) late gastrula, (L,M,U,V) prisme/early pluteus (36 hours), (W) pluteus (48 hours). Ectodermal and mesodermal expression domains are indicated by arrows in K.
D
E
V
E
LO
P
M
E
N
showed that the bilateral ectodermal regions expressing fgfA are located between the dorsal and ventral regions and therefore coincide with the lateral domains where the PMCs aggregate to form bilateral clusters before differentiation (Fig. 1C and data not shown). Furthermore, at the early gastrula stage, intense expression of fgfA was observed in the ectodermal regions in which the PMCs had accumulated to form the bilateral clusters (Fig. 1H).
During gastrulation, the pattern of fgfA expression prefigures the branching pattern of the skeleton: expression of fgfA was detected in 5- to 6-cell-wide regions of the ectoderm near the regions where one of the three arms (the anonymous rod) of the tri-radiate spicule rudiment branches to form the body rods and the post-oral (or anal) rod (Horstadius, 1973). At this stage, intense expression of fgfA was detected within the PMCs located in the center of the newly formed PMC clusters (Fig. 1I-K). During gastrulation, novel domains of expression appeared near the thickened animal plate, on either side of the neurogenic ectoderm (Fig. 1I,K). These are regions in which another of the three arms of the tri-radiate rudiment (the dorsoventral connecting rod) will branch to give rise to the anterolateral rods. Expression of the fgfA gene in these four ectodermal domains persisted at the late gastrula and prism stages but became undetectable in plutei (Fig. 1L,M). In the early pluteus larva, expression of fgfA transcripts was restricted to the region surrounding the stomodeum and to four clusters of PMCs comprising three to four cells located at the rod tip of the growing spicules that sustain the oral arms (Fig. 1L,M). These clusters probably correspond to the plug of PMCs associated with the growth zones of the two pairs of arm rods (Ettensohn and Malinda, 1993; Wolpert and Gustafson, 1961).
FGFR2 is expressed in migrating PMCs
WhereasfgfA (Fig. 1E,F) and FGFR1 (Lapraz et al., 2006; McCoon et al., 1996; McCoon et al., 1998) were abundantly expressed in the ectoderm at blastula and gastrula stages, fgfr2 transcripts were expressed exclusively within the PMCs (Fig. 1Q-V). Consistent with the northern blot analysis, expression of fgfr2 started at the beginning of PMC ingression and continued to be restricted to these cells during gastrulation and morphogenesis. At the gastrula stage, fgfr2 transcripts were detected in all subpopulations of PMCs, including the bilateral clusters, and the oral and aboral chains (Fig. 1S,T and data not shown). However, at the prism stage, a spatial restriction of fgfr2transcripts was observed in PMCs located in regions in which the oral rods of the skeleton will branch (Fig. 1U), close to regions expressing fgfA (Fig. 1L,M). fgfr2expression was no longer detected at the pluteus stage (Fig. 1W). Experiments using dissociated embryos (data not shown) showed that this gene, like most other PMC marker genes studied so far, was expressed in a cell-autonomous manner and therefore that its expression is part of the cell-autonomous program of the PMCs. Thus, fgfA and FGFR2 are expressed during similar periods and partially overlapping regions, suggesting that they may act as a couple ligand-receptor.
AnfgfA synexpression group comprising genes encoding the FGF/MAP kinase modulator Sprouty and the transcription factors Pea3 and Pax2/5/8
In the course of an in situ hybridization screen, we identified three additional genes expressed at specific stages in bilateral regions of the ectoderm in a pattern strikingly similar to that of fgfA (Fig. 2). The first gene encodes a protein related to the FGF/MAP kinase modulator Sprouty, which is thought to act in a negative-feedback regulatory loop during FGF and EGF signaling (Casci et al., 1999; Hacohen et al., 1998; Kramer et al., 1999; Sivak et al., 2005). The second encodes the
Ets domain transcription factor Pea3 (Polyoma enhancer activator 3) (Raible and Brand, 2001; Roehl and Nusslein-Volhard, 2001) and the third is the paired domain transcription factor pax2/5/8(Czerny et al., 1997). From late mesenchyme blastula/early gastrula to pluteus stages, expression of sproutylargely followed that of fgfA in bilateral regions of the ectoderm, in the PMC clusters, and at the tip of the growing arms of the larva (Fig. 2A-H), consistent with the idea that sproutyexpression is dependent on FGF signaling, as shown in other systems (Mason et al., 2006). Similarly, during gastrulation, pea3was expressed in four ectodermal domains that can be superimposed over the four domains of fgfA at this stage (Fig. 2K). However, both pea3 and sproutywere strongly expressed in the endoderm, whereasfgfA Fig. 2. The fgfA synexpression group and effects of treatments with lithium chloride or nickel chloride on expression of fgfA andpax2/5/8.(A-D) Schematic representation of fgfA expression in sea urchin. (E-P) Spatial expression of sprouty(E-H), pea3(I-L), pax2/5/8 (M-P). (Q-Zb) Morphology and effects on fgfA and pax2/5/8expression of treatments that perturb animal-vegetal or oral-aboral polarity; (Q-T) 60 hour embryos; (U-Zb) 24 hour embryos.
D
E
V
E
LO
P
M
E
N
[image:5.612.314.560.59.503.2]transcripts were absent from this tissue. Expression of pax2/5/8, like that of fgfA, was strictly zygotic and started abruptly at late mesenchyme blastula/early gastrula stage in two lateral domains of the ectoderm, at the intersection between the ectoderm-endoderm and oral-aboral boundaries (Fig. 2M,N). Thus, pax2/5/8together with pea3and sprouty, belongs to the fgfA synexpression group. However, in contrast to fgfA, sproutyand pea3, which are expressed both in the ectoderm and in the PMC clusters, pax2/5/8 was expressed exclusively in the ectoderm overlying the PMC clusters.
Remarkable similarities in the expression patterns of sprouty, pax2/5/8 andpea3 continued to be observed at the prism and pluteus stages, but whereasfgfA expression was restricted to a small group of PMCs at the tip of the budding arms (Fig. 2D), pax2/5/8was expressed exclusively within the ectoderm overlying these isolated PMCs (Fig. 2P), and sprouty (Fig. 2H) and pea3(Fig. 2L) were expressed both in the PMCs and in the overlying ectoderm. Taken together, these observations show that several genes encoding proteins known to act in the FGF signaling pathway in vertebrates, including Sprouty (Casci et al., 1999; Hacohen et al., 1998; Kramer et al., 1999), Pax2/5/8 (Hans et al., 2004; Reifers et al., 1998) and Pea3 (Raible and Brand, 2001; Roehl and Nusslein-Volhard, 2001) are probable downstream targets of FGF signaling during morphogenesis of the skeleton in the sea urchin.
fgfA and pax2/5/8 are de-repressed in most of the ectoderm in the absence of Nodal signaling
To gain insight into the function and regulation of fgfA expression, we first examined its expression in embryos treated with reagents that perturb patterning along the animal-vegetal and dorsal-ventral axes (Fig. 2Q-Zb). In embryos treated with lithium, the PMCs migrated towards the animal pole and arranged into a ring at the level of the displaced ectoderm-endoderm boundary (Fig. 2Q). In these embryos, fgfA and pax2/5/8were expressed in the animal pole region immediately above the aggregated PMCs (Fig. 2U,Y). By contrast, in embryos radialized by treatment with NiCl2, which causes circumferential expression of Nodal (Duboc et al., 2004), the PMCs formed multiple clusters around the basis of the archenteron (Fig. 2R) (Armstrong et al., 1993b; Hardin et al., 1992). In all the NiCl2-treated embryos, fgfA was expressed radially in two rings located at the level of the ectoderm-endoderm boundary and below the animal pole region (Fig. 2V, n>200), whereas pax2/5/8was expressed in a single belt of cells at the level of the endoderm-ectoderm boundary (Fig. 2Z, n>200). These observations show that treatments that affect the bilateral symmetry of the embryo, such as vegetalization by lithium or ventralization by NiCl2, are associated with dramatic perturbations of the expression of fgfA and pax2/5/8, causing them to be expressed radially near the sites where the PMCs differentiate into spicule rudiments.
The TGF- Nodal is a crucial determinant of dorsal-ventral polarity of the sea urchin embryo and is required for establishment of bilateral symmetry (Duboc et al., 2005; Duboc et al., 2004). We therefore examined fgfA andpax2/5/8 expression in embryos in which Nodal signaling was exacerbated or abrogated. Strikingly, overexpression of nodalby microinjection of mRNA into the egg did not promote but abolishedfgfA and pax2/5/8expression (Fig. 2W,Za, n>100), suggesting that Nodal signaling leads to repression of fgfA expression. As described above, in NiCl2-treated embryos, fgfA was excluded from the equatorial region located between the two rings of cells expressing fgfA (Fig. 2V). This medial region that does not express fgfA following NiCl2 treatment most likely corresponds to the belt of cells that ectopically expresses Nodal in these embryos (see Duboc et al., 2004) (Fig. 2Cn), in agreement with
the finding that misexpression of Nodal represses fgfA expression. Reciprocally, in most embryos in which translation of nodalmRNA was blocked by microinjection of a morpholino oligonucleotide, fgfA and pax2/5/8were expressed radially in a large equatorial belt of cells that surrounded the embryo but not in the animal pole territory (Fig. 2X,Zb, n=70). These results show that in the absence of Nodal, fgfA and pax2/5/8are expressed ectopically in most of the ectoderm. They suggest that Nodal establishes the bilateral expression pattern of fgfA by repressing its expression in the ventral ectoderm and probably by inducing a repressor of fgfA expression in the dorsal territory.
Overexpression of fgfA disrupts morphogenesis
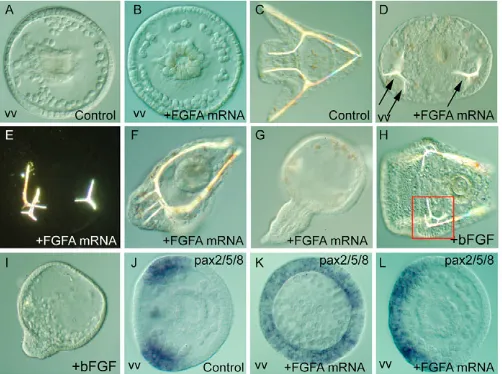
To test the potential of FGFA to regulate morphogenesis of the sea urchin embryo, we overexpressed it by microinjection of mRNA into the egg. Microinjection of fgfA transcripts strongly perturbed morphogenesis and differentiation of mesenchymal cells (Fig. 3). At the gastrula stage, whereas in the control embryos the PMCs had formed a regular ring with two clusters, in the FGFA-injected embryos the pattern of PMCs was much more irregular, some embryos displaying a radial organization (Fig. 3A,B). Also, we noted that the number of PMCs was frequently above the number of 32 cells normally present in P.lividus (Fig. 3A,B). At 48 hours, a supernumerary spicule was present in about 30% of the embryos (n>500), and the remaining embryos frequently displayed abnormal branching of the spicules (Fig. 3D-F). Abnormal skeletogenesis was correlated with ectopic expression of pax2/5/8 in the injected embryos (Fig. 3J-L n=35/45), suggesting that pax2/5/8 is a downstream target of FGFA signaling.
[image:6.612.314.565.461.648.2]Overexpression of fgfA also caused exogastrulation in a small fraction (1 to 10%, depending on the batch) of the injected embryos (Fig. 3G, n>500). The same defects in skeletogenesis and gastrulation were observed following treatment of embryos with purified recombinant human bFGF (also known as NUDT6 – Human Gene Nomenclature Database) (treatment at 50 ng/ml caused
Fig. 3. Patterning defects caused by overexpression of fgfA in sea urchin.Embryos overexpressing fgfA or treated with bFGF were observed at the early gastrula stage (A,B) or at 48 hours (C-H) or fixed at the mesenchyme blastula stage for in situ hybridization with a pax2/5/8probe (I-L). fgfA overexpression induces radial (K) or broadened (L) expression of pax2/5/8. See text for details. vv, vegetal view. Arrows indicate the positions of the spicule rudiments; red square
indicates the presence of an ectopic spicule.
D
E
V
E
LO
P
M
E
N
exogastrulation in 5-10% of the larvae and abnormal skeletogenesis in 20-40% of the larvae (Fig. 3H,I, n>200). These results suggest that FGFA signaling may regulate differentiation of mesenchymal cells as well as cellular movements during gastrulation.
Inhibiting FGFA signaling delays gastrulation and disrupts patterning and differentiation of the PMCs
To analyse in more detail the roles of the FGFA ligand, we attempted to block its function by microinjecting antisense morpholino oligonucleotides (Fig. 4). AsfgfA is a zygotic gene,
[image:7.612.51.407.198.736.2]we tried to interfere with splicing of newly synthesized transcripts with a morpholino directed against an exon-intron boundary (Fig. 4A). Sequence analysis of the splicing products generated in embryos injected with the fgfA morpholino revealed that the outcome of targeting the exon2-intron2 junction in the 3-exon fgfA transcript is deletion of exon 2 (Fig. 4Aa,Ab). As this deletion removes 35 codons in the middle of the region coding for the core FGF domain (see Fig. S1 in the supplementary material), this morpholino is predicted to cause a strong loss-of-function situation. Injection of the fgfA splice morpholino resulted in a highly penetrant (95%; n>2000) and severe phenotype
Fig. 4. Loss of FGFA function disrupts skeletogenesis and gut morphogenesis in sea urchin. (A) Controls of the efficiency of the fgfA-splice and fgfA-ATG
morpholinos. (a) Scheme of the intron-exon organization of the fgfA pre-mRNA, indicating the positions of the target sequence and of the primers used to characterize the mRNA products generated in the presence of this morpholino. (b) RT-PCR analysis of control uninjected and embryos injected with the fgfA-splice morpholino at 0.5, 1 or 1.5 mM. The PCR product in control embryos had the expected size (101 codons, 303 bp) and predicted sequence. The PCR product amplified from the fgfA-splice morphants had the expected size (66 codons, 198 bp) and sequence for a transcript deleted from exon 2. (c)fgfA-ATG-Mo but not fgfA -splice-Mo, specifically blocks in vitro translation of a synthetic fgfA transcript. PCS2-fgfA was in vitro translated in the presence of fgf9A -ATG-Mo or fgfA-splice at the indicated concentrations (1.6, 8, 40 M) and the products were analysed by PAGE and autoradiography.
(Ba-o) Phenotypes caused by microinjection of the fgfA-splice-Mo (f-j) or fgfA-ATG-Mo (k-o) in the egg. (Ca-l) Rescue experiment. Eggs were first injected with fgfA-splice-Mo together with RLDX then subsequently re-injected at the one- or two-cell stage with a synthetic mRNA encoding FGFA together with an FLDX.
D
E
V
E
LO
P
M
E
N
characterized by delayed ingression of the primary mesenchyme cells and invagination of the archenteron and disrupted patterning and differentiation of skeletogenic precursors and gut (Fig. 4Ba-Bj). A population of about 30 mesenchymal cells always eventually formed in these embryos and a small archenteron invaginated, but with a considerable delay not observed when control morpholinos were injected (Fig. 4Bg,Bh and see Fig. S2 in the supplementary material). However, the PMCs that formed did not start the characteristic sequence of migration observed in the control embryos and remained as a disorganized population near the vegetal pole. These PMCs maintained a round shape and failed to extend cellular processes typical of wandering PMCs. As a consequence, the bilateral clusters of PMCs, the aboral PMC ring, and the spicule rudiments did not form and these embryos lacked a skeleton (Fig. 4Bi,Bj). Most of the FGFA-spliceMO injected embryos also showed severe defects in patterning of the gut (Fig. 4Bi,Bj). The archenteron that formed in these embryos neither became tripartite nor opened on a stomodeum (Fig. 4Bj). Similar phenotypes were observed with a morpholino directed against the translational start site (MoFGFA-ATG) or with a morpholino directed against the 5⬘UTR, arguing for specificity (Fig. 4Bk-Bo).
To further demonstrate the specificity of the splice morpholino, we performed a rescue experiment (Fig. 4C). Eggs were first injected with the FGFA morpholino targeted against the splice junction then subsequently re-injected at the one- or two-cell stage with a synthetic mRNA encoding FGFA. Whereas in most of the FGFA morphants, PMC migration and gastrulation were delayed and differentiation of spicules was prevented (Fig. 4Cb,Cd), by contrast a significant fraction (40%; n=77) of embryos injected successively with the morpholino and the synthetic RNA started to gastrulate at the same time as the controls and formed spicules on each side of the archenteron, although these did not elongate as much as in control embryos (Fig. 4Cc,Ce).
Expression of FGFA in the vegetal hemisphere is required for skeleton formation
As fgfA is expressed early in part of the animal hemisphere and later in tissues derived from the vegetal pole, such as the PMC clusters and overlying ectoderm, we analysed the respective roles of this ligand in the animal and vegetal domains by making chimeras. Eggs were injected with the fgfA-spliceMo morpholino together with a lineage tracer and allowed to develop up to the 16-32-cell stage; then the animal and vegetal regions were separated and recombined with their complementary halves derived from wild-type embryos (Fig. 5A). Chimeras in which the fgfA morpholino was present in the animal hemisphere developed into almost normal pluteus larvae, with a skeleton and tripartite archenteron (100%, n=7). The only defect observed in these larvae was incomplete development of the anterolateral arms and spicules consistent with previous expression of fgfA in two regions of the animal hemisphere where the anterolateral arms and spicules form (Fig. 5Bc-Bf). Thus fgfA is not required in the ectoderm derived from the animal region for gastrulation or formation of most of the skeletal rudiments. By contrast, when the function of fgfA was inhibited in the vegetal hemisphere, the resulting chimeras displayed a phenotype very similar to that observed following injection of the morpholino into the egg (Fig. 5Bg-Bj): skeletal differentiation was blocked, the archenteron remained poorly differentiated and the stomodeum did not form (n=6/6). These findings indicate that fgfA function is required in tissues derived from the vegetal hemisphere for skeletogenesis and morphogenesis of the gut.
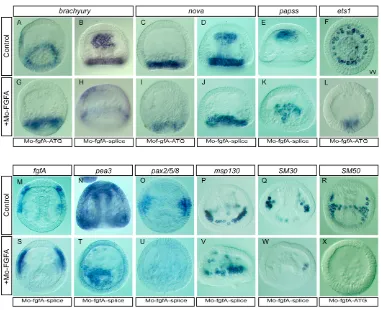
FGFA is required for expression of pax2/5/8and pea3in the ectoderm and of genes encoding spicule matrix proteins in the PMCs
[image:8.612.315.562.58.328.2]As inhibition of fgfA function interferes with invagination and differentiation of the archenteron, we examined the expression of nova and bhmt, two endodermal markers that start to be expressed in the vegetal plate at mesenchyme blastula stage (Röttinger et al., 2006), of brachyury, which is expressed dynamically at the ectoderm-endoderm boundary (Croce et al., 2001a; Gross and McClay, 2001), and of papss, which is expressed in the SMC territory (Röttinger et al., 2004), in the fgfA morphants (Fig. 6). Apparently normal levels of expression of brachyury (Fig. 6A,B,G,H, n>70) and slightly reduced expression of nova(Fig. 6C,D,I,J, n>100) and bhmt(data not shown) were still observed at the mesenchyme blastula and gastrula stage in most embryos in which FGF function had been blocked. These results indicate that FGF signaling is probably not required for mesendoderm specification per se, but most likely for the cellular movements of gastrulation and for invagination of the archenteron. Similarly, the expression of papss(Fig. 6E,K, n>200) and the early expression of ets1, skeTand alx1, (Fig. 6F,L and data not shown, n>70 for each marker) three transcription factors required for PMC specification (Croce et al., 2001b; Ettensohn et al., 2003; Kurokawa et al., 1999), were unaffected by FGFA morpholinos, suggesting that FGFA signaling participates in regulating PMC differentiation, although not their specification. We then examined the expression of gene markers in the ectoderm, such as otp (Cavalieri et al., 2003; Di Fig. 5. FGFA function is required in the vegetally derived tissues for skeleton formation and morphogenesis of the gut in sea urchin.(A) Experimental design. The animal and vegetal halves of wild-type (gray) and fgfA-splice morpholino-injected embryos (red) were recombined as shown. (Ba,b) Control uninjected embryos. Side views. (c-f) Chimeras in which fgfA function is inhibited in the animal half develop into pluteus larvae with reduced oral lobes and arms (arrow in e). (g-j) Inhibition of fgfA function in the vegetal half prevents skeletogenesis and disrupts gut morphogenesis.
D
E
V
E
LO
P
M
E
N
Bernardo et al., 1999), fgfA, pax2/5/8 and pea3, in the FGF morphants. Expression of the homeobox gene otp, which has been implicated previously in skeletogenesis, was largely unaffected (data not shown, n>40) following downregulation of FGFA. Remarkably, FGFA expression was always much stronger in the FGFA morphants compared with controls, suggesting that FGFA regulates its own expression through a negative-feedback loop (Fig. 6M,S, n>100). By contrast, expression of pax2/5/8and pea3in bilateral regions of the ectoderm (Fig. 6N,T,O,U) was abolished in most (95% n>200) of the FGFA morphants. Furthermore, the expression of two out of three genes examined encoding spicule matrix glycoproteins synthesized during skeletogenesis, msp130, SM30 and SM50 (Anstrom et al., 1987; Benson et al., 1987; George et al., 1991; Guss and Ettensohn, 1997), was either abolished or barely detectable
(n>200), whereas the third remained expressed at a high level (Fig. 6P-R,V-X) (n>150). Most of these experiments were initially performed using the FGFA-ATG morpholino and subsequently repeated using the FGFA-splice morpholino with identical results.
FGFA signaling largely accounts for activation of ERK in the lateral ectoderm, and ERK is required for expression of sproutyand pax2/5/8in these domains
[image:9.612.50.431.56.366.2]We have shown previously that during gastrulation the MAP kinase ERK is activated in the PMC clusters and overlying ectoderm (Röttinger et al., 2004). The domains of activation of ERK coincide with the territories expressing fgfA and sproutyduring gastrulation, raising the possibility that FGFA signaling may be responsible for
Fig. 6. Molecular analysis of FGFA loss of function in sea urchin.(A-X) The effects of loss of FGFA function on the gene expression program of the endoderm SMCs, ectoderm or PMCs were analysed by in situ hybridization with the indicated probes. See text for details.
Fig. 7. FGFA is required for activation of ERK in lateral ectodermal regions whereas ERK is required for expression of sproutyand pax2/5/8 in sea urchin.(A-D) Control or MoFGF-injected embryos were processed for whole-mount immunolocalization of activated ERK using an anti MAPK-P antibody (A,C) or for in situ hybridization using a sproutyprobe (B,D). (E-J) Embryos at the mesenchyme blastula stage were treated or not with the MEK inhibitor U0126 for 6 hours and processed for in situ hybridization using the indicated probes.
D
E
V
E
LO
P
M
E
N
[image:9.612.53.507.545.692.2]activation of ERK in these bilateral domains. In most embryos, blocking translation of the fgfA transcript strongly reduced activation of ERK within the lateral ectoderm at gastrula stages (Fig. 7A,C). Similarly, expression of sprouty within the lateral ectoderm was abolished in most of the FGFA morphants whereas expression in the endoderm was still present (Fig. 7B,D; n>200). To ask if the FGFA-dependent expression of sproutyand pax2/5/8requires the ERK signaling pathway, we treated embryos with the MEK inhibitor U0126 immediately following ingression of the PMCs and analysed expression of these genes at the early gastrula stage. fgfA was strongly expressed in the lateral ectoderm following treatment with the inhibitor (Fig. 7E,H; n>50). By contrast, expression of sprouty (Fig. 7F,I; n>150) and pax2/5/8(Fig. 7G,J; n>200) was abolished, which is consistent with these genes being downstream transcriptional targets of FGF signaling.
Partially overlapping functions of FGFR1 and FGFR2 in gastrulation and PMC patterning
Inhibition of FGFR1 using two different antisense morpholino oligonucleotides (Fig. 8E-H and data not shown) or with a dominant-negative form of FGFR1 (Fig. 8I,L) strongly delayed the initiation of gastrulation, consistent with our previous observation that FGFR1 is strongly expressed in precursors of the PMCs and in the vegetal plate before the onset of gastrulation (Lapraz et al., 2006). Surprisingly, at 48 hours, a significant recovery of archenteron formation and differentiation of the spicules was observed in most of the embryos, but these spicules remained rudimentary and the embryos did not acquire the typical elongated pluteus shape (Fig. 8H,L). In embryos injected with antisense morpholino oligonucleotides against fgfr2 transcripts, PMC ingression and archenteron invagination occurred on schedule (Fig. 8M,N,Q,R), but in most cases these PMCs did not acquire the regular spatial organization characteristic of these cells (Fig. 8N,R). Small skeletal rudiments nevertheless formed in these embryos, but in most of the larvae they did not elongate as much as in the controls and remained as small tri-radiated spicules (Fig. 8O,P,S,T). FGFR2 signaling thus appears to be required, in addition to FGFR1, for normal patterning and differentiation of the PMCs. When the morpholino oligonucleotides against FGFR1 and FGFR2 were co-injected, the resulting phenotype was more severe than with each morpholino: PMC ingression and gastrulation were strongly delayed, and no spicule rudiment formed (Fig. 8U-X), as described above for the fgfA morphants.
To characterize the respective roles of FGFR1 and FGFR2 in gastrulation and skeletogenesis, we examined the expression of fz5/8, bhmt, pax2/5/8and SM30in embryos injected with MoFGFR1 and MoFGFR2, singly or together (Fig. 9). Vegetal pole expression offz5/8was perturbed following interference with FGFR1 but not with FGFR2 (Fig. 9A-D). Expression of bhmtin the endoderm was only slightly affected by inhibition of FGFR1 or of FGFR2, consistent with the idea that FGF signaling does not regulate endomesoderm specification (Fig. 9E-H). By contrast, and as anticipated from the FGFR1 expression pattern, pax2/5/8 expression in the ectoderm was abolished in all the embryos injected with FGFR-Mo (Fig. 9J). Unexpectedly, pax2/5/8expression was also lost in all embryos injected with FGFR2-Mo (Fig. 9K). This result could be explained if reciprocal FGF signaling between the PMCs and the ectoderm is required to maintain ectodermal pax2/5/8 expression.
In 48 hour larvae in which either FGFR1 or FGFR2 function was blocked, expression of the biomineralization marker SM30 was downregulated but still detectable (Fig. 9N,O), consistent with the
presence of small spicule rudiments in these embryos. By contrast, co-injection of the two morpholinos resulted in a complete loss of SM30 expression (Fig. 9P). We conclude that FGFR1 and FGFR2 both participate in the regulation of skeletogenesis and have partially redundant function in regulating the gene expression in the ectoderm and in the skeletogenic differentiation program of the PMCs.
DISCUSSION
FGF and VEGF as regulators of PMC migration and differentiation
[image:10.612.315.561.57.423.2]In this article, we have presented several lines of evidence showing that an FGFA/FGFR pathway regulates migration and differentiation of the PMCs. First, we showed that fgfA is expressed before the onset of gastrulation in a pattern that prefigures the bilateral clusters of PMCs, suggesting that it may be one of the elusive chemoattractants for the migrating PMCs. Furthermore, we found that concomitantly with the onset of fgfA expression, the migrating PMCs start to express FGFR2. Finally, we showed that a dramatic effect of inhibiting fgfA or its two putative receptors is inhibition of migration and differentiation of the PMCs and, as a consequence, absence of skeleton.
Fig. 8. Roles of FGFR1 and FGFR2 in gastrulation and
skeletogenesis in sea urchin.(A-D) Control embryos. (E-X) Embryos injected with antisense morpholino oligonucleotides directed against FGFR1 (E-H) or mRNA encoding a dominant-negative form of FGFR1 (I-L) or with antisense morpholino oligonucleotides against FGFR2 (M-T) or co-injected with both FGFR1-Mo and FGFR2-Mo (U-X).
D
E
V
E
LO
P
M
E
N
Previous studies had shown that the ectoderm plays a crucial role in steering the PMCs after their ingression and that it regulates the skeletogenic gene expression program in these cells (Ettensohnn and Winklbauer, 2004; Hardin, 1996; McClay et al., 1992; McClay et al., 2004). Micromeres isolated at the 16-cell stage can differentiate in vitro into spicules, and this property has been interpreted as reflecting the cell-autonomous programming of the PMCs (Okazaki, 1975). However, normal seawater cannot support spicule formation unless it is supplemented with horse serum, suggesting that isolated PMCs lack some components normally provided by the other cells. FGFA is an excellent candidate for the elusive growth factor present in the serum. When extra PMCs are transplanted into the blastocoel of recipient embryos, resulting in two or three times the normal number of skeletogenic precursors being present, a skeleton of normal size forms, indicating that the size and scale of the spicules depends on spatial cues provided by other cell types (Armstrong et al., 1993b; Ettensohn, 1990b). The finding thatfgfA regulates morphogenesis of the spicules and is first expressed in the ectoderm overlying the PMC clusters is consistent with these observations. Finally, treatments with NiCl2, which radialize sea urchin embryos, cause development of supernumerary spicule primordial, probably by extending the spatial cues normally present in ventrolateral regions of the embryo (McClay et al., 1992). The radial extension of fgfA expression in NiCl2-treated embryos could explain these effects.
Numerous studies in vertebrates have documented direct chemotactic effects of FGFs on migrating mesenchymal cells. Migrating mesencephalic neural crest cells are attracted by FGF2 and FGF8 (Kubota and Ito, 2000), and these same factors act as attractants for mesenchymal cells during limb bud development (Webb et al., 1997). During gastrulation in the chick, mesodermal cells emerging from the primitive streak follow different trajectories guided by FGF signals from neighboring tissues (Yang et al., 2002). Experiments
using implantation of beads soaked with different FGFs showed that streak cells are attracted by FGF4 and repelled by FGF8. Axonal navigation and angiogenesis provide some of the best examples of FGF-dependent regulation of cell migration. Axonal navigation and angiogenesis depend upon attractive and repulsive guidance cues provided by the cellular environment and interpreted by the growth cones of axons or the migrating endothelial precursors (Dickson, 2002; Tessier-Lavigne and Goodman, 1996). A classical example of axonal navigation is provided by the axons of the spinal motoneurons that leave the spinal cord. After following a common ventral path, these axons subsequently diverge and follow specific pathways to innervate different organs, such as the limbs, sympathetic ganglia or axial musculature. Recent studies have demonstrated that FGF family members including FGF9 are produced by the dermomyotome and selectively attract the axons of a subpopulation of spinal cord motoneurons called MMCm (Shirasaki et al., 2006), which selectively express FGFR1, whereas the other subclasses of neurons lack this receptor. The role of FGFs in this process is remarkably similar to the role of FGF in guidance of primary mesenchyme cells in the sea urchin embryo. In both cases, the ligand is produced by the target cells (ectodermal cells in the sea urchin embryo and dermomyotome in mouse) and it appears to act on a long range to attract the migrating cells, which specifically express the receptor.
Another interesting parallel could be drawn between skeletogenesis in the sea urchin embryo and angiogenesis in vertebrates. Neovascularization is a complex process that involves guided migration of endothelial cells and the combined activities of different RTKs (Cross and Claesson-Welsh, 2001). FGF was the first growth factor purified with angiogenic properties (Shing et al., 1984), a finding rapidly followed by the discovery of VEGF. A recent study has described the essential role of VEGF/VEGFR signaling in PMC patterning and skeletal morphogenesis (Duloquin et al., 2007). The spatial expression of the FGFA and VEGF and of FGFR2 and VEGFR are largely congruent. However, these two signals are independent and not functionally redundant and thus both are required for correct migration and differentiation of the primary mesenchyme.
FGF signaling and branching morphogenesis
FGF signaling plays a key role in regulating the formation of the ramified respiratory system of Drosophila and vertebrates. In Drosophila, a core FGF signaling pathway including Btl/FGFR and its FGF ligand, Branchless (Bnl), is used repeatedly to control the successive rounds of branching (Metzger and Krasnow, 1999). In this process, FGF expressed by clusters of cells in the tracheal sac plays the role of a chemoattractant for the migrating tracheal cells. Similarly, in vertebrates, FGF10 regulates the branching pattern of the lung, as shown by the absence of lung in the Fgf10-knockout mice (Min et al., 1998) and by the severe inhibition of branching observed following expression of a dominant-negative FGF receptor in the bronchial epithelium (Peters et al., 1994).
[image:11.612.49.301.62.321.2]In sea urchin embryos, previous studies had suggested that signals from the ectoderm are required to direct both the growth, the branching pattern and the final size of the spicules (Armstrong et al., 1993a; Ettensohn, 1990b; Ettensohn and Malinda, 1993; Ettensohn and McClay, 1986; Hardin et al., 1992; Katow et al., 2000; Malinda and Ettensohn, 1994; McClay et al., 1992; Peterson and McClay, 2003). For example, spicules formed in vitro from isolated micromeres are linear and lack the complex branches observed in vivo (Okazaki, 1975). They are also much longer than the spicules present in normal embryos, suggesting that isolated PMCs lack signals that regulate growth of the spicules.
Fig. 9. Molecular analysis of FGFR1 and FGFR2 loss of function in sea urchin.(A-D) Mesenchyme blastula, (E-L) mid gastrula, (M-P) early pluteus. In situ hybridization using fz58(A-D), bhmt(E-H), pax258(I-L) and SM30(M-P) probes.
D
E
V
E
LO
P
M
E
N
Despite the fact that the skeleton of the sea urchin larva does not form from an epithelium as the lung of vertebrates and the tracheae of flies, but from a syncytium, we found that, fgfA, sprouty, pea,ets1 and fgfr2are expressed selectively in all regions where the spicules will branch, allowing formation of the oral arms of the pluteus larva. This suggests that the FGF signaling pathway regulates formation of the complex three-dimensional structure that serves as a skeleton for the embryo and reinforces the idea that the function of this pathway in regulating formation of ramified organs and structures is highly conserved between species.
FGF as a signal regulating growth of the spicules
Local PMC-ectoderm interactions are required for skeletal rod elongation, as shown by photoablation experiments of the growing arms (Ettensohn, 1990a). Curiously, fgfA, ets1, pax2/5/8and sprouty are all expressed in the regions where the arms of the pluteus larva will form but in a different register. Expression of fgfA andets1is mainly restricted to the isolated PMCs located just at the tip of the growing arms, while pax2/5/8 and sprouty are detected in the ectoderm surrounding the tip of the arm rods. This suggests that the interplay between ectoderm and mesenchyme cells continues at later stages and that FGFA and Pax2/5/8 may also be involved in regulating skeletal rod elongation.
FGFR2 and reciprocal FGF signaling between the PMCs and the ectoderm
Studies on lung morphogenesis and outgrowth of the limb bud suggest that FGF signaling between the epithelial and mesenchymal compartments is reciprocal (Lewandoski et al., 2000; White et al., 2006; Zhang et al., 2006). In other words, the epithelium signals to the mesenchyme, which signals back to the epithelium. For example, during limb bud outgrowth in the mouse embryo, both FGF8 in the mesenchyme and FGF9 in the apical ectodermal ridge regulate outgrowth of the bud. Reciprocal signaling between the ectoderm and mesenchymal cells probably also occurs in the sea urchin embryo, as we found that blocking FGFR2 signaling in the PMCs eliminates pax2/5/8expression from the overlying ectoderm. The signal released by the PMCs that is required to maintain pax2/5/8in the overlying ectoderm is presently not known. AsfgfA is first expressed within the ectoderm, then within the PMCs of the ventrolateral clusters, this signal could be fgfA itself. Alternatively, it could be another member of the FGF family, as in the case of vertebrates. Experiments using chimeras in which micromeres derived from FGFA morpholino-injected embryos will be transplanted into control embryos could help to address this issue.
FGF signaling and the regulation of gastrulation
The role of FGF signals in gastrulation is also well documented (Bottcher and Niehrs, 2005; Sivak and Amaya, 2004; Thisse and Thisse, 2005). Studies in Xenopushave shown that FGF signaling regulates cell motility, lamellipodia formation and cellular polarization. In addition, it is required for gastrulation through the expression of downstream genes such as sprouty and of the neurotrophin-receptor-like molecule Nrh (Christofori, 2003; Chung et al., 2005; Nutt et al., 2001; Sivak et al., 2005). Our finding that FGF signaling is crucially required for gastrulation is in line with previous observations showing that FGFR1 is expressed at high levels in the vegetal pole region before and during gastrulation (Lapraz et al., 2006). It is also consistent with previous findings showing that treatments with the growth factor inhibitor suramin severely interfere with gastrulation when applied during blastula stages onward (Katow and Aizu, 2002) and that MEK inhibition by
U0126 causes exogastrulation in the Strongylocentrotus (Fernandez-Serra et al., 2004; Kumano and Foltz, 2003). However, FGFA is probably not the only FGF ligand involved in the regulation of gastrulation, as its inhibition does not eliminate expression of sproutyand pea3within the vegetal plate and archenteron.
Taken together, our findings show that FGF signals are essential components of the developmental program of the sea urchin embryo, regulating several essential processes such as oriented cell migration and differentiation of skeleton, as well as gastrulation. The sea urchin embryo, which is simple, readily accessible to experimental perturbations and transparent, will hopefully help to unravel the fundamental regulatory mechanisms underlying these processes.
We thank Giovanni Spinelli for the SM30 and SM50 probes, Meinrad Busslinger and Thomas Czerny for the full-length P.lividus pax2/5/8cDNA, Christian Gache for the Frz5/8 cDNA probe, Morgane Poulain for her participation in the initial stages of this project and our colleagues for help and support, and particularly Evelyn Houliston and Hitoyoshi Yasuo for fruitful discussions and careful reading of the manuscript. We thank Laurent Gilletta and David Luquet for taking care of the animals. This work was supported by the Association pour la Recherche sur le Cancer (ARC), the Agence Nationale de la Recherche (ANR), the CNRS, the Université of Paris 6, the European consortium Marine Genomics Europe and the Genoscope. E.R. was supported by a fellowship from the ARC and FRM foundations.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/2/353/DC1
References
Anstrom, J. A., Chin, J. E., Leaf, D. S., Parks, A. L. and Raff, R. A.(1987). Localization and expression of msp130, a primary mesenchyme lineage- specific cell surface protein in the sea urchin embryo. Development101, 255-265. Armstrong, N., Hardin, J. and McClay, D. R.(1993a). Cell-cell interactions
regulate skeleton formation in the sea urchin embryo. Development119, 833-840.
Armstrong, N., Hardin, J. and McClay, D. R.(1993b). Cell-cell interactions regulate skeleton formation in the sea urchin embryo. Development119, 833-840.
Beane, W. S., Gross, J. M. and McClay, D. R.(2006). RhoA regulates initiation of invagination, but not convergent extension, during sea urchin gastrulation. Dev.
Biol.292, 213-225.
Bellosta, P., Iwahori, A., Plotnikov, A. N., Eliseenkova, A. V., Basilico, C. and Mohammadi, M.(2001). Identification of receptor and heparin binding sites in fibroblast growth factor 4 by structure-based mutagenesis. Mol.Cell.Biol.21, 5946-5957.
Benson, S., Sucov, H., Stephens, L., Davidson, E. and Wilt, F.(1987). A lineage-specific gene encoding a major matrix protein of the sea urchin embryo spicule. I. Authentication of the cloned gene and its developmental expression.
Dev.Biol.120, 499-506.
Bottcher, R. T. and Niehrs, C.(2005). Fibroblast growth factor signaling during early vertebrate development. Endocr.Rev. 26, 63-77.
Casci, T., Vinos, J. and Freeman, M.(1999). Sprouty, an intracellular inhibitor of Ras signaling. Cell96, 655-665.
Cavalieri, V., Spinelli, G. and Di Bernardo, M.(2003). Impairing Otp homeodomain function in oral ectoderm cells affects skeletogenesis in sea urchin embryos. Dev.Biol.262, 107-118.
Christofori, G.(2003). Split personalities: the agonistic antagonist Sprouty. Nat.
Cell Biol.5, 377-379.
Chung, H. A., Hyodo-Miura, J., Nagamune, T. and Ueno, N.(2005). FGF signal regulates gastrulation cell movements and morphology through its target NRH.
Dev.Biol.282, 95-110.
Croce, J., Lhomond, G. and Gache, C.(2001a). Expression pattern of Brachyury in the embryo of the sea urchin Paracentrotus lividus. Dev.Genes Evol. 211, 617-619.
Croce, J., Lhomond, G., Lozano, J. C. and Gache, C.(2001b). ske-T, a T-box gene expressed in the skeletogenic mesenchyme lineage of the sea urchin embryo. Mech.Dev. 107, 159-162.
Croce, J., Duloquin, L., Lhomond, G., McClay, D. R. and Gache, C.(2006). Frizzled5/8 is required in secondary mesenchyme cells to initiate archenteron invagination during sea urchin development. Development133, 547-557. Cross, M. J. and Claesson-Welsh, L.(2001). FGF and VEGF function in
angiogenesis: signalling pathways, biological responses and therapeutic
inhibition. Trends Pharmacol.Sci. 22, 201-207.
D
E
Czerny, T., Bouchard, M., Kozmik, Z. and Busslinger, M.(1997). The characterization of novel Pax genes of the sea urchin and Drosophila reveal an ancient evolutionary origin of the Pax2/5/8 subfamily. Mech.Dev. 67, 179-192. Decker, G. L. and Lennarz, W. J.(1988). Skeletogenesis in the sea urchin embryo.
Development103, 231-247.
Di Bernardo, M., Castagnetti, S., Bellomonte, D., Oliveri, P., Melfi, R., Palla, F. and Spinelli, G.(1999). Spatially restricted expression of PlOtp, a
Paracentrotus lividus orthopedia-related homeobox gene, is correlated with oral ectodermal patterning and skeletal morphogenesis in late-cleavage sea urchin embryos. Development126, 2171-2179.
Dickson, B. J.(2002). Molecular mechanisms of axon guidance. Science298, 1959-1964.
Duboc, V., Röttinger, E., Besnardeau, L. and Lepage, T.(2004). Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev.
Cell6, 397-410.
Duboc, V., Röttinger, E., Besnardeau, L., Lapraz, F. and Lepage, T.(2005). Left-right asymmetry in the sea urchin embryo is regulated by Nodal signalling on the right side. Dev.Cell8, 1-12.
Duloquin, L., Lhomond, G. and Gache, C.(2007). Localized VEGF signaling from ectoderm to mesenchyme cells controls morphogenesis of the sea urchin embryo skeleton. Development134, 2293-2302.
Ettensohn, C. A.(1990a). Cell interactions in the sea urchin embryo studied by fluorescence photoablation. Science248, 1115-1118.
Ettensohn, C. A.(1990b). The regulation of primary mesenchyme cell patterning.
Dev.Biol.140, 261-271.
Ettensohn, C. A. and McClay, D. R.(1986). The regulation of primary mesenchyme cell migration in the sea urchin embryo: transplantations of cells and latex beads. Dev.Biol.117, 380-391.
Ettensohn, C. A. and Malinda, K. M.(1993). Size regulation and
morphogenesis: a cellular analysis of skeletogenesis in the sea urchin embryo.
Development119, 155-167.
Ettensohn, C. A. and Winklbauer, R.(2004). Cell-substrate interactions during deuterostome gastrulation. In Gastrulation(ed. C. Stern), pp. 317-328. London: Cold Spring Harbor Laboratory Press.
Ettensohn, C. A., Illies, M. R., Oliveri, P. and De Jong, D. L.(2003). Alx1, a member of the Cart1/Alx3/Alx4 subfamily of Paired-class homeodomain proteins, is an essential component of the gene network controlling skeletogenic fate specification in the sea urchin embryo. Development130, 2917-2928.
Fernandez-Serra, M., Consales, C., Livigni, A. and Arnone, M. I.(2004). Role of the ERK-mediated signaling pathway in mesenchyme formation and differentiation in the sea urchin embryo. Dev.Biol.268, 384-402. George, N. C., Killian, C. E. and Wilt, F. H.(1991). Characterization and
expression of a gene encoding a 30.6-kDa Strongylocentrotus purpuratus spicule matrix protein. Dev.Biol.147, 334-342.
Gross, J. M. and McClay, D. R.(2001). The role of Brachyury (T) during gastrulation movements in the sea urchin Lytechinus variegatus. Dev.Biol.239, 132-147.
Guindon, S., Lethiec, F., Duroux, P. and Gascuel, O.(2005). PHYML Online-a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res.33, W557-W559.
Guss, K. A. and Ettensohn, C. A.(1997). Skeletal morphogenesis in the sea urchin embryo: regulation of primary mesenchyme gene expression and skeletal rod growth by ectoderm-derived cues. Development124, 1899-1908. Hacohen, N., Kramer, S., Sutherland, D., Hiromi, Y. and Krasnow, M. A.
(1998). sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell92, 253-263.
Hans, S., Liu, D. and Westerfield, M.(2004). Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development131, 5091-5102.
Hardin, J.(1996). The cellular basis of sea urchin gastrulation.Curr.Top.Dev.Biol. 33, 159-262.
Hardin, J., Coffman, J. A., Black, S. D. and McClay, D. R.(1992). Commitment along the dorsoventral axis of the sea urchin embryo is altered in response to NiCl2. Development116, 671-685.
Horstadius, S.(1973). Experimental Embryology of Echinoderms. Oxford: Clarendon Press.
Katow, H. and Aizu, G.(2002). Essential role of growth factor receptor-mediated signal transduction through the mitogen-activated protein kinase pathway in early embryogenesis of the echinoderm. Dev.Growth Differ. 44, 437-455.
Katow, H., Nakajima, Y. and Uemura, I.(2000). Primary mesenchyme cell-ring pattern formation in 2D-embryos of the sea urchin. Dev.Growth Differ. 42, 9-17.
Kominami, T. and Takata, H.(2004). Gastrulation in the sea urchin embryo: a model system for analyzing the morphogenesis of a monolayered epithelium.
Dev.Growth Differ. 46, 309-326.
Kramer, S., Okabe, M., Hacohen, N., Krasnow, M. A. and Hiromi, Y.(1999). Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development126, 2515-2525.
Kubota, Y. and Ito, K.(2000). Chemotactic migration of mesencephalic neural crest cells in the mouse. Dev.Dyn. 217, 170-179.
Kumano, M. and Foltz, K. R.(2003). Inhibition of mitogen activated protein kinase signaling affects gastrulation and spiculogenesis in the sea urchin embryo. Dev.Growth Differ. 45, 527-542.
Kurokawa, D., Kitajima, T., Mitsunaga-Nakatsubo, K., Amemiya, S., Shimada, H. and Akasaka, K.(1999). HpEts, an ets-related transcription factor implicated in primary mesenchyme cell differentiation in the sea urchin embryo.
Mech.Dev. 80, 41-52.
Lapraz, F., Röttinger, E., Duboc, V., Range, R., Duloquin, L., Walton, K., Wu, S. Y., Bradham, C., Loza, M. A., Hibino, T. et al.(2006). RTK and TGF-beta signaling pathways genes in the sea urchin genome. Dev.Biol. 300, 132-152.
Lewandoski, M., Sun, X. and Martin, G. R.(2000). Fgf8 signalling from the AER is essential for normal limb development. Nat.Genet.26, 460-463.
Malinda, K. M. and Ettensohn, C. A.(1994). Primary mesenchyme cell migration in the sea urchin embryo: distribution of directional cues. Dev.Biol.164, 562-578.
Malinda, K. M., Fisher, G. W. and Ettensohn, C. A.(1995). Four-dimensional microscopic analysis of the filopodial behavior of primary mesenchyme cells during gastrulation in the sea urchin embryo. Dev.Biol.172, 552-566. Mason, J. M., Morrison, D. J., Basson, M. A. and Licht, J. D.(2006). Sprouty
proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol.16, 45-54.
McClay, D. R., Armstrong, N. A. and Hardin, J.(1992). Pattern formation during gastrulation in the sea urchin embryo. Dev.Suppl. 1992, 33-41.
McClay, D. R., Gross, J. M., Range, R., Peterson, R. E. and Bradham, C.(2004). Sea urchin gastrulation. In Gastrulation(ed. C. Stern), pp. 123-137. London: Cold Spring Harbor Laboratory Press.
McCoon, P. E., Angerer, R. C. and Angerer, L. M.(1996). SpFGFR, a new member of the fibroblast growth factor receptor family, is developmentally regulated during early sea urchin development. J.Biol.Chem. 271, 20119-20125.
McCoon, P. E., Blackstone, E., Angerer, R. C. and Angerer, L. M.(1998). Sea urchin FGFR muscle-specific expression: posttranscriptional regulation in embryos and adults. Dev.Biol.200, 171-81.
Metzger, R. J. and Krasnow, M. A.(1999). Genetic control of branching morphogenesis. Science284, 1635-1639.
Miller, J., Fraser, S. E. and McClay, D.(1995). Dynamics of thin filopodia during sea urchin gastrulation. Development121, 2501-2511.
Min, H., Danilenko, D. M., Scully, S. A., Bolon, B., Ring, B. D., Tarpley, J. E., DeRose, M. and Simonet, W. S.(1998). Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156-3161.
Nutt, S. L., Dingwell, K. S., Holt, C. E. and Amaya, E.(2001). Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 15, 1152-1166.
Okazaki, K.(1975). Spicule formation by isolated micromeres of the sea urchin embryo. Am.Zool.15, 567-581.
Ornitz, D. M.(2000). FGFs, heparan sulfate and FGFRs: complex interactions essential for development. BioEssays 22, 108-112.
Ornitz, D. M. and Itoh, N.(2001). Fibroblast growth factors. Genome Biol.2, REVIEWS3005.
Peters, K., Werner, S., Liao, X., Wert, S., Whitsett, J. and Williams, L.(1994). Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 13, 3296-3301.
Peterson, R. E. and McClay, D. R.(2003). Primary mesenchyme cell patterning during the early stages following ingression. Dev.Biol.254, 68-78.
Raible, F. and Brand, M.(2001). Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development.
Mech.Dev. 107, 105-117.
Reifers, F., Bohli, H., Walsh, E. C., Crossley, P. H., Stainier, D. Y. and Brand, M. (1998). Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis.
Development125, 2381-2395.
Roehl, H. and Nusslein-Volhard, C.(2001). Zebrafish pea3 and erm are general targets of FGF8 signaling.Curr.Biol.11, 503-507.
Röttinger, E., Besnardeau, L. and Lepage, T.(2004). A Raf/MEK/ERK signaling pathway is required for development of the sea urchin embryo micromere lineage through phosphorylation of the transcription factor Ets. Development
131, 1075-1087.
Röttinger, E., Besnardeau, L. and Lepage, T.(2006). Expression pattern of three putative RNA-binding proteins during early development of the sea urchin Paracentrotus lividus. Gene Expr.Patterns6, 864-872.
Sanchez-Heras, E., Howell, F. V., Williams, G. and Doherty, P.(2006). The fibroblast growth factor receptor Acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J.Biol.
Chem. 281, 35208-35216.
Shing, Y., Folkman, J., Sullivan, R., Butterfield, C., Murray, J. and Klagsbrun,