Pediatric
Heart
Transplantation
at Stanford:
Results
of a 15-Year
Experience
David Baum, MD; Daniel Bernstein, MD; Vaughn A. Starnes, MD;
Philip Oyer, MD; Paul Pitlick, MD; Edward Stinson, MD; and
Norman Shumway, MD
From the Stanford University School of Medicine, Stanford University, Stanford, California
ABSTRACT.
The long-term results of pediatric hearttransplantation were evaluated in 53 patients, aged 0.25
to 18.94 years, who received transplants at Stanford
University Medical Center between 1974 and 1989.
In-dications for transplantation were idiopathic
cardiomy-opathy (68%), congenital heart disease (21%),
endocar-dial fibroelastosis (8%), and doxorubicin cardiomyopathy
(3%). Immunosuppression was achieved with
combina-tions of cyclosporine, prednisone, and azathioprine.
Thirty-seven of 42 recipients leaving the hospital after transplantation were alive and in New York Heart As-sociation class I at study’s end. Cumulative survival was 79% at 1 year, 76% at 3 years, and 69% at 5 years. Fourteen recipients have survived more than 5 years (5.1
to 12.4 years). Hospital readmission for illness has been
infrequent, decreasing from 6.8 days to 0.9 days per year
over 5 years. Eleven patients have required no
rehospi-talization. Posttransplant deaths were due to infection
(19%), rejection (4%), pulmonary hypertension (4%),
coronary artery disease (2%), and lymphoproliferative
disease (2%). Retransplantation was required for
intrac-table rejection in 4 patients and advanced coronary artery
disease in 2. Hypertension and elevated blood urea
nitro-gen and creatinine levels were common in individuals
receiving cyclosporine. Growth was often impaired in
prepubertal children receiving daily prednisone. Based on this 15-year experience, it is concluded that heart
transplantation represents a reasonable alternative for selected young patients with end-stage cardiac disease.
Pediatrics 1991;88:203-214; pediatric heart transplant.
Pediatric heart transplantation is now widely considered standard medical therapy for children
Received for publication Sep 17, 1990; accepted Jan 22, 1991.
Presented, in part, at the annual meeting of the Society for
Pediatric Research and the American Pediatric Society, Ana-heim, CA, April 27-30, 1987.
Reprint requests to (D. Baum) Dept of Pediatrics, Stanford University School of Medicine, Stanford, CA 94305.
PEDIATRICS (ISSN 0031 4005). Copyright © 1991 by the
American Academy of Pediatrics.
with end-stage heart disease and no therapeutic
alternative.’ Transplantation has been used in the
management of children with both acquired and
congenital heart disease and performed successfully
as early as the neonatal period.5’6’5’#{176} Although there are published results of pediatric heart
transplan-tation from several institutions,’6 the numbers of
patients reported by other transplant centers have been small and the length of follow-up has been 5 years or less. The purpose of this report is to describe the experience gained at Stanford since the inception of the Pediatric Heart Transplant
Program approximately 15 years ago. Using these
observations, we evaluated the long-term outlook of children with cardiac transplants.
SUBJECTS AND METHODS
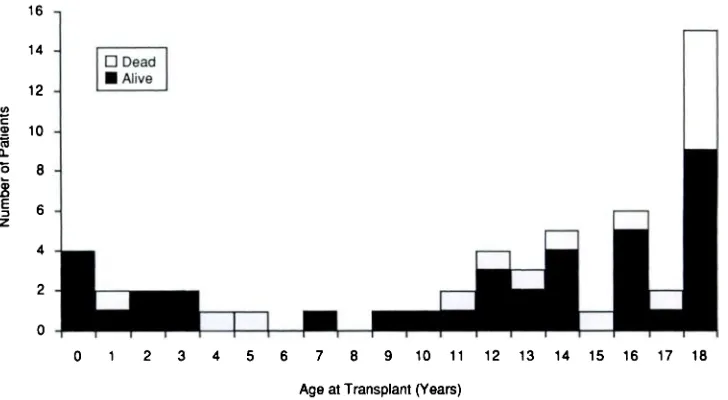
Fifty-three patients, aged 0.25 to 18.94 years (Fig 1), who received heart transplants at Stanford Uni-versity Medical Center between August 19, 1974, and June 30, 1989, are the subjects of this report. Thirty-one were male and 22 female. Thirty-six (68%) had idiopathic cardiomyopathy, 11 (21%) had congenital heart disease, 4 (8%) had endocar-dial fibroelastosis, and 2 (4%) had cardiomyopathy
resulting from doxorubicin administration.
Acceptance to the Pediatric Heart Transplant
Program was based on the following criteria: (1)
existing end-stage heart disease for which no other accepted medical or surgical alternative was avail-able; (2) absence of systemic disease, infection, stroke, or recent pulmonary infarction; (3)
pulmo-nary vascular resistance of less than 7 to 8
resist-ance units per square meter of body surface area
12
10
8
6
4
2
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
Age at Transplant (Years)
Fig 1. Age distribution of patients receiving heart transplants. Total patients in each age group are shown in black bars and deaths are shown in white.
16
U) C a a a.
0
.
E z
14
o Dead
#{149}Alive
evidence of strong motivation for the transplant as assessed by both physicians and a social worker
member of the transplant team. Tn addition to
consent from the parents, consent/assent was
ob-tamed from the children whenever feasible.
End-stage heart disease was diagnosed in
pa-tients with acquired or congenital heart disease
using the following criteria: (1) severe congestive
heart failure unresponsive to anticongestive ther-apy; (2) repeated hospitalizations despite an opti-mal medical regimen; or (3) serious ventricular arrhythmias in the setting of cardiac dysfunction.
Patients meeting any one of these criteria were
added to the active transplant list.
Echocardiographic information was useful in
judging disease severity and timing for transplan-tation. For individuals with left ventricular
frac-tional shortening greater than 20%, listing was
usually postponed unless there were other
mitigat-ing factors. With a shortening fraction between
10% and 20%, other clinical circumstances played a major role in making the decision. For patients
with a fractional shortening of less than 10%,
im-mediate listing was considered, with less emphasis
placed on other criteria. Because of the risk of
embolization, the presence of a mural thrombus on
echocardiogram was an indication for anticoagula-tion and early listing.
There were additional factors that played a role in the timing of transplantation. Tf a prospective
candidate was found to have elevated pulmonary
vascular resistance at initial evaluation, but not of
sufficient severity to preclude heart
transplanta-tion, early listing was considered. This was done in
hope of preventing progression to a point where
heart transplantation would be inadvisable. Since
adequate preoperative nutrition improves the
chance of survival after transplantation, patients developing significant weight loss or growth failure were listed earlier. Lastly, early listing was
consid-ered in cases where there would be difficulty in
donor acquisition. For example, patients who on
routine screening were found to be sensitized
against a series Of random donors were considered for early listing. The group at highest risk for this
scenario has been patients who have had prior
cardiac surgery.
Immunosuppression regimens varied during the
period included in this report. Prior to December
1980, 9 patients received azathioprine and
pred-nisone (NOCYCLO) for maintenance
immuno-suppression. Subsequently, 44 patients were given
cyclosporine and prednisone (CYCLO).
Twenty-nine of these also received azathioprine. Prior to
1987 antithymocyte globulin induction therapy was used postoperatively, whereas after 1987 the
mono-clonal antibody, OKT3, was used for the same
purpose.11 Cyclosporine was not subsequently added to the regimen in those who initially received
NOCYCLO therapy, nor was it withdrawn in any
patient receiving CYCLO therapy. However, the
dosages of the immunosupressant drugs were
al-tered with time. The current dosages of prednisone, azathioprine, and cyclosporine for maintenance
im-munosuppression are shown in Table 1.
Acute rejection was monitored and confirmed by
endomyocardial biopsy12 in all patients, regardless of age. Surveillance biopsies were begun within 7 to
10 days of transplant. The femoral approach was
used in most although the jugular vein was used in
TABLE 1.
Curren t Maintenanc e Immunosuppression*Drug (Months Dose, mg/ Target Range
Posttransplant) kg/d
Cyclosporine
<6 mo 10 ± 5.0 100-200 ng/mL
6-60 mo 10 ± 5.0 75-175 ng/mL
>60 mo 5 ± 2.5 75-175 ng/mL
Azathioprine 1.75 ± 0.25 WBC count
4000-5000/mm3
Prednisone
<6 mo 0.5 ± 0.2
>6 mo 0.2 ± 0.1 Attempt weaning * Drug doses are expressed as means ± SD for all patients. Serum cyclosporine levels were determined by fluores-cence polarization immunoassay (TDx, Abbott
Labora-tories, Chicago, IL). Total daily cyclosporine dose was
divided into three doses in patients younger than 3 years
old and two doses in patients older than age 3 years. Weaning of prednisone was monitored by endomyocar-dial biopsy. WBC, white blood cell.
After discharge from the hospital surveillance biop-sies were performed no less frequently than every
3 to 6 months. The response to rejection therapy
was also monitored by endomyocardial biopsy.
Diagnosis of acute rejection required histologic
examination of three or more pieces of tissue.’2 A
sparse perivascular and endocardial infiltrate,
en-docardial and/or interstitial edema, with no
evi-dence of myocardial necrosis was considered char-acteristic of mild rejection. Moderate rejection was
diagnosed on observing increased perivascular and
endocardial mononuclear cell infiltrate and
my-ocyte necrosis. Severe rejection was indicated by the presence of a more widespread inflammatory infiltrate, interstitial hemorrhage, and more exten-sive myocyte necrosis. The diagnosis of resolving/
resolved rejection was made on determination of
decreased or absent infiltrate, active fibrosis, and early scar formation.
Early postoperative acute rejection with evidence of myocardial necrosis was treated with intravenous methylprednisolone (15 mg/kg per day) for 3 days. For refractory rejection, methylprednisolone
ther-apy was combined with antithymocyte globulin or
OKT3. Acute rejection appearing after the first year posttransplant was usually treated with high doses
of oral prednisone (1.5 mg/kg per day) for 3 days
with subsequent tapering to maintenance
predni-sone doses over a 2-week period. In a few, methyl-prednisolone was used. In the last 2#{189}years of this study total lymphoid irradiation was administered to two young patients with sustained acute rejection unresponsive to the previous measures.13 Retrans-plantation was performed in four cases for rejection
unresponsive to medical therapy. The current
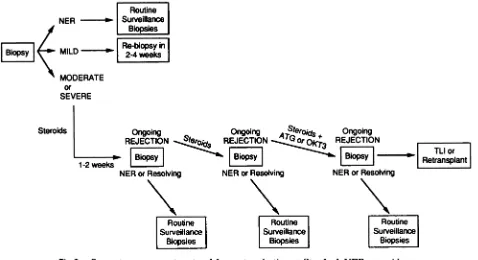
pro-tocol for the management of acute rejection is illus-trated in Fig 2.
Clinical follow-up was carried out at weekly in-tervals in the first month posttransplant and then extended to a maximum of 3-month intervals there-after. In those individuals living at great distances,
regular examinations were performed by local
pe-diatricians, internists, and cardiologists. Whenever possible, biopsies were performed locally, and tissue slides reviewed at Stanford. Cardiac catheteriza-tion, coronary angiography, and in-depth clinical
assessments were performed annually. For the most
part these annual studies took place at Stanford.
Actuarial data were analyzed using the
Cutler-Ederer method.’4 Absolute data regarding numbers of infection and rejection episodes per unit time (eg, linear rates) were quantified in 3-month
inter-vals and described as episodes per 100
patient-days.15 Group data and their variation were
ex-pressed as means ± one standard deviation.
Be-cause of the small size of the NOCYCLO group,
statistical comparisons between the NOCYCLO
and CYCLO groups were not attempted.
RESULTS
Survival
The data reported represent 179.8 patient-years
of clinical experience. Forty-two (79%) of 53
pa-tients were discharged home following transplan-tation and 37 (70%) were living and out of hospital as of June 30, 1989. The cumulative survival at 1 year was 79%; at 3 years, 76%; and at 5 years, 69%
(Fig 3). Fourteen survived more than 5 years (5.1
to 12.4 years), and 13 of these are still alive and at home. The exception is our first pediatric patient, who died 11.4 years after receiving his initial allo-graft.
Quality of Life
The clinical status of surviving patients was
dramatically improved after transplant. Markedly symptomatic children with limited exercise
toler-ance became active, asymptomatic individuals. The
activity level for all postoperative patients is New
York Heart Association class T. Those children
underweight because of cardiac illness experienced marked nutritional improvement following heart transplantation.
Once discharged from the hospital, children
re-quired remarkably few rehospitalizations for
Routine
Surveillance
Biopsies
100
60
0
I
Routine INER
I
SurveillanceI
I
BiopsiesI
I
Biopsy MILD Re-biopsy inI
______
I
2-4weeksMODERATE
or SEVERE
Steroids Ongoing Ongoing Jteroj #{247} Ongoing
I
. REJECTIONBIOpSY] REJECTIONBIOPSYJ REJECTION[Biopsy______
J
I
__________
__________
TLI orI
Retransplaj1-2 weeks ________
NER or Resolving NER orResolving NER orResolving
___________
I
Routine 1Surveillance
I
__________ Biopsies
J
__________Routine
Surveillance
Biopsiej
Fig 2.
Current management protocol for acute rejection at Stanford. NER, no evidence of rejection; MILD, mild rejection; MODERATE, moderate rejection; SEVERE, severe rejection; OKT3, murine anti-human T-lymphocyte antibodies; ATG, antithymocyte globulin; TLT, total lymphoid irradiation.40
20
0 2 4 6 8 10 12
Years Post-Transplant
Fig 3.
Cumulative survival of pediatric heart transplant patients (mean ± 1SD). Figures in parentheses represent number of patients.prolonged hospitalizations by a small group of
pa-tients with serious complications. Eleven patients
(27% of those discharged from the hospital)
re-quired no rehospitalizations for illness, with follow-up ranging from 44 days to 9.5 years.
Cardiac Catheterization
Annual cardiac catheterization demonstrated
hemodynamic data within the normal range (Table
2), with the exception of one young man who
re-ceived a transplant because of dilated
cardiomyop-athy at age 16 years. At his first annual study
hemodynamic data were consistent with a
restric-tive cardiomyopathy. At his second annual study,
coronary artery disease was detected in addition to an increase in restrictive signs and he underwent retransplantation shortly thereafter.
Mortality
Preoperative. Seven patients (12%) accepted to
the program between January 1, 1978, and June 30,
1989, died before a donor heart could be obtained
(comparable data are unavailable prior to 1978).
Six died between 1 and 30 days after acceptance and one after 125 days ofwaiting. For the remaining
patients who did receive a transplant, 26.4 ± 3.4
(range: 1 to 88) days were required to obtain a
suitable allograft. The time required to obtain a
suitable donor organ for children has remained
relatively constant over the 15 years ofthe program.
Postoperative. There were 16 postoperative
deaths (Table 3). Eleven (69%) took place during
the initial hospitalization and occurred within 6
months of the first transplant. Six of these took
place during the first month. Eight were due to
infection, 1 to rejection, and 2 to pulmonary hyper-tension and graft failure. No patients died between
TABLE 3.
Relationship Between Duration of Follow-up and Cause of Death in Pediatric Heart Transplant PatientsDays Posttransplant
(I)
C
a
0
0
0
a 0. U)
C
I
U-C
0
$ a
C
a 2 a a.
-11-.
-1/-7 12
#{149}1-12
TABLE 2. Cardiac Catheterization Results in Pediatric Heart Transplant Recipients
(Mean ± 1 SD)
Years Posttransplant
ly
Pulmonary artery mean pressure (mm Hg) 12.3 ± 4.4 10.5 ± 3.7
Right ventricle systolic pressure (mm Hg) 23.1 ± 4.5 23.2 ± 3.2
Right ventricle end-diastolic pressure (mm Hg) 4.5 ± 3.9 4.0 ± 2.2
Left ventricle systolic pressure (mm Hg) 123.7 ± 16.3 127.7 ± 30.5
Left ventricle end-diastolic pressure (mm Hg) 8.8 ± 4.9 8.8 ± 2.7
Cardiac index (L/min/m2) 3.1 ± 0.7 3.2 ± 1.1
Pulmonary vascular resistance (U/rn2) 1.0 ± 0.5 1.1 ± 0.8
Systemic vascular resistance (U/rn2) 18.8 ± 5.8 18.9 ± 2.8
<30 30-90 90-180 >1000
Infection 3 4 1 2
Rejection 1 0 0 1
Graft failure 2 0 0 0
Coronary disease 0 0 0 1
Malignancy 0 0 0 1
deaths (31%), occurring more than 30 months after
transplant. Two were secondary to infection, and 1
each was the result of rejection, lymphoproliferative
disease, and coronary artery disease. There was no
correlation between patient age or sex and overall mortality.
Seven (63%) of the 11 children with congenital heart disease died. Of these deaths only two,
occur-ring in patients with pulmonary vascular disease
and graft failure, could be attributed to the congen-ital cardiac anomalies. The remainder were second-ary to infection and coronary artery disease.
Rejection
Acute rejection was one of the two most common
serious complications of heart transplantation in
children. Symptoms of rejection were uncommon,
especially in patients receiving cyclosporine. In the
few cases where symptoms did appear in our
pedi-atric patients, they were usually secondary to either impaired systolic function or arrhythmia.
Acute rejection was most frequent in the first 3
postoperative months (Fig 4). Seventy percent of
patients experienced at least one episode of acute rejection during the first 3 postoperative months. Thereafter, the rejection rate fell, stabilizing at a low level after 6 months. Of those recipients surviv-ing 5 years or longer, none was rejection-free.
Four patients (8%) underwent retranspiantation for sustained acute rejection unresponsive to
med-ical therapy. In this small group the frequency of
0L11kk
Years Post Transplant
100
75
1
::
0
0 1 2 3 4 5 6 7
Years Post-Transplant
Fig
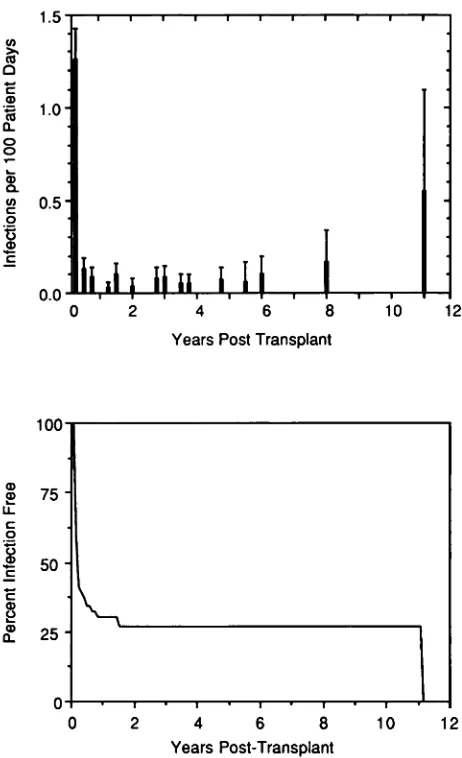
4. Linear rate of acute rejection episodes (top) andpercent of recipients remaining rejection-free (bottom).
acute rejection again increased immediately follow-ing retransplantation and thereafter declined.
Infection
Although less frequent than rejection, infection
attributed to immunosuppression caused far more
morbidity and mortality. Like acute rejection,
se-rious infection was most frequent in the first 3
months after transplant (Fig 5). Subsequently, the
re-1.5
1.0
0.5
0.0
0
z L L
2 U)
>, (5
C
a Cs a.
0
0
a 0. U)
C
0 13 0
C
a a, U.
C
0
C
C
a, 2 a, a.
4 6 8
Years Post Transplant
10
0 2 4 6 8 10 12
Years Post-Transplant
Fig 5.
Linear rate of infection episodes (top) and per-cent of patients remaining infection-free (bottom).mained low. Within 3 months of transplant, 62%
of patients had at least one episode of infection (Fig 5). As was the case with acute rejection, only a few
additional patients experienced infection for the
first time in the months that followed.
Bacteria were responsible for 38 infections. En-terococci, Kiebsiella, Pseudornonas, Serratia, and
Staphylococcus were most frequent. There was a
single nocardial infection. The respiratory tract was the usual primary site of infection although blood, urinary tract, and the central nervous system were often involved.
Viral infections also were frequent. The majority
of common childhood viral infections were well
tolerated. Laboratory diagnosis was not made in
recipients with minor childhood respiratory and
gastrointestinal illness of suspected viral origin.
However, all significant viral infections (n = 30)
were confirmed by isolation and/or seroconversion
and were shown to be caused by herpesviruses.
Thirteen were due to cytomegalovirus, of which
seven were detected only by seroconversion. Herpes simplex, almost invariably oral and benign, was the etiologic agent in 11 cases. There were four herpes
zoster and two Epstein-Barr virus infections.
Except for oral herpes simplex, the viral
infec-tions predominantly involved the respiratory tract or were disseminated. Cytomegalovirus infections
were the most severe, playing a major role in six
serious infections, of which half resulted in death. Fungi were less common etiologic agents, al-though disproportionately frequent sources of seri-ous infection. Of 10 such infections, 5 were due to
2 Aspergillus, 4 to Candida, and 1 was secondary to an unclassified yeast. Seven of the fungal infections were located in the respiratory tract, 2 were dissem-mated, and 1 involved the gastrointestinal tract. There were 2 protozoan infections. One was caused
by Pneumocystis carinii and the other by
Toxo-plasma gondi.
Infection was the cause of death in 10 patients. Eight infection-related deaths occurred within the
first 6 months of transplant. A ninth occurred
within 3 months of retransplantation. One other
death from infection occurred 5 years after
trans-plant and was related to hemodialysis and renal
failure. Fungi (n = 5) and viruses (n = 4) were most
frequently involved.
Coronary Artery Disease
Coronary atherosclerosis of variable severity was found in 8 (15%) of our 53 patients. Of those with
coronary disease, 6 were male and 2 were female,
roughly the same sex distribution found in all 53
patients. The diagnosis of coronary artery disease
was made by pathologic examination of the
allo-grafts obtained from 4 patients after
retransplan-tation or death. The disorder was recognized in
these four allografts 25 days, 59 days, 2 years, and 4.3 years after transplantation. Angiographic
evi-dence of coronary disease was found in 7 (21%) of
34 patients surviving 2 or more years (Table 4).
Three of these had subsequent pathologic
confir-mation of the abnormality. The earliest
angio-graphic detection of coronary disease occurred 2
years posttransplant. No evidence of coronary
ar-tery disease was found in any patient receiving a
disease. One male teenager who refused retrans- 80 plantation died from a myocardial infarction.
0 20 40 60 80 100
Months post Transplant
60
BUN
(mg/dl) 40
0 NOCYCLO
S
CYCLO 200
5
4
Creatinine 3
(mg/dl)
0 NOCYCLO 2
S
0
0 20 40 60 80 100
Months post Transplant
Fig 6.
Plasma blood urea nitrogen (BUN) and creati-nine levels in patients receiving cyclosporine and pred-nisone (CYCLO) and those receiving azathioprine andprednisone (NOCYCLO) (mean ± 1 SD).
rienced slow, continued increases in blood urea
nitrogen and creatinine levels.
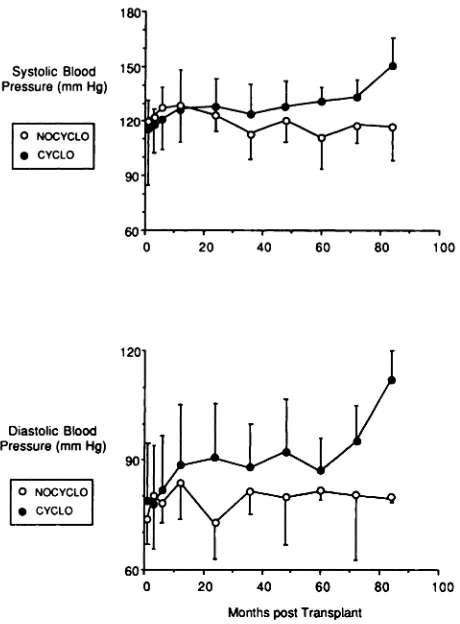
Systolic and diastolic blood pressures were
ele-vated in the group receiving CYCLO therapy. All
but one of this group required at least one
anti-hypertensive medication. Some required two or
three antthypertensive drugs. One ofthe individuals
in the CYCLO group had a hypertensive crisis but
none had a cerebral vascular accident. In contrast,
the use of an antihypertensive medication was
Un-TABLE
4. Coronary Artery Disease After Pediatric Heart Transplantation* PatientNo.
Months Posttransplant
12 24 36 48 60 72 84 124
74 0 0 0 1 2 2 2 3
200 0 0 0 1 1 1 2
216 0 0 0 0 1 1 3
221 0 2 2 3
277 0 0 0 1 1
344 0 0 1
368 0 3
* Degree of luminal obstruction: 0 = no disease; 1 = less than 30%; 2 greater than 70%.
= 30-70%; 3 =
Tumors
Tumors were found in six patients (11%).
Symp-tomatic lymphoproliferative disease was diagnosed
clinically in three patients. Tumors were
unex-pected secondary findings at autopsy in three oth-ers. Two of the latter had localized lymphoprolif-erative disease and one had a small hepatic carci-noma.
Of the symptomatic patients, one infant with
generalized lymphoproliferative disease was treated
by reducing immunosuppressive therapy. In
addi-tion, he was given a 6-month course of acyclovir
because of laboratory evidence of associated
Ep-stein-Barr viral infection. He is currently alive and free of tumor, 28 months after diagnosis. One ado-lescent male developed central nervous system lym-phoproliferative disease and was treated with radia-tion therapy. He is alive, free of lymphoproliferative disease, and functioning normally 10 years later.
The other symptomatic patient was the only
mdi-vidual who died from malignancy. His lymphopro-liferative disease appeared at the muscular site of antithymocyte globulin injections. It was
unrespon-sive to therapy and became widespread before
death. Neither of the children receiving transplants for doxorubicin cardiomyopathy has demonstrated
evidence of recurrent malignancy at 2 years and 12/3
years follow-up.
Renal Function and Blood Pressure
Alterations of renal function (Fig 6) and blood pressure (Fig 7) were related to cyclosporine admin-istration. In the nine patients who received
trans-plants prior to 1980 and were given NOCYCLO
immunosuppression therapy, blood urea nitrogen
and creatinine levels were normal throughout
ewe-20 40 60 80 100
Systolic Blood Pressure (mm Hg)
0 NOCYCLO . CYCLO
Diastolic Blood Pressure (mm Hg)
0 NOCYCLO . CYCLO
40 60
Months post Transplant
Fig 7. Systolic and diastolic blood pressure in patients
receiving cyclosporine and prednisone (CYCLO) and
those receiving azathioprine and prednisone (NOCY-CLO) (mean ± 1 SD).
usual in the small group given NOCYCLO
immu-nosuppression.
Growth
Growth impairment was found in 11 (79%) of 14
children who received transplants before age 14
years and who, in addition, received daily
predni-sone for more than a year. Three children whose
height was less than the fifth percentile at the time
of operation continued to grow at their previous
slow rates. Deceleration in the rate of growth was observed as early as 6 months after initiating pred-nisone. The severity of the growth rate abnormality varied from patient to patient.
Other Complications
Long-term steroid administration was associated with additional side effects. Variable cushingoid features were evident in all children receiving
pred-nisone. Increased appetite and excessive weight
gain were common, and severe obesity was observed in four patients. The changes in physical appear-ance were particularly troublesome for teenagers
and occasionally became sufficiently severe to
re-quire psychologic counseling. Radiologic evidence
of bone demineralization was noted and aseptic
necrosis of weight-bearing joints appeared in three adolescent patients. Pathologic fractures occurred in two of these.
Cyclosporine also was associated with other
trou-blesome complaints. Headache and fine tremor
were noted, and seizures occurred in two patients.
Variable degrees of hypertrichosis appeared in all
children receiving cyclosporine. Depilatory agents were necessary in some teenage girls. Gingival hy-perplasia was observed and, in a few, periodontal surgery was necessary. Very young children devel-oped mild facial changes which were characterized
by mandibular prognathism, prominence of the
su-praorbital ridges, and thickening of the nares and lips.
Behavioral difficulty interfered with medical
therapy in a small number. Three adolescent males independently discontinued their medications with
resulting acute rejection. None demonstrated a
predisposition to noncompliant behavior pretrans-plant. With counseling, all reinitiated their medical regimens and survived. One adolescent girl became severely depressed and has required prolonged psy-chotherapy.
Permanent pacemakers were implanted in four
patients (8%). All were inserted between 17 and 57
days posttransplant. Pacemaker generators (VVI)
were placed in three because of sinus and/or nodal bradycardia with heart rates less than 40/mm. The fourth received a VVI pacemaker because of
dizzi-ness associated with a sinus rhythm and a heart
rate of 60/mm.
DISCUSSION
The 15-year experience with pediatric heart
transplantation at Stanford demonstrates that
chil-dren with end-stage heart disease derived great
benefit from this therapeutic modality. Since most
patients were not expected to live more than 6 to
12 months, their life expectancy on average was
markedly prolonged. Cumulative survival in Stan-ford’s pediatric patients suggests that 5 additional years of life can be expected in 70% and that more than 12 years is a reasonable possibility for some.
These results in children compare favorably with
survival in Stanford’s adult patients16 and in chil-dren followed up for shorter periods at other cen-ters.4’6’7”#{176}The Stanford pediatric experience is en-couraging and tends to dispel the concern that life
expectancy in children receiving cardiac grafts is
shorter than that in adult patients.
recovered quickly and were discharged from the hospital within a few weeks of operation.4”#{176} Young
patients gained strength rapidly and in a few
months were capable of activities appropriate for
normal individuals of comparable age.”2’7”7
Pa-tients remained normally active and participated in their usual activities as long as they remained free
of significant complications. Although exercise
hemodynamic data in pediatric heart transplant
patients are not yet available, data obtained at rest
during cardiac catheterization suggest normal
hemodynamic function and provide a basis for
an-ticipating good exercise capacity. With a renewed feeling of well-being, appetite increased and
nutri-tional status improved.’8 Another manifestation of
general improvement was the pronounced reduction in illness-related hospitalization. In the 5 years following cardiac transplantation, illness of suffi-cient severity to require rehospitalization initially averaged less than 7 days per year per patient and, in general, decreased as survival lengthened. In one quarter of our patients, the posttransplant course was sufficiently benign so that no illness-related rehospitalization was required. Thus, for patients incapacitated, and often debilitated with end-stage heart disease, the quality of life was markedly
im-proved by heart transplantation. However, heart
transplantation was not risk-free. There were com-plications related to graft rejection and its manage-ment that for some became significant liabilities.
In organ transplantation, rejection and its
se-quelae are the primary factors affecting graft sur-viva!.2”9
j
is one of the two leading complicationsof heart transplantation in children.2’4 The
fre-quency of acute rejection is greatest in the early postoperative period as is the case with adult heart recipients.2”9’2#{176} At Stanford 70% of children expe-rienced at least one episode of acute rejection during
the first 3 postoperative months. Thereafter, the
rejection rate fell and only a few additional patients annually experienced acute rejection for the first time. Nevertheless, of those surviving 5 years, all had experienced at least one episode of acute rejec-tion. This pattern is similar to that seen in adult heart transplant patients.20’2’
Symptoms of acute rejection rarely appeared,
especially after cyclosporine was added to the
immunosuppressive regimen in 1980.722 Because
many recipients developing rejection are
asympto-matic, it is necessary to maintain a high index of
suspicion, particularly in the early months follow-ing transplantation. Also, many episodes of symp-toms that could have been attributed to graft rejec-tion were found to have other causes, such as infec-tion. Thus, we believe myocardial biopsy is the only
definitive method for diagnosing acute rejection in pediatric patients.
Even though rejection was usually responsive to
therapy, medical treatment was inadequate in some. Serious difficulties most often occurred in the early
months after transplantation. One death and all
four retransplants for medically refractory rejection took place during this early period. There was only
one late death due to rejection. Thus, although
mortality due to acute rejection was only 4%, the
problem was greater when one takes into account
the need for retransplantation due to sustained
unresponsive rejection.
Improved management appears to have reduced
the risk of acute rejection.2’2#{176} Whereas graft loss
occurred in 3 of 9 individuals given NOCYCLO
immunosuppression, only 3 of 44 grafts were lost
among those given CYCLO therapy. Furthermore,
since total lymphoid irradiation and OKT3 were
added to the antirejection armamentarium in 1986
and 1987, respectively, there have been no deaths
or need for retransplantation because of acute
re-jection. Although controlled prospective studies
have not been done in children, retrospective
ex-perience suggests that with (1) appropriate
main-tenance immunosuppression, (2) a high index of
suspicion, (3) regular endomyocardial biopsy, and
(4) the aggressive use of rescue therapy, mortality from acute rejection and the need for retransplan-tation can be minimized.
Graft rejection was not the only source of
post-operative difficulty. Rejection management was an
even greater source of undesirable side effects. The
effects of immunosuppression itself and the side
effects of drugs used in suppressing the immune
system were responsible for these negative effects.
Infection was the most serious complication of
immunosuppression, causing more deaths and
se-rious morbidity than all other complications com-bined. This experience differs from that of Green
et al,23 who found acute rejection to be a more
common cause of death than infection. This differ-ence may reflect lower overall immunosuppression in that series. Like rejection, the incidence of
infec-tion was greatest in the initial 3 months after
transplantation,7’23 regardless of whether it was the recipient’s initial allograft or a retransplant. It is
because of the high risk of rejection during this
early period that immunosuppression is maximized, making patients particularly vulnerable to infec-tion.24 Over time, as the risk of rejection falls and
immunosuppression is gradually reduced, the rate
to Stanford pediatric heart transplant patients, the
common childhood illnesses were well tolerated by
the young recipients.’#{176}’25
Viruses, particularly the herpes group, and
bac-teria were the microorganisms most frequently
en-countered. Cytomegalovirus was the most virulent
viral agent, contributing to four deaths. Aside from its virulence, cytomegalovirus has been identified
as a factor associated with increased incidence of
rejection, graft atherosclerosis, and increased risk of superinfection with opportunistic organisms, es-pecially fungal agents.26’27 As a result, graft failure and mortality are increased with cytomegaloviral infection. Cytomegaloviral infection transmitted
via donor organs is likely to continue as long as
there is a shortage of available donors and allografts carrying viruses are used.23 The only feasible alter-native is an effective prophylactic antiviral agent. In this latter regard, one of the newer antiviral drugs26 or hyperimmune globulintm may prove
use-ful. Although infrequent, fungal infections were
serious in that they were the least responsive to
therapy. They were a particular problem in the
presence of cytomegaloviral infection.
The appearance of tumors is another
complica-tion of immunosuppression. In adult heart
trans-plant recipients, the incidence of neoplastic disor-ders has been reported as 5.5%, with lymphoprolif-erative disease comprising about one half of these tumors.29 In the 53 Stanford pediatric patients, the
incidence was 11% and all tumors except one were
due to lymphoproliferative disease.
Coronary artery disease is a known complication of heart transplantation in adults and is observed in a third to one half of adult recipients surviving 5 years.3#{176}32Many believe it is a form of
immuno-logically mediated vascular injury secondary to
chronic rejection.33 Because cardiac allografts lack neural innervation, patients usually do not experi-ence anginal pain. Without myocardial infarction,
heart failure, or arrhythmia, the disease may go
unrecognized clinically, making periodic coronary
angiography a necessity. Although reports are few,
it is clear that the disorder occurs in children and can cause myocardial infarction and death.6’34 The disorder was found in 15% of Stanford’s pediatric group. It was sufficiently severe to cause death in one patient and result in retransplantation in two others. Distribution between males and females was similar to that of the entire pediatric recipient
group, showing no male predilection as it does in
the natural atherosclerotic process.
Whereas similarities existed between Stanford
pediatric patient and adult populations, there was one striking difference. With follow-up as long as 5
years, we found no evidence of coronary artery
disease in any of the 24 patients receiving
trans-plants before age 14 years. On the other hand,
coronary abnormalities were found in teenagers. Coronary artery disease was observed at pathologic examination within 2 months of transplantation
and by angiogram after 2 years of operation.
Al-though the explanation for this age-dependent
dif-ference is unknown, there are possibilities that
deserve consideration. Graft atherosclerosis has
been observed in young children at other centers.
Inasmuch as graft atherosclerosis may in part be
immunologically mediated, this difference may be
partly related to differences in immunosuppression
among the various pediatric programs. It is also
possible that coronary arteries in the very young are less susceptible to immunologic injury or that
the immune system is quantitatively deficient in
the young. It will be of considerable consequence to determine whether these young patients remain free of coronary artery disease and, if they do, to ascertain the determining factors.
Impaired renal function, reflected by elevated plasma blood urea nitrogen and creatinine levels, was common in pediatric patients given
cyclospor-me. Similar chronic nephropathy has been found
in adult heart transplant recipients receiving cyclo-sporine.35 Progression to renal failure requiring di-alysis or renal transplantation is rare,36 and the majority of cases of renal dysfunction resolve when cyclosporine dosage is reduced or the drug is dis-continued.3739 This overall clinical pattern is sim-ilar to that seen in Stanford’s adult allograft
recip-ients. Hypertension also was common and
persist-ent in children receiving cyclosporine therapy.
Blood pressure elevation developed rapidly and be-came sufficiently severe to require sodium restric-tion, a diuretic, and often, one or more
antihyper-tensive agents for control. However, only one
pa-tient experienced a hypertensive crisis and none
had a cerebral vascular accident. Previous studies in adults40’4’ also have shown that hypertension is a frequent complication of cyclosporine therapy,
especially when given in conjunction with
ste-roids.42
Linear growth is inhibited in both pediatric
car-diac and renal transplant patients.”43’ This has
been ascribed to the growth-retarding effects of
chronic steroid administration. However, growth
impairment is not universal among pediatric pa-tients and some grow normally even while receiving steroids.4’7
The emotional stress of heart transplantation is
particularly difficult for teenagers.45 Drug-induced changes in physical appearance may be problematic
for young persons to whom body image is a matter
in-dependence may develop an aversion to the
com-plexities of medical management. The threat of
graft rejection can become extremely stressful for
individuals who are particularly fearful of death.
These matters gain considerable importance to
ad-olescent recipients and can lead to depression and
rebelliousness. Psychologic counseling is occasion-ally necessary for management.
Candidates accepted for heart transplantation may die before a suitable cardiac allograft is found.
At Stanford, 11% of patients accepted to the
pro-gram were lost while waiting for a donor. Steps
have been taken to diminish the number of
preop-erative deaths. Graft procurement and preservation
have been improved. Mechanical devices useful as
bridges to transplant are under development. Public
awareness concerning the need for donors has
in-creased. Nevertheless, the problem remains and is
a matter that families must understand before mak-ing the decision to proceed with heart transpian-tation.
CONCLUSIONS
The overall experience among Stanford’s
pedi-atric heart transplant recipients has been very en-couraging. The possibility for long-term survival is high and despite certain limitations the quality of
life for surviving patients is good. Therefore, we
believe that the net result is sufficiently beneficial
to recommend heart transplantation for selected
young patients with end-stage cardiac disease and no medical alternative.
ACKNOWLEDGMENTS
This work was supported, in part, by a grant from Alpha Phi Alumnae.
We are indebted to Patricia Gamberg, RN, Joan Miller,
RN, and Dr Maria Wallington for their assistance with
this study and to Winnie Noble for her assistance with
the manuscript.
REFERENCES
1. Starnes VA, Stinson EB, Oyer PE, et al. Cardiac
transplan-tation in children and adolescents. Circulation. 1987;
76(suppl V):V43-V47
2. Pennington DG, Sarafian J, Swartz M. Heart
transplants-tion in children. J Heart Transplant. 1985;4:441-445
3. Bailey L, Nehlsen-Cannarella SL, Doroshow RW, et al.
Cardiac allotransplantation in newborns as therapy for
hy-poplastic left heart syndrome. N EnglJ Med. 1986;315:949-951
4. Pahi E, Fricker FJ, Trento A, et al. Late follow-up of
children after heart transplantation. Transplant Proc.
1988;20(suppl 1):743-746
5. Radley-Smith R, Yacoub MH. Heart and heart-lung
trans-plantation in children. Circulation. 1987;76(suppl IV):! V-24
6. Addonizio L, Hsu D, Fuzesi L, Smith C, Rose E. Optimal timing of pediatric heart transplantation. Circulation. 1989; 80(suppl III):84-89
7. Dunn JM, Cavarocchi NC, Balsara RK, et al. Pediatric heart
transplantation at St Christopher’s Hospital for Children.
J Heart Transplant. 1987;6:334-342
8. Bailey L, Concepcion W, Shattuck H, Huang L. Method of
heart transplantation for treatment of hypoplastic left heart
syndrome. J Thorac Cardiovasc Surg. 1986;92:1-5
9. Mavroudis C, Harrison H, Klein JB, et al. Infant orthotopic
cardiac transplantation. J Thorac Cardiovasc Surg. 1988;
96:912-924
10. Bailey LL, Assaad AN, Trimm RF, et al. Orthotopic
trans-plantation during early infancy as therapy for incurable
congenital heart disease. Ann Surg. 1988;208:279-286
11. Starnes VA, Oyer PE, Stinson EB, Dein JR, Shumway NE.
Prophylactic OKT3 used as induction therapy for heart
transplantation. Circulation. 1989;80(suppl III):79-83
12. Billingham ME. Diagnosis of cardiac rejection by
endomy-ocardial biopsy. J Heart Transplant. 1982;1:25-30
13. Strober S, Dhillon M, Schubert M, et a!. Acquired immune
tolerance to cadaveric renal allografts: a study of three
patients treated with total lymphoid irradiation. N Engi J
Med. 1989;321:28-33
14. Cutler 5, Ederer F. Maximum utilization of the life table in
analyzing survival. J Chronic Dis. 1958;8:699-712
15. Glantz S. Primer of Biostatistics. 2nd ed. New York, NY:
McGraw-Hill International Book Co; 1987;191-244
16. Fowler M, Schroeder J. Current status of cardiac transplan-tation. Mod Concepts Cardiouasc Dis. 1986;55:37-41
17. Baum D, Stinson E, Oyer P, et al. Do long term results
justify pediatric heart transplantation? Pediatr Res. 1987;
21:186A
18. Grady KL, Herold LS. Comparison of nutritional status in
patients before and after heart transplantation. J Heart
Transplant. 1988;7:123-127
19. Kaye MP. The International Heart Transplantation
Regis-try. J Heart Transplant. 1984;3:278-279
20. Hunt SA. Complications of heart transplantation. J Heart Transplant. 1983;3:70-74
21. Baumgartner W, Augustine S, Borkon AM, Gardner TJ,
Reitz BA. Present expectations in cardiac transplantation. Ann Thorac Surg. 1987;43:585-590
22. Bhargava H, Donner RM, Sanchez G, Dunn JM, Zaeri N,
Cavarocchi N. Endomyocardial biopsy after heart transplan-tation in children. J Heart Transplant. 1987;6:298-302
23. Green M, Wald ER, Fricker FJ, Griffith BP, Trento A.
Infections in pediatric orthotopic heart transplant
recipi-ents. Pediatr Infect Dis J. 1989;8:87-93
24. Mason JW, Stinson EB, Hunt SA, Schroeder JS, Rider AK.
Infections after cardiac transplantation: relation to rejection therapy. Ann Intern Med. 1976;85:69-72
25. Hofflin JM, Potasman I, Baldwin JC, Oyer PE, Stinson EB, Remington JS. Infectious complications in heart transplant
recipients receiving cyclosporine and corticosteroids. Ann
Intern Med. 1987;106:209-216
26. Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE,
Stinson EB, Shumway NE. Cytomegalovirus infection is
associated with cardiac allograft rejection and
atheroscle-rosis. JAMA. 1989;261:3561-3609
27. Rand K, Pollard R, Merrigan T. Increased pulmonary
su-perinfections in cardiac transplant patients undergoing
pri-mary cytomegalovirus infection. N Engi J Med. 1978;
298:951-953
28. Syndman DR, Werner CG, Heinze-Lacey B, et al. Use of
cytomegalovirus immune globulin to prevent
cytomegalovi-rus disease in renal-transplant recipients. N Erigi J Med.
1987;317:1049-1054
29. Krikorian JG, Anderson JL, Bieber CP, Penn I, Stinson EB.
Malignant neoplasms following cardiac transplantation.
JAMA. 1978;240:639-643
30. Billingham ME. Cardiac transplant atherosclerosis.
31. Gao SZ, Schroeder JS, Alderman EL, Hunt SA, Silverman
JF, Wiederhold V. Clinical and laboratory correlates of
accelerated coronary artery disease in the cardiac transplant patient. Circulation. 1987;76(suppl V):V-56-61
32. Pascoe EA, Barnhart GR, Carter WHJ, et al. The prevalence
of cardiac allograft arteriosclerosis. Transplantation. 1987; 44:838-839
33. Gao SZ, Alderman EL, Schroeder JS, Silverman JF, Hunt
SA. Accelerated coronary vascular disease in the heart
trans-plant patient: coronary arteriographic findings. J Am Coil
Cardiol. 1988;12:334-340
34. Pahl E, Fricker F, Armitage J, et al. Coronary
arterioscle-rosis in pediatric heart transplant survivors: limitation of
long-term survival. J Pediatr. 1990;116:177-183
35. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M.
Cyclosporine-associated chronic nephropathy. N Engi J
Med. 1984;311:699-705
36. Rottembourg J, Mattei MF, Cabrol A, et al. Renal function
and blood pressure in heart transplant recipients. J Heart
Transplant. 1985;4:404-408
37. Trento A, Griffith BP, Hardesty RL, Kormos RL,
Thomp-son ME, Bahnson HT. Cardiac transplantation: improved
quality of survival with a modified immunosuppressive
pro-tocol. Circulation. 1987;76(suppl V):V-48-51
38. Bolman RMI, Elick B, Olivari MT, Ring WS, Arentzen CE.
Improved immunosuppression for heart transplantation. J
Heart Trarzspkint. 1985;4:315-318
39. Moyer T, Post GR, Sterioff 5, Anderson CF. Cyclosporine
nephrotoxicity is minimized by adjusting dosage on the basis ofdrug concentration in blood. Mayo Clin Proc. 1988;63:241-247
40. Bellet M, Carbrol C, Sassano P, L#{233}gerP, Corvol P, M#{233}nard
J. Systemic hypertension after cardiac transplantation:
ef-fect of cyclosporine on the renin-angiotensin-aldosterone system. Am J Cardiol. 1985;56:927-931
41. Curtis JJ, Luke RG, Jones P, Diethelm AG. Hypertension
in cyclosporine-treated renal transplant recipients is sodium
dependent. Am J Med. 1988;85:134-137
42. Forman SJ, Textor SC, Carlson JE. Prednisone potentiates
cyclosporine induced blood pressure changes in
normoten-sive bone marrow transplant recipients. Kidney
mt.
1987;31:297
43. Tejani A, Butt KMH, Rajpoot D, et al. Strategies for
opti-mixing growth in children with kidney transplants.
Trans-plantation. 1989;47:229-233
44. Uzark K, Crowley D, Callow L, Bove E. Linear growth after
pediatric heart transplantation. Circulation. 1988;78(suppl II):!I-492
45. Lawrence KS, Fricker FJ. Pediatric heart transplantation: quality of life. J Heart Transplant. 1987;6:329-333
46. Goldstein GD, Gollub 5, Gill B. Cutaneous complications of
heart transplantation. J Heart Transplant. 1986;5:143-147
MEETING ANNOUNCEMENT
“Banking Human Milk: Current Implications for Use” is the title of the 7th
Annual Meeting of the Human Milk Banking Association of North America,
Inc. The meeting will be held November 15, 1991 in Washington, D.C., with
the Community Human Milk Bank of Georgetown University Hospital hosting.
Featured speaker is Ruth Lawrence, M.D., who will discuss the medical
suita-bility of banked human milk. Continuing education credits have been applied
for physicians, nurses, dietitians, and lactation consultants. Invitations are extended to those interested in presenting a poster or abstract relating to human
milk research. Registration will be limited by available space. For further
information, please contact: Lois D. W. Arnold, MPH, IBCLC, Executive
Director, HMBANA, P0 Box 370464, West Hartford, CT 06137-0464.
1991;88;203
Pediatrics
Stinson and Norman Shumway
David Baum, Daniel Bernstein, Vaughn A. Starnes, Philip Oyer, Paul Pitlick, Edward
Pediatric Heart Transplantation at Stanford: Results of a 15-Year Experience
Services
Updated Information &
http://pediatrics.aappublications.org/content/88/2/203
including high resolution figures, can be found at:
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or in its
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
1991;88;203
Pediatrics
Stinson and Norman Shumway
David Baum, Daniel Bernstein, Vaughn A. Starnes, Philip Oyer, Paul Pitlick, Edward
Pediatric Heart Transplantation at Stanford: Results of a 15-Year Experience
http://pediatrics.aappublications.org/content/88/2/203
the World Wide Web at:
The online version of this article, along with updated information and services, is located on
American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.