International Journal of Medical Science and Current Research (IJMSCR)
Available online at: www.ijmscr.com
Volume2, Issue 1,Page No: 15-27
January-February 2019
15
Detection of colibactin genes of E. coli in patients with colorectal polyps as a predictive
sign of colorectal cancer
Zahraa Falah Al-Fatlawi, M.Sc., Ali Abdul Hussein S. AL-Janabi, postdoctoral
Dept. of Microbiology, College of Medicine, University of Karbala, Karbala-Iraq
*Corresponding Author:
Professor Ali Abdul Hussein S. AL-Janabi
Dept. of Microbiology, College of Medicine, University of Karbala, Karbala-Iraq
Type of Publication: Original Research Paper Conflicts of Interest: Nil
ABSTRACT
Introduction: Colorectal cancer (CRC) is a malignant epithelial tumour of the colon or rectum. Most of CRC can develop from colorectal polyp. Colibactin genes are suggested to associate with conversion of colorectal polyp to CRC.
Patients and Methods: A total of 100 subjects distributed between 50 patients with colorectal polyps and 50 healthy individuals as a control group were involved in a cohort control study.Rectal swabs were collected from involved subjects for isolation of E. coli.
ClbA and clbP genes were chosen as the main colibactin genes diagnosed in E. coli.
Results: Colibactin genes were mostly diagnosed in all patients with colon polyps, while they less found in those with rectal polyps. They also found without a single distribution in 13 patients with neoplastic polyp. Females with inflammatory polyp and hamartomatous polyp as two types of non-neoplastic polyp contained both of genes. The distribution of colibactin genes among healthy individuals was variable. A high percentage of colibactin genes were found in patients who needed 7-9 months and 1-2 years for developing of neoplastic polyp.
Conclusion: A more frequency presence of colibactin genes in patients with colon polyp may make them more susceptible for development of colorectal cancer, especially in early development stages. Neoplastic polyps and the inflammatory and hamartomatous
polyps of non-neoplastic type are also contenting colibactin genes..
Keywords: colibactin, colorectal polyp, coloreactal cancer, E. coli.
INTRODUCTION
Colorectal cancer (CRC) is one of the gastrointestinal malignant tumours developing in the colon or rectum. It started in large intestinal tissues as a benign form can turn over to malignant tumour when it penetrated through the muscularis mucosa into submucosa [1]. CRC is a widely disease in all of the world with an incidence of 1.36 million and about 50,630 deaths due to its progression or complication based on GLOBOCAN series of the international agency for research on cancer 2012 [2]. It recorded to be the second most common cancer in the Arabic Gulf Co-operation Council (GCC) states as registered for ten years (1998-2007)[3]. In one of Iraqi cities (Karbala), it found to be the most common third type of cancers among males and fourth among females during the eight years (2008-2015)[4].
Colorectal polyp is an enlargement with overgrowth cells in the wall tissues of the large intestinal which may develop later to the malignant stage called CRC [5]. It can be histologically classified into neoplastic and non-neoplastic [6] when a non- neoplastic polyp involved hyperplastic polyps, inflammatory polyps, and hamartomatous polyp [5].
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
Pag
e
16
induction, genotoxins production, or conversion of procarcinogenic dietary factors into carcinogens [9-11]. This bacterium has the ability to cause many another extraintestinal diseases due to its content of 54-kb polyketide synthases pks pathogenicity island that encodes the assembly line system for the synthesis of colibactin as a peptidepolyketide hybrid genotoxin [12-13]. Thus, production of gentotoxins
by E. coli B2, especially colibactin encoded by pks
island, is an important mechanism associated with CRC development [11].
Colibactins are secondary genotoxic metabolites of cyclomodulins group created by human commensal and extraintestinal pathogenic E. coli [14]. Pks cluster genes responsible for producing of colibactin was first identified in 2006 by Nougayréde and colleagues. The pks island consists of a total of 19 genes (clbA to clbS)[15]. ClbA and clbP genes are the most important types of pks island of E. coli
required for the synthesis of colibactin [16].
Colibactin genes were investigated among patients with colorectal polyps as a causative agent for CRC.
Patients and Methods
Patients
A total of 100 subjects distributed between 50 patients with colorectal polyps (age range 3-80 years) and 50 healthy individuals (age range 5-75 years) as a control group were involved in a cohort control study. The group of patients, including 33 males (age range 5-80 years) and 17 females (age range 3-65 years), while those in control group distributed between 30 males (age range 5-75 years) and 20 females (age range 15-82 years). Colorectal polyps were investigated in both of patients and control by the histopathologists staff of the Centre of the digestive system of Al-Sadder hospitals through making a colonoscopy and histopathological examination of biopsy samples during admitted the involved subjects into the Centre in Al-Najaf province from December 2017 to January 2018. A healthy group was chosen after obtaining a negative result for polyps or other intestinal diseases by colonscopical examining. Patients were not taking any antibiotic for at least three days before sample collection and those under antibiotic treatment were excluded. Exclusion also included patients suffering from any other types of colorectal diseases.
Isolation of bacteria
Rectal swabs were collected from involved subjects. Collected specimens were cultured on EMB media (Himedia, India) as a specific medium for E. coli and incubated at 37°C for 24 h. Primary diagnosis of bacteria was depended on the visual notice of green metallic sheen colour of growing colonies and on morphological characters of isolated bacteria. Complete diagnosis of E. coli was performed by using Api 20E system for Enterobacteriaceae (BioMérieux, France).
Detection of colibactin genes and amplification conditions
A subculture of bacteria was prepared by inoculating the isolated strains in Müller-Hinton broth (Himedia, India) and incubated at 37°C for 24 h. Bacterial genomic DNA was extracted by PrestoTM Mini g DNA Bacteria Kit (Geneaid Biotech Ltd., USA).
ClbA and clbP genes were chosen as the main colibactin genes in E. coli. Primers and PCR conditions for amplifying of these genes were performed as mentioned by McCarthy et al. (2015) with some modification in PCR conditions [16].
Statistical Analysis
Data of all tests were expressed as mean ± SD. The values were analyzed statistically by one-way ANOVA by using the Excel application of Window 7. The minimum level of (p) value was < 0.05 concerts as a significant level.
Results
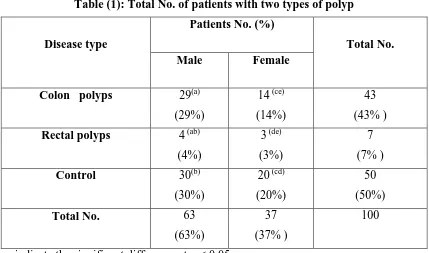
Colon polyp (43%) found to be the more polyp type than rectal polyp (7%) among involved patients with a significant difference at p <0.05 from a control. Males showed a high percentage of the colon (29%) and rectal polyps (4%) than females (14%, 3%, respectively) (table 1). Almost all patients with colon polyps contained clbA and clbP genes and no one of them had only one gene. The absence of genes was also recorded in 65.11% of patients. Most of the genes were found among males (10 patients) at the age range (30-39 years and 50-59 years) than in females (5 patients) (table 2). Meanwhile, patients with rectal polyps revealed less content from clbA
and clbP genes (3 patients) compared with patients
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
Pag
e
17
individuals that used as a control was variable (table 4).
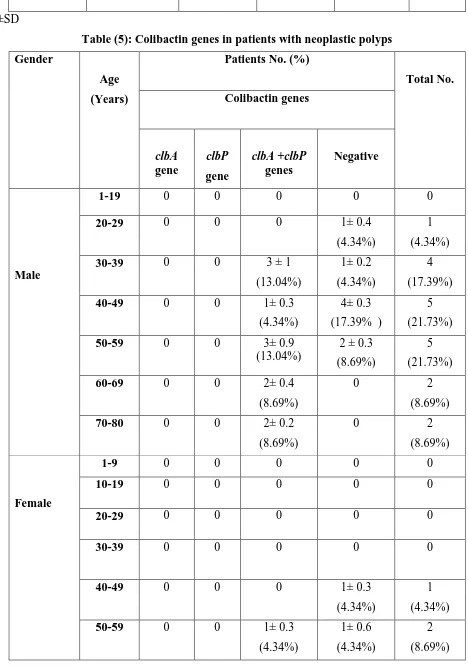
Colibactin genes were found in 13 patients (56.52%) with neoplastic polyp from a total of 23 patients, especially among males at age ranged 30-39 years and 50-59 years. All of isolated genes were occurred together without any single distribution (table 5). Meanwhile, no patients with hyperplastic polyp had any of these genes. Otherwise, females with inflammatory polyp or hamartomatous polyp showed the presence of both genes (2 and 3 patients, respectively). The absence of colibactin genes was also recorded among 21 patients (77.77%) with non-neoplastic polyps (table 6).
A high percentage of colibactin genes (8%) were found in patients who needed 7-9 months and 1-2 years for developing of neoplastic polyp. Meanwhile, they only found in 4 patients with non-neoplastic polyp who developed in 1-3 months. The clbA gene was singly found in patients who need 1-2 years (2%) for development of non-neoplastic polyp (table 7).
Discussion
Colorectal cancer (CRC) is a malignant disease induced by a number of hereditary and ecological factors [17]. Most CRC can originate from colorectal polyps by transformation of adenoma to carcinoma or it emerges from the gradual accumulation of pathogenic transformations (genetic mutation) and environmental factors [18-19].
Colon polyps found to be the more polyp type among involved patients with a significant difference from rectal polyp of both genders. This result is also illustrated by other studies. The percentage of colonic polyp (12.7%) in India, especially in right colon (6.9%) was noted to be higher than in the rectum (4.6%) [20]. Polyps in the colon also noted higher in Saudi Arabia (36.6%), especially in the sigmoid colon than in the rectum area (21%) [21]. On the other hand, rectum polyp cases could be higher in some cases as found in South Asia when the rectum poly represented 33.5%, while sigmoid colon was only 22.9% [22].
Among involved patients, the distribution of colorectal polyp showed a higher percentage in male than in females. The high percentage is also recorded in other different countries such as in Saudi Arabia (66.5%) [21], India (76.4%) [20], U.S.A. [23] and in
Iran [24]. However, Wickramasinghe et al. (2014) found that there is no statistically significant correlation between malignant colorectal polyp and either of age, gender, site, or histology [22].
Escherichia coli is a non-spore forming bacterium
with a Gram negative, rod, motile and facultative anaerobic [25]. It considers an important bacterium of Enterobacteriaceae family living as a normal flora in the parts of the human intestine [7]. Some strains
of E. coli related to B2 phylogenic group harbor a
polyketid synthase island (PKS) which diagnosed in 55.3% of colorectal cancer patients [26]. The genes of PKS are the main responsible for producing of colibactin [12]. Colibactin is secondary genotoxic metabolites of cyclomodulins group created by gut microbiota and extraintestinal pathogenic E. coli
[27]. Intracellular functions of such compound are activating of DNA damage, chromosomal instability, mutations, or cell cycle arrest [14]. Colorectal cancer is an advanced step of genetic function of colibactin as proved in a mouse model [28]. The most important genes of colibactin synthesis are clbA and clbP genes of the pks island of E. coli [16].
A high percentage of our involved patients with colon polyps contend both of clbA and clbP genes than in patients with rectal polyps. A mutation in
clbP gene (ΔclbP) in pks-positive E. coli showed less efficiency to develop a colon tumour than a wild type
of E. coli which may related to the ability of
colibactin producing wild type to increase carcinogenic capacity than a mutated type [29]. It also found that targeting clbP gene will block the deleterious effect of colibactin toxin in vitro and leads to a significant decrease in tumour numbers in vivo [15].
Colibactin genes in specimens of colon and rectal polyps were mostly found in middle or old age range of males compared with early age of females. An increased level of mucosa-associated and internalized
E. coli was observed in the colon cancer at age 75
years compared with normal tissue [30]. The pks positive-E. coli showed a strong contact with adenomatouse polyp at age range 40-85 years compared with no attachment of this bacterium on normal mucosa of control individuals [31].
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
Pag
e
18
be the most common type of polyp that can develop to CRC. It found in 60.9% of patients with colorectal polyp in Iran during five years (2005-2011) [24]. It also represented 74.1% of colorectal polyps based on a retrospective study during eight years (2006-2013) in Saudi Arabia [21]. The similar study was also performed in India, which revealed that adenomatous polyp during two years (2014-2016) was higher than other types of colorectal polyps [20].
Colibactin genes were mostly found at middle ages of adenomatouse patients. Most of studies recorded that adenomatouse polyp is common among male at old age as mentioned by Jain et al. (2017) who found that adenomatouse polyp more common in patients at the age above of 60 years [20]. Also adenomatouse found to be increased in male at age 42-85 years [24]. However, the incidence of it increased with age [22].
Colibactin genes of pks-positive E. coli are often associated with the contact of this bacterium with adenomatouse tissues of CRC cases. Such type of bacterium which belongs to B2 phylogroup found in high percentages (86%) of CRC [32] and also in 82% of patients with colonic adenoma and colorectal carcinoma [33]. The mechanisms used by E. coli to trigger the development of CRC could be progressive by inflammation induction, genotoxins production, or conversion of procarcinogenic dietary factors into carcinogens [9-11].
Inflammatory polyp was the most common type of non-neoplastic polyps among our involved patients who contain colibactin genes, especially in females at early ages. This result was also mentioned by Jain et al. (2017) when the inflammatory polyp was highly recognized at age below 40 years in India [20], while it found higher at age between 32 years to 76 years in patients of South Iran [24]. However, several studies illustrated that prevalence of inflammatory polyp was less than other types of non-neoplastic polyps. It recorded to be the lowest one of non-neoplastic types after hyperplastic, and juvenile polyps [21, 24].
Colorectal polyps as an elevated projects from the mucosa layer of the colon and/or rectum region is usually needed a time to progress into different shape and size. Thus, the presence of E. coli and its contents of colibactin genes could be affected by such time of development. Most of colibactin genes found in high percentages among our patients who needed 1-3 months to develop either of neoplastic or
non- neoplastic polyps. Hofstad et al. (1996) found that colorectal polyps, especially in the rectum and sigmoid colon, increased in size about 75-90% for each year during three years. They also mentioned that the size of adenomatouse polyp increased in patient's ages between 50-60 years more than in older ages and the same increasing in those with multiple adenomas compared with those with a single adenoma [34]. Progression of CRC from adenomatouse polyp is usually a multistep process which needed 10-15 years relating to the alterations in functions of several suppressor genes which lead to abnormalities of cell regulation [35].
Increasing the spending time of pks positive E. coli in the intestinal region of patients with colorectal polyps could play an important role in the development of CRC. Nowrouzian and Oswald (2012) found that long-term colonizers of E. coli strains that belong to phylogroup B2 in the gut of Swedish infants increased the probability to have the pks island than either intermediate-term colonizers or transient strains and also lead to enhance the induction of genomic mutations in the host intestine [36]. This could be evidence that the presence of E. coli
producing colibactin at long-term persistence in the colon might be related to the induction of genomic transformations in the host digestive system.
Conclusion
Colibactin genes more frequently found in patients with colon polyps compared with rectal polyps, especially in early development stages. They also were higher in neoplastic polyps than in non-neoplastic polyps. Inflammatory polyp and hamartomatous polyp were the most types of non-neoplastic polyp contents of colibactin genes.
Ethical statements:
Conflict of interesting: The authors have no conflict of interesting
Funding: There was no funding for this research
Ethical considers: All of involved individuals were volunteers with writhing concerns.
References
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
Pag
e
19
2- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray E. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International J cancer. 2015; 136: E359-E386.
3- Al- Madouj AN, Eldali A, AI-Zahrani AS. Ten-year cancer incidence among nationals of the GCC States 1998–2007. Gulf center for cancer control and prevention. King Faisal Specialist Hospital and Research Center. Saudi Arabia. 2011.
4- AL-Janabi AA, Naseer ZH, Hamody TA. Epidemiological study of cancers in Iraq-Karbala from 2008 to 2015. International J Medical Research & Health Sciences. 2017; 6: 79-86.
5- Shussman N, Wexner SD. Colorectal polyps and polyposis syndromes. Gastroenterology Report. 2014; 2:1-15.
6- Gordon PH, Nivatvongs S. Principles and practice of surgery for the colon, rectum, and anus. Quality Medical Pub. Missouri. 1999.
7- Welich RA. The genus Escherichia. Prokaryotes. 2006; 6:60-71.
8- Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli
phylo‐typing method revisited: improvement of specificity and detection of new phylo‐groups. Environmental Microbiology Reports. 2013; 5: 58-65.
9- Lax A J. Opinion: Bacterial toxins and cancer—a case to answer?. Nature Reviews Microbiology. 2005; 3;343-349.
10- Khan S. Potential role of Escherichia coli DNA mismatch repair proteins in colon cancer. Critical Reviews in Oncology/Hematology. 2015; 96: 475-482.
11- Johnson JR, Johnston B, Kuskowski MA, Nougayrede J, Oswald E. Molecular epidemiology and phylogenetic distribution of
the Escherichia coli pks genomic island. J
Clinical Microbiology.2008; 46: 3906-3911.
12- Nougayrède J, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C,
Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006; 313: 848-851.
13- Cuevas-Ramos G, Petiti CR, Marcq I, Boury M, Oswald E, Nougayrède J. Escherichia coli
induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proceedings of the National Academy of Sciences. 2010; 107: 11537-11542.
14- Balskus EP. Colibactin: understanding an elusive gut bacterial genotoxin. Natural Product Reports. 2015; 32:1534-1540.
15- Faïs T, Delmas J, Cougnoux A, Dalmasso G, Bonnet R. Targeting colorectal cancer-associated bacteria: A new area of research for personalized treatments. Gut Microbes. 2016; 7: 329-333.
16- McCarthy AJ, Martin P, Cloup E, Stabler RA, Oswald E, Taylor PW. The genotoxin colibactin is a determinant of virulence in
Escherichia coli K1 experimental neonatal
systemic infection. Infection and Immunity. 2015; 83: 3704-3711.
17- Binefa G, Rodríguez-Moranta F, Teule À, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterology. 2014; 20: 6786-6808.
18- Witold K, Anna K, Maciej T, Jakub J. Adenomas–genetic factors in colorectal cancer prevention. Reports of Practical Oncology & Radiotherapy. 2018; 23: 75-83.
19- Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clinical Gastroenterology and Hepatology. 2009; 7: 682-688.
20- Jain M, Vij M, Srinivas M, Michael T, Venkataraman J. Spectrum of colonic polyps in a South Indian Urban cohort. J Digestive Endoscopy. 2017; 8: 119-122.
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
Pag
e
20
22- Wickramasinghe DP, Samaranayaka SF, Lakmal C, Mathotaarachchi S, Lal CK, Keppetiyagama C, Samarasekera DN. Types and patterns of colonic polyps encountered at a tertiary care center in a developing country in South Asia. Analytical Cellular Pathology.
2014; ID 248142:1-4.
http://dx.doi.org/10.1155/2014/248142.
23- Diamond SJ, Enestvedt BK, Jiang Z, Holub JL, Gupta M, Lieberman DA, Eisen GM. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointestinal Endoscopy. 2011; 74:1-10.
24- Geramizadeh B, Keshtkar-Jahromi M. Pathology of colorectal polyps, a study from South of Iran. Annals of Colorectal Research. 2013; 1: 60-62.
25- Zinnah MA, Bari MR, Islam MT, Hossain MT, Rahman MT, Haque MH, Babu SA, Ruma RP, Islam MA. Characterization of Escherichia coli
isolated from samples of different biological and environmental sources. Bangladesh J Veterinary Medicine. 2007; 5:25-32.
26- Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R. High prevalence of mucosa-associated E. coli
producing cyclomodulin and genotoxin in colon cancer. PlOS ONE. 2013; 8, e56964:1-10. doi:e56964:1-10.1371/journal.pone.0056964.
27- Putze J, Hennequin C, Nougayríde JP, Zhang W, Homburg S, Karch H, Bringer MA, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, Dobrindt U. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infection and Immunity. 2009. 77: 4696-4703.
28- Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Déchelotte P, Bonnet M, Pezet D, Wodrich H, Darfeuille-Michaud A, Bonnet R. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014; 0:1-11. doi:10.1136/gutjnl-2013-305257.
29- Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, Sun X, Mohamadzadeh M, Liu X, Martin P, Wang GP, Oswald E, Fodor A, Jobin C. Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Research. 2017; 77: 2620-2632.
30- Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Déchelotte P, Bonnet R, Petzet D, Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clinical Cancer Research. 2014; 20: 859-867.
31- Sarshar M, Scribano D, Marazzato M, Ambrosi C, Aprea MR, Aleandri M, Pronio A, Longhi C, Nicoletti M, Zagaglia C, Palamara T, Conte MP. Genetic diversity, phylogroup distribution and virulence gene profile of pks positive
Escherichia coli colonizing human intestinal
polyps. Microbial Pathogenesis. 2017; 112: 274-278.
32- Raisch J, Buc E, Bonnet M, Sauvanet P, Vazeille E, de Vallée A, Déchelotte P, Darcha C, Pezet D, Bonnet R, Bringer M, Darfeuille-Michaud A. Colon cancer-associated B2
Escherichia coli colonize gut mucosa and
promote cell proliferation. World J Gastroenterology. 2014; 20: 6560-6572.
33- Swidsinski A, Khilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber J, Lochs H. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998; 115: 281-286.
34- Hofstad B, Vatn MH, Andersen SN, Huitfeldt HS, Rognum T, Larsen S, Osnes M. Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. Gut. 1996; 39: 449-456.
35- Winawer SJ. Natural history of colorectal cancer. American J Medicine. 1999; 106:3S-6S.
36- Nowrouzian FL, Oswald E. Escherichia coli
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Pag
e
21
Table (1): Total No. of patients with two types of polyp
Total No. Patients No. (%)
Disease type
Female Male
43
(43% ) 14 (ce)
(14%) 29(a)
(29%) Colon polyps
7
(7% ) 3 (de)
(3%) 4 (ab)
(4%) Rectal polyps
50
(50%)
20 (cd)
(20%) 30(b)
(30%) Control
100 37
(37% ) 63
(63%) Total No.
Similar letters indicate the significant difference at p < 0.05
Table (2): Colibactin genes in patients with colon polyps
Total No. Patients No. (%)
Age
(Years)
Gender
Colibactin genes
Negative clbA +clbP
genes clbP
gene clbA
gene
0 0
0 0
0 1-19
Male
6
(13.95%) 6 ± 0.9
(13.95%) 0
0 0
20-29
6
(13.95%) 3 ± 0.7
(6.97%) 3 ± 1
(6.97%) 0
0 30-39
8
(18.60%) 7 ± 1
(16.27%) 1± 0.8
(2.32%) 0
0 40-49
6
(13.95%) 3 ± 0.6
(6.97%) 3 ± 0.6
(6.97%) 0
0 50-59
1
(2.32% ) 0
1± 0.2
(2.32%) 0
0 60-69
2 0
2 ± 0.7 0
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
Pag
e
22
(4.65% ) (4.65%)
1
(2.32%) 0
1± 0.5
(2.32%) 0
0 1-19
Female
2
(4.65%) 0
2 ± 0.3
(4.65%) 0
0 20-29
4
(9.30%) 4 ± 1
(9.30%) 0
0 0
30-39
2
(4.65%) 2± 0.3
(4.65%) 0
0 0
40-49
3
(6.97%) 2 ± 0.4
(4.65%) 1± 0.4
(2.32%) 0
0 50-59
2
(4.65%) 1± 0.4
(2.32%) 1± 0.3
(2.32%) 0
0 60-69
0 0
0 0
0 70-80
43 28
(65.11%) 15
(34.88%) 0
0 Total No.
Mean ±SD
Table (3): Colibactin genes in patients with rectal polyps
Total No. Patients No. (%)
Age
(Years)
Gender Colibactin genes
Negative clbA +clbP
genes clbP
gene
clbA gene
1
(14.28%) 1± 0.2
(14.28%) 0
0 0
1-9
Male
0 0
0 0
0 10-19
0 0
0 0
0 20-29
1
(14.28%) 1± 0.3
(14.28%) 0
0 0
30-39
1 1± 0.2
0 0
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
Pag
e
23
(14.28%) (14.28%)
0 0
0 0
0 50-59
1
(14.28%) 0
1± 0.3
(14.28%) 0
0 60-69
0 0
0 0
0 70-80
3
(42.85%) 0
2± 0.9
(28.57%) 0
1± 0.7
(14.28%) 1-9
Female 10-19 0 0 0 0 0
0 0
0 0
0 < 20
7 3
(42.85%) 3
(42.85%) 0
1
(14.28%) Total No.
Mean ±SD
Table (4): Colibactin genes in healthy individuals
Total No. Individual No. (%)
Age
(Years) Gender
Colibactin genes
clbA +clbP genes clbP
gene
clbA gene
1
(25%) 0
0 1± 0.5
(25%) 1-19
Male
1
(25%) 0
0 1 ± 0.3
(25%) 20-29
0 0
0 0
>30
0 0
0 0
1-39
Female 2
(50%) 2 ± 0.8
(50%) 0
0 40-49
0 0
0 0
>50
4 2
0 2
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
Pag
e
24
(50%) (50%)
Mean ±SD
Table (5): Colibactin genes in patients with neoplastic polyps
Total No. Patients No. (%)
Age
(Years) Gender
Colibactin genes
Negative clbA +clbP
genes clbP
gene clbA
gene
0 0
0 0
0 1-19
Male
1
(4.34%) 1± 0.4
(4.34%) 0
0 0
20-29
4
(17.39%) 1± 0.2
(4.34%) 3 ± 1
(13.04%) 0
0 30-39
5
(21.73%) 4± 0.3
(17.39% ) 1± 0.3
(4.34%) 0
0 40-49
5
(21.73%) 2 ± 0.3
(8.69%) 3± 0.9
(13.04%) 0
0 50-59
2
(8.69%) 0
2± 0.4
(8.69%) 0
0 60-69
2
(8.69%) 0
2± 0.2
(8.69%) 0
0 70-80
0 0
0 0
0 1-9
Female
0 0
0 0
0 10-19
0 0
0 0
0 20-29
0 0
0 0
0 30-39
1
(4.34%) 1± 0.3
(4.34%) 0
0 0
40-49
2
(8.69%) 1± 0.6
(4.34%) 1± 0.3
(4.34%) 0
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
Pag
e
25
1
(4.34%) 0
1± 0.1
(4.34%) 0
0 60-69
0 0
0 0
0 70-80
23 10
(43.47%) 13
(56.52%) 0
0 Total No.
Mean ±SD
Table (6): Colibactin genes in patients with non-neoplastic polyps
Total Patient No. (%)
Age
(Years) Gender
Polyp type Colibactin genes
Negative clbA +clbP
genes clbP
gene clbA
gene
0 0
0 0
0 1-80
Male Hyperplastic
polyp
0 0
0 0
0 1-19
Female
1
(3.70%) 1± 0.4
(3.70%) 0
0 0
20-29
1
(3.70%) 1± 0.1
(3.70%) 0
0 0
30-39
0 0
0 0
0 40-80
0 0
0 0
0 1-19
Male Inflammatory
polyp
3
(11.11%) 3± 0.7
(11.11%) 0
0 0
20-29
3
(11.11%) 3± 0.5
(11.11%) 0
0 0
30-39
2
(7.40%) 2± 0.4
(7.40%) 0
0 0
40-49
1
(3.70%) 1± 0.3
(3.70%) 0
0 0
50-59
0 0
0 0
0 60-80
2
(7.40%) 0
1± 0.3
(3.70%) 0
1± 0.1
(3.70%) 1-9
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
Pag
e
26
1
(3.70%) 0
1± 0.4
(3.70%) 0
0 10-19
0 0
0 0
0 20-29
2
(7.40%) 2± 0.9
(7.40%) 0
0 0
30-39
1
(3.70%) 1± 0.5
(3.70%) 0
0 0
40-49
1
(3.70%) 1± 0.3
(3.70%) 0
0 0
50-59
1
(3.70%) 1± 0.2
(3.70%) 0
0 0
60-69
0 0
0 0
0 70-80
1
(3.70%) 1± 0.4
(3.70%) 0
0 0
1-9 Male
Hamartomatous polyp (Juvenile)
0 0
0 0
0 10-19
2
(7.40% ) 2± 0.7
(7.40% ) 0
0 0
20-29
0 0
0 0
0 30-39
1
(3.70%) 1± 0.2
(3.70%) 0
0 0
40-49
0 0
0 0
0 50-80
1
(3.70% ) 0
1± 0.2
(3.70%) 0
0 1-9
Female
0 0
0 0
0 10-19
2
(7.40%) 0
2 ± 0.7
(7.40%) 0
0 20-29
1
(3.70%) 1± 0.2
(3.70%) 0
0 0
30-39
0 0
0 0
0 40-80
27 21
(77.77%) 5
(18.51%) 0
1
(3.70%) Total No.
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Pag
e
27
Table (7): Relationship between colibactin genes and the duration time of the polyp development
Total No. Patient No. (%)
Duration time
(Years)
Polyp type
Colibactin genes
Negative clbA
+clbP genes clbP
gene
clbA gene
8
(16%) 5± 2
(10%) 3± 1
(6%) 0
0 1-3
months
Neoplastic polyp
5
(10%) 4± 1.8
(8%) 1± 0.2
(2%) 0
0 4-6
months
4
(8%) 0
4± 1.2
(8%) 0
0 7-9
months
6
(12%) 2± 0.8
(4%) 4± 1
(8%) 0
0 1-2 years
18
(36%) 14± 2
(28%) 4± 0.7
(8%) 0
0 1-3
months
Non-neoplastic polyp
7
(14%) 6± 1.6
(12%) 1± 0.2
(2%) 0
0 4-6
months
0 0
0 0
0 7-9
months
2
(4%) 1± 0.4
(2%) 0
0 1± 0.2
(2%) 1-2 years
50 32
(64%) 17
(34%) 0
1
(2%) Total No.