HIGH – PERFORMANCE LIQUID CHROMATOGRAPHY METHOD VALIDATION AND DEVELOPMENT STRATEGY FOR RIFABUTIN
Full text
Figure
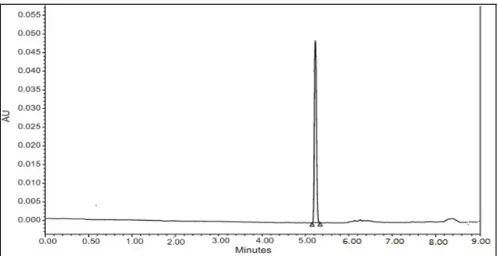

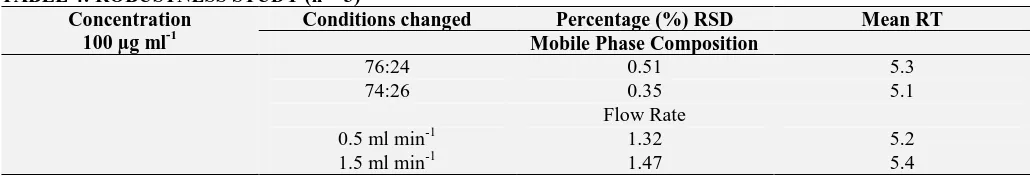
Related documents
Walachowski S, Dorenlor V, Lefevre J, Lunazzi A, Eono F, Merbah T, Eveno E, Pavio N, Rose N (2014) Risk factors associated with the presence of hepatitis E virus in livers
This study aimed to develop and pilot test an educa- tional video containing information regarding the in- formed consent process for trauma patients undergoing surgery, develop
Patients with inflammatory bowel disease (IBD) may have increased risk of developing CDI, along with worse outcomes, higher rates of colectomy and higher rates of recurrence
The purpose of this study is to characterize the very eld- erly population, who received emergency general surgery, and examine their surgical outcomes including identifica- tion
suis cells were detected on PAECs incubated with the negative control preparation (Figure 5d).. To assess whether the presence
In this paper we describe how a participatory, multi- method, continuous informed consent process developed by researchers, study participants and community stake- holders during
Aldous EW, Mynn JK, Banks J, Alexander DJ: A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a
However, the nanosilver-coated orthodontic bracket can be utilized during fixed orthodontic treatment with caution, because the foreign particles which are brown-black granules