Low Molecular Weight Heparin in the Treatment of Venous and
Arterial Thromboses in the Premature Infant
Lisa A. Michaels, MD*; Michael Gurian*; Thomas Hegyi, MD‡; and Richard A. Drachtman, MD*
ABSTRACT. Objective. Thrombosis in the preterm newborn is a growing problem, a result of improved survival of the smallest and sickest infants. Treatment with low molecular weight heparin (LMWH) has poten-tial advantages, including predictable pharmacokinetics, subcutaneous administration, and minimal monitoring. However, studies with LMWH in term infants demon-strate the need for higher doses as compared with older children and adults. Physiologic differences suggest the need for gestational age–appropriate treatment strate-gies. Because of the relatively small numbers of infants affected each year, large-scale prospective studies have not been feasible. With the goal of establishing treatment guidelines within our own institution, we reviewed ret-rospectively our experience with LMWH for the treat-ment of thrombosis in the preterm infant.
Methods. Medical and pharmacy records of the
inten-sive care nursery were used to identify preterm infants with venous and arterial thrombosis. Chart documenta-tion, orders, pharmacy records, and radiologic studies were used to develop a retrospective database to assess efficacy and safety of the treatment. Main outcome mea-sures were the dose of LMWH required for therapeutic levels, anti-factor Xa levels achieved, bleeding complica-tions, resolution of thrombosis, additional thromboem-bolic events, and death from all causes.
Results. Ten preterm infants (mean gestational age:
26 weeks) who were treated with LMWH were identified. Mean patient weight at diagnosis of thrombosis was 1215 g (range: 565–1950 g). All 10 patients had either a current or recent history of a central venous or arterial catheter. Mean starting dose of enoxaparin was 1.25 mg/kg per 12 hours (range: 0.8 –2 mg/kg). Therapeutic anti-factor Xa levels were achieved in only 5 patients. Mean time to therapeutic range was 33 days (range: 14 – 63 days). The mean dose of enoxaparin required to achieve therapeutic levels was 2.27 mg/kg per 12 hours (dose range: 2.0 –3.5 mg/kg per 12 hours). Clot resolution was observed in all but 2 patients, both of whom died of complications of their thromboembolic events. No bleed-ing events that necessitated a change in treatment strat-egy occurred.
Conclusions. Higher doses of LMWH are required in
the preterm infant as compared with the healthy term neonate. Once therapeutic levels are achieved, continued
regular monitoring and dose adjustments are required to maintain anticoagulation in therapeutic range.Pediatrics 2004;114:703–707;anticoagulation, low molecular weight heparin, enoxaparin, thrombosis, premature, neonate, low birth weight infant.
ABBREVIATIONS. UFH, unfractionated heparin; ATIII, anti-thrombin III; LMWH, low molecular weight heparin; ICN, inten-sive care nursery; IVH, intraventricular hemorrhage.
T
he majority of thrombotic events in the pediat-ric population occur in neonates and infants who are younger than 2 years.1 Advances in neonatal care have led to increased survival of in-fants who are born as early as 23 weeks’ gestation. These improvements have been facilitated by a reli-ance on indwelling catheters, which have resulted in an increased incidence of associated intravascular and intracardiac thrombus formation.No guidelines currently exist for the management of neonatal arterial and venous thromboembolic events as treatments are largely extrapolated from experience in adults and older children. Although suggested doses have been published for anticoagu-lation2,3 and thrombolysis4 in term infants, experi-ence in the premature infant is lacking. Recognition of physiologic disparities in the blood of the prema-ture and term neonate emphasizes the need for ges-tational age–appropriate treatment strategies. Devel-opmental differences in pharmacokinetics have an impact on choices of anticoagulants and how they are used and the doses required.5 Unfractionated heparin (UFH) is difficult to use in preterm infants because of the need for continuous infusion, frequent monitoring, and physiologically reduced levels of anti-thrombin III (ATIII). Fibrinolytic agents may in-crease the risk of intracranial hemorrhage and may not be optimally effective as a result of physiologi-cally lower levels of plasminogen.6Finally, surgical thrombectomy may be particularly risky in the ex-tremely low birth weight infant.
Since 1996, we have used low molecular weight heparin (LMWH) in the intensive care nursery (ICN) for the treatment of arterial and venous thrombosis. LMWH was chosen because of the difficulty of ad-ministering and monitoring other anticoagulants in this age group. Potential advantages of LMWH in-clude predictable pharmacokinetics, subcutaneous administration, and minimal monitoring. However, despite these benefits, there are no clear guidelines and few published data on how best to use this
From the Divisions of *Pediatric Hematology and Oncology and ‡Neona-tology, Bristol Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey, New Brunswick, New Jersey.
Accepted for publication Mar 5, 2004. DOI: 10.1542/peds.2004-0178
medication in the sick, premature neonate. In an attempt to establish treatment guidelines within our own institution, we reviewed retrospectively our ex-perience with LMWH in the premature infant.
METHODS
Inpatient medical records of the ICN at the St Peter’s University Hospital (an affiliated teaching hospital of the Robert Wood John-son Medical School) for the period of July 1996 to August 2002 were reviewed to identify critically ill infants with thromboembo-lism. Pharmacy records were reviewed for the same period to identify infants who were treated with LMWH. Medical and phar-macy records, echocardiographic and radiologic reports, and lab-oratory results were used to develop a retrospective database to assess efficacy and safety of the treatment.
Main outcome measures were the dose of LMWH required for therapeutic levels, anti-factor Xa levels achieved, bleeding compli-cations, resolution of thrombosis, additional thromboembolic events, and death from all causes. Therapeutic dosing of LMWH was defined as an anti-factor Xa level between 0.5 and 1.0 U/ml, and prophylactic dosing was defined as an anti-factor Xa range of 0.1 to 0.4 U/ml consistent with previously published sources.7
Permission to review the medical records was obtained from the University of Medicine and Dentistry of New Jersey Robert Wood Johnson Medical School-Robert Wood Johnson University Hospital Institutional Review Board and the Committee for the Protection of Human Subjects in Research at the St Peter’s Uni-versity Hospital and Health System (Institutional Review Board #W-4075).
RESULTS
Thirteen neonates with catheter-associated throm-bosis were identified. Of these 13 patients, 11 were treated with LMWH, 1 was treated with UFH and human tissue plasminogen activator, and 1 was treated with observation alone. All 11 infants who were treated with LMWH received enoxaparin. One infant was term and excluded from additional anal-ysis. Characteristics of the 10 premature neonates who were treated with enoxaparin are shown in Table 1. Gestational ages were 24 to 34 weeks with a mean gestational age of 26 weeks. The age distribu-tion of infants who received anticoagulant treatment ranged from 1 to 60 days (mean: 32 days). At the start of anticoagulation, mean patient weight was 1215 g (range: 565–1950 g).
In all cases, diagnosis of arterial or venous throm-bosis was made on the basis of radiologic evaluation. In 9 patients, the diagnosis was made by demonstrat-ing an echo dense mass in the heart or superior vena cava during an echocardiogram evaluation. In 1 pa-tient, an aortic thrombus was seen on abdominal ultrasound. Ultrasound and echocardiogram evalu-ations were ordered for several specific indicevalu-ations, including evaluation for a patent ductus arteriosis, worsening respiratory status, heart failure, superior vena-caval syndrome, and suspected thrombosis as a result of thrombocytopenia. All 10 patients had ei-ther a current or a recent history of a central venous or arterial catheter. None had testing for an inherited or acquired thrombophilia.
Before starting anticoagulation, all patients had cranial ultrasonography. Nine patients were throm-bocytopenic at diagnosis, with 8 requiring transfu-sions to maintain a platelet count ⬎50 000/mm3. Therapy was monitored with weekly echocardio-grams and head ultrasounds.
Anticoagulation was with enoxaparin. Because of TABLE
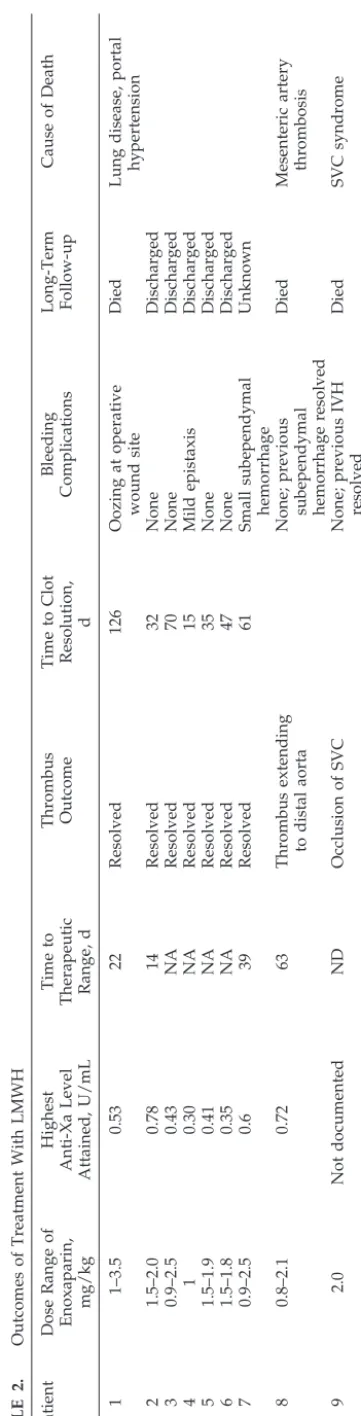
the small doses required, a 30-mg/0.3 mL vial of enoxaparin was diluted with sterile water to a con-centration of 20 mg/mL then refrigerated until use. Injections were given with a 25-G tuberculin needle, and sites were rotated to avoid skin breakdown. Blood was drawn 4 hours after an injection to sure anti-factor Xa activity. The frequency of mea-surement was at the discretion of the treatment team and differed for each patient. During this treatment period, anti-factor Xa levels were not available at the treating hospital. Frozen plasma samples were sent to the Blood Center of Southeastern Wisconsin (Mil-waukee, WI) for measurement. Patient outcomes are shown in Table 2.
Bleeding
Four patients had a history of intracranial bleed-ing, including 1 with a grade III intraventricular hemorrhage (IVH), and 1 of retinal hemorrhage be-fore starting anticoagulation. Resolution of hemor-rhage was observed in all 5 patients. During treat-ment, 1 patient developed a small subependymal hemorrhage (anti-factor Xa level: 0.43 U/mL) and another an episode of epistaxis (anti-factor Xa level: 0.18 U/mL). In both cases, anticoagulation was con-tinued without additional adverse events. In a third patient, postoperative wound bleeding (anti-factor Xa level: 0.50 U/mL) necessitated stopping LMWH for 36 hours.
Efficacy
Clot resolution was observed in all but 2 patients. Both of the patients with clot persistence or progres-sion died of complications of their thromboembolic events. In 1 patient with worsening clot (patient 9), the anti-factor Xa was not known (enoxaparin dose: 2 mg/kg). In the second (patient 8), a therapeutic anti-factor Xa level was attained 63 days after start-ing treatment (enoxaparin: 2.1 mg/kg; anti-factor Xa: 0.72 U/mL), then decreased because of patient growth.
Level of Anticoagulation
Mean starting dose of enoxaparin was 1.25 mg/kg per 12 hours (range: 0.8 –2.0 mg/kg). A total of 28 measurements of anti-factor Xa were documented among the 10 patients. Therapeutic anti-factor Xa levels were achieved in 5 patients. Mean time to therapeutic range was 33 days (range: 14 – 63 days). Four of the remaining 5 patients had documented anti-factor Xa levels in the prophylactic range (0.1– 0.4 U/mL). Anti-factor Xa levels could not be found for the last patient. Only 2 patients did not attain a level of 0.2 U/mL or higher after the first dose of LMWH. The observed dose ranges to achieve thera-peutic and prophylactic anti-factor Xa levels are shown in Table 3. The data were divided to deter-mine whether the required doses needed to achieve either therapeutic or prophylactic levels varied with patient weight. The mean dose for infants who weighed 500 to 1000 g compared with those who weighed 1000 g or greater was similar for both ther-apeutic (P⫽.4; standard deviation: 0.5) and
prophy-lactic (P⫽ .3; standard deviation: 0.3) levels. TABLE
DISCUSSION
Newborns constitute the single largest group of children who are diagnosed with thromboembolic complications. Although an increasingly common complication in the tertiary care nursery, thrombosis remains a relatively rare event. The incidence of symptomatic thrombosis is ⬃5.1 per 100 000 live births8and 2.4 per 1000 neonatal intensive care ad-missions.9 Treatment options include supportive care, anticoagulation, thrombolysis, and surgery. Several treatment registries currently exist,8–10 but none has been sufficient to determine the benefits of any one particular option over the others. Manage-ment decisions seem to be highly individualized, reflecting local preferences and the heterogeneous nature of the specific events reported. How best to treat these sick neonates remains an unanswered question. Because of the relatively small numbers of infants affected each year, determining the optimal therapeutic approach to these patients inevitably will require large, multicenter, collaborative studies.
Among infants with thrombosis, mortality rates are the highest for those with aortic or central venous line–associated thrombosis involving the right atrium or superior vena cava.9All of our patients in this study had thromboses in 1 of these locations, driving the decision to start anticoagulation. Our decision to use LMWH was made because subcuta-neous injection obviates the requirement of dedi-cated intravenous access and there is less need for laboratory monitoring as compared with UFH. We chose to avoid thrombolytic therapy due to concerns of precipitating hemorrhagic complications, because most patients were thrombocytopenic and required platelet transfusion and several had a history of in-tracranial or retinal hemorrhage or had recent sur-gery.
Dosing recommendations for LMWH in term in-fants have been published.2,3 In infants who weigh ⬍5 kg and are younger than 2 months, the current recommended starting dose of enoxaparin is 1.5 mg/kg per 12 hours, as compared with the adult recommended dose of 1 mg/kg per 12 hours. Studies with UFH and LMWH in young children and in the porcine model11 demonstrate that younger patients require higher doses as a result of accelerated clear-ance of these drugs and from lower levels of ATIII.12 ATIII levels are lower in preterm than in compara-tively aged term infants,13and renal clearance mech-anisms are not yet fully developed,14so it is probable that UFH and LMWH doses will be different in this particular group.
The possibility that preterm infants require higher doses of LMWH was suggested by a report of 2
neonates who were⬍28 weeks’ gestational age with multiple medical problems and extensive central line–related thrombosis who required⬎3 mg/kg per 12 hours of enoxaparin to achieve the targeted anti-factor Xa.15 In our study, the average dose required to achieve therapeutic levels was 2.2 mg/kg per 12 hours; however, 1 patient required 3.5 mg/kg per 12 hours and a second infant’s anti-factor Xa remained subtherapeutic despite 2.5 mg/kg per 12 hours. Only 5 of the 10 patients had therapeutic levels docu-mented during the treatment period. On the basis of this experience, we have modified our practice to start anticoagulation with enoxaparin at 2 mg/kg per 12 hours. In many sick preterm infants, this starting dose will not be sufficient to achieve target anti-factor Xa levels, thus emphasizing the need for ag-gressive monitoring in the first days of treatment to avoid long delays in achieving adequate anticoagu-lation.
Although 1 of the benefits of using LMWH is a decreased need for monitoring, it is evident that ongoing measurement of anti-factor Xa levels is still necessary in this population. Once therapeutic levels are achieved, continued regular monitoring and dose adjustments are still needed. In this study, only 1 patient (patient 2) had anti-factor Xa levels stable in the therapeutic range 100% of the time, but this pa-tient required the shortest course of anticoagulation of all of the infants studied. Primary reasons identi-fied for anti-factor Xa levels dropping outside the therapeutic range were 1) infrequent monitoring and 2) rapid patient growth. As a result of these findings, we recommend that anti-factor Xa levels be reas-sessed once a week at a minimum—still a consider-able improvement over UFH, which requires daily or more frequent monitoring. Simple correction of the dose of LMWH for changes in weight may not be adequate, as there is evidence that LMWH may ac-cumulate in the body over time.7
The incidence of intracranial hemorrhage among infants with a birth weight⬍1500 g is 25%,16raising questions regarding the safety of anticoagulation in the ICN. The lack of aggressive anticoagulation seen in our study may in part reflect unease by the neo-natologists. Such concern is reasonable in the face of a possible increased risk of IVH in low birth weight infants who receive UFH to maintain patency of vascular catheters.17–19 Although no strong conclu-sions regarding safety of LMWH in the ICN can be made from our data, it can be pointed out that no patients incurred bleeding events that necessitated a change in treatment plan. One patient did develop a subependymal bleed during treatment, but the lesion was small and did not change during the remainder of the course of therapy. Three patients had ultra-sound findings of concurrent intracranial hemor-rhage at the start of anticoagulation, including 1 with superior vena caval occlusion and grade III IVH. None of these 3 patients experienced progression of their intracranial hemorrhage, and all had resolution of their abnormal ultrasound findings while receiv-ing LMWH. Notably, 2 of these patients did attain anti-factor Xa levels in therapeutic range. In contrast, 2 patients with either undocumented or
subthera-TABLE 3. Distribution of Dose Requirements for Premature Infants Who Received Therapeutic and Prophylactic Enoxaparin
Anti-Factor Xa Level (U/mL)
Prophylactic Dose (n⫽14)
Therapeutic Dose (n⫽8) Observed dose range,
mg/kg per 12 h
0.8–1.9 2.0–3.5
Observed mean dose, mg/kg per 12 h
peutic anti-factor Xa levels died of clot progression, illustrating the risks associated with nonaggressive treatment of neonatal arterial and venous thrombo-embolic events.
The strength of the conclusions presented in this article are constrained by both the small number of patients observed and the retrospective study de-sign. Despite these limitations, our findings are sim-ilar to those recently published in a prospective co-hort study of unselected newborn infants who were treated with LMWH. Among 15 infants who were
⬍37 weeks’ gestation, the average dose of enoxapa-rin required to maintain therapeutic range was 2.1 mg/kg per 12 hours, and therapeutic levels were attained in only 50% of measurements.20The results of these studies suggest that our clinical experience with LMWH in the preterm infant is consistent with that encountered at other institutions. We propose that these observations provide a starting point for future randomized collaborative treatment studies to define better the appropriate dosing and treatment protocols for preterm neonates with thrombosis.
ACKNOWLEDGMENT
This project was supported in part by an Unrestricted Educa-tional Grant from Aventis.
REFERENCES
1. Andrew M, David M, Adams M, et al. Venous thromoboembolic com-plications (VTE) in children: first analyses of the Canadian Registry of VTE.Blood.1994;83:1251–1257
2. Massicotte P, Adams M, Marzinotto V, Brooker LA, Andrew M. Low molecular weight heparin in pediatrics patients with thrombotic disease: a dose finding study.J Pediatr.1996;128:313–318
3. Massicotte P, Julian JA, Marzinotto V, et al. Dose-finding and pharma-cokinetic profiles of prophylactic doses of a low molecular weight heparin (reviparin-sodium) in pediatric patients.Thromb Res.2003;109: 93–99
4. Farnoux C, Camard O, Pinquier D, Hurtaud-Roux MF, et al. Recombi-nant tissue-type plasminogen activator therapy of thrombosis in 16 neonates.J Pediatr.1998;133:137–140
5. Williams MD, Chalmers EA, Gibson BES. Guidelines: the investigation and management of neonatal hemostasis and thrombosis.Br J Haematol. 2002;119:295–309
6. Andrew M, Brooker L, Leaker M, Paes B, Weitz J. Fibrin clot lysis by thromboembolic agents is impaired in newborns due to a low plasmin-ogen concentration.Thromb Haemost.1992;68:325–330
7. Hirsh J.Low Molecular Weight Heparins. Monograph. Hamilton, Ontario, Canada: Decker Periodicals Inc; 1994
8. Nowak-Gottl U, von Kries R, Gobel U. Neonatal symptomatic throm-boembolism in Germany: two year survey.Arch Dis Child Fetal Neonatal Ed.1997;76:F163–F167
9. Schmidt B, Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry.Pediatrics.1995;96:939 –943 10. Von Ommen CH, Heijboer H, Buller HR, Hirasing RW, Heijmans SA,
Peters M. Venous thromboembolism in childhood: a prospective two year registry in The Netherlands.J Pediatr.2001;139:676 – 681 11. Andrew M, Ofosu F, Brooker L, et al. The comparison of the
pharma-cokinetics of a low molecular weight heparin in the newborn and adult pig.Thromb Res.1989;56:529 –539
12. Andrew M. Developmental hemostasis: relevance to hemostatic prob-lems during childhood.Semin Thromb Hemost.1995;2:341–356 13. Andrew M, Paes B, Johnston M. Development of the hemostatic system
in the neonate and young infant.Am J Pediatr Hematol Oncol.1990;12: 95–104
14. Alcore J, McNamera PJ. Pharmacokinetics in the newborn.Adv Drug Del Rev.2003;29:667– 686
15. Dix D, Andrew M, Marzinotto V, et al. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136:439 – 445
16. Phillip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemor-rhage in preterm infants: declining incidence in the 1980’s.Pediatrics. 1989;84:797– 801
17. Lesko SM, Mitchell AA, Epstein MF, Louis C, Giacola GP, Shapiro S. Heparin use as a risk factor for intraventricular hemorrhage in low birth weight infants.N Engl J Med.1986;314:1156 –1160
18. Malloy MH, Cutter GR. The association of heparin exposure with intraventricular hemorrhage among very low birth weight infants.J Perinatol.1995;15:185–191
19. Chang GY, Lueder FL, DiMichele DM, Radkowski MA, McWilliams LJ, Jansen RD. Heparin and the risk of intraventricular hemorrhage in premature infants.J Pediatr.1997;131:362–366
20. Streif W, Goebel G, Chan AKC, Massicotte MP. Use of low molecular mass heparin (enoxaparin) in newborn infants: a prospective cohort study of 62 patients. Arch Dis Child Fetal Neonatal Ed. 2003;88: F365–F370
OUR SICK SOCIETY
“Today, in some suburbs, breast implants are a popular gift for high school graduates.”
Goodman E.Boston Globe. October 31, 2003
DOI: 10.1542/peds.2004-0178
2004;114;703
Pediatrics
Lisa A. Michaels, Michael Gurian, Thomas Hegyi and Richard A. Drachtman
Thromboses in the Premature Infant
Low Molecular Weight Heparin in the Treatment of Venous and Arterial
Services
Updated Information &
http://pediatrics.aappublications.org/content/114/3/703
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/114/3/703#BIBL
This article cites 19 articles, 5 of which you can access for free at:
Subspecialty Collections
sub
http://www.aappublications.org/cgi/collection/hematology:oncology_
Hematology/Oncology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2004-0178
2004;114;703
Pediatrics
Lisa A. Michaels, Michael Gurian, Thomas Hegyi and Richard A. Drachtman
Thromboses in the Premature Infant
Low Molecular Weight Heparin in the Treatment of Venous and Arterial
http://pediatrics.aappublications.org/content/114/3/703
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.