ARTICLE
Obesity and Excessive Daytime Sleepiness in
Prepubertal Children With Obstructive Sleep Apnea
David Gozal, MD, Leila Kheirandish-Gozal, MD
Kosair Children’s Hospital Research Institute and Division of Pediatric Sleep Medicine, Department of Pediatrics, University of Louisville, Louisville, Kentucky
The authors have indicated they have no financial relationships relevant to this article to disclose.
What’s Known on This Subject
EDS in children with OSA has not been well characterized. In addition, differences in parental reports of EDS for obese and nonobese children emerge in clinical practice.
What This Study Adds
EDS, defined with objective criteria such as the MSL test, is significantly more prevalent among obese children with habitual snoring, compared with nonobese snoring children.
ABSTRACT
INTRODUCTION.The epidemic of childhood obesity has prompted remarkable changes in
the relative proportions of symptomatic overweight or obese children being referred for evaluation of habitual snoring. However, it remains unclear whether obesity modifies the relative frequency of daytime symptoms such as excessive daytime sleepiness.
METHODS.Fifty consecutive, nonobese, habitually snoring, otherwise-healthy children
(age range: 6 –9 years) and 50 age-, gender-, and ethnicity-matched obese children (BMI zscore: ⬎1.67) underwent an overnight polysomnographic evaluation, fol-lowed by a multiple sleep latency test the following day.
RESULTS.The mean obstructive apnea/hypopnea index values for the 2 groups were
similar (nonobese: 12.0⫾1.7 episodes per hour of total sleep time; obese: 10.9⫾1.5 episodes per hour of total sleep time). However, the mean sleep latency for obese children was significantly shorter (12.9 ⫾ 0.9 minutes) than that for nonobese children (17.9 ⫾ 0.7 minutes). Furthermore, 21 obese children had mean sleep latencies of ⱕ12.0 minutes, compared with only 5 nonobese children. Although significant associations emerged between mean sleep latency, obstructive apnea/ hypopnea index, proportion of total sleep time with oxygen saturation of⬍95%, and respiratory arousal index for the whole cohort, the slopes and intersects of the linear correlation of mean sleep latency with any of these polygraphic measures were consistently greater in the obese cohort.
CONCLUSIONS.The likelihood of excessive daytime sleepiness for obese children is
greater than that for nonobese children at any given level of obstructive sleep apnea
severity and is strikingly reminiscent of excessive daytime sleepiness patterns in adults with obstructive sleep apnea.
Pediatrics2009;123:13–18
T
HE PREVALENCE ANDseverity of overweight and obesity in children and adolescents have demonstrated dramatic increases throughout the world in recent decades.1–3 For example, the prevalence of childhood overweightdoubled among children 6 to 11 years of age and tripled among children 12 to 17 years of age in the United States between 1980 and 2000.4,5 Since its initial description, obstructive sleep apnea (OSA) has emerged as a highly
prevalent condition in the pediatric age range, affecting 2% to 3% of school-aged children.6–11Although the primary
pathophysiological mechanism involved in pediatric OSA involves hypertrophy of adenoid and tonsillar tissues in the upper airway,12 it has become apparent that obese children are at increased risk for OSA.13–17 Indeed, by using a
case-control study design, Redline et al18examined risk factors for sleep-disordered breathing in children 2 to 18 years
of age and found that the risk among obese children was increased fourfold to fivefold. Although it is important to emphasize that not all obese children have OSA, the risk of OSA increased 12% for every increase in BMI of 1 kg/m2 beyond the mean BMI for age and gender. Similar trends demonstrating an increased risk of OSA among obese and overweight children have been reported from around the world.
It has been suggested that excessive daytime sleepiness (EDS) is relatively infrequent in children with OSA and probably depends on the perceptions of caretakers, because children are unlikely to describe such symptoms. Indeed, Carroll et al19suggested that only a small minority of these children (7%) presented with symptoms consistent with
EDS. In recent years, however, questionnaires that include more-specific questions on behaviors associated with EDS www.pediatrics.org/cgi/doi/10.1542/ peds.2008-0228
doi:10.1542/peds.2008-0228
Key Words
excessive daytime sleepiness, obesity, hypoxia, sleep apnea, sleep fragmentation
Abbreviations
EDS— excessive daytime sleepiness MSL—mean sleep latency TST—total sleep time OSA— obstructive sleep apnea OAHI— obstructive apnea/hypopnea index
RAI—respiratory arousal index SaO2—arterial oxygen saturation
Accepted for publication Mar 24, 2008
Address correspondence to David Gozal, MD, Kosair Children’s Hospital Research Institute, University of Louisville School of Medicine, 570 S Preston St, Suite 204, Louisville, KY 40202. E-mail: david.gozal@louisville.edu
indicate that the frequency of EDS may be much higher, in the range of 40% to 50%.20 When sleepiness was
measured objectively by using the multiple sleep latency test,⬃13% to⬃20% of children who fulfilled the crite-ria for OSA displayed EDS.21,22 Furthermore, despite a
relatively small number of obese children in the initially reported cohort of 54 children with OSA, the presence of obesity seemed to increase the likelihood of EDS.21It has
become increasingly apparent in the clinical setting that, despite a substantial degree of overlap, the manifesta-tions of EDS may differ somewhat in nonobese children versus obese children. Inattention and hyperactivity emerged as the primary behavioral correlates of EDS for nonobese children (ie, very low modified Epworth Sleepiness Scale scores22), whereas obese children
dem-onstrated increased Epworth Sleepiness Scale scores, and tiredness and falling asleep in school, during car travel, or while watching television were noted fre-quently (D.G., unpublished observations, 2007). There-fore, we hypothesized that habitually snoring, obese children would be more likely to display increased sleep propensity (as measured with the multiple sleep latency test), compared with nonobese children with similar degrees of respiratory disturbance during sleep.
METHODS
Subjects
Habitually snoring children (6 –9 years of age) with ad-enotonsillar hypertrophy who were evaluated at the University of Louisville Pediatric Sleep Medicine Center because of habitual snoring and suspected OSA were recruited into the study. The study was approved by the University of Louisville Human Research Committee. Parental informed consent and child assent, in the pres-ence of a parent, were obtained. Children were excluded if they had any chronic medical condition, were receiv-ing medications known to affect sleep, were known to have narcolepsy or idiopathic hypersomnia, or had any genetic or craniofacial syndromes.
Overnight Polysomnography and Multiple Sleep Latency Test A standard, overnight, multichannel, polysomnographic evaluation was performed in the sleep laboratory, as described previously.23 Sleep architecture was assessed
by using standard techniques.24The proportion of time
spent in each sleep stage was expressed as a proportion of total sleep time (TST). Central, obstructive, and mixed apneic events were counted. Obstructive apnea was de-fined as the absence of airflow with continued chest wall and abdominal movement for the duration of ⱖ2 breaths.23,25Hypopnea was defined as a decrease in
oro-nasal flow of ⱖ50%, with a corresponding decrease in pulse oxygen saturation of ⱖ4% and/or arousal.23 The
obstructive apnea/hypopnea index (OAHI) was defined as the number of episodes of apnea and hypopnea per hour of TST. Arousals were defined as recommended in the American Sleep Disorders Association task force re-port26,27and included respiratory-related (occurring
im-mediately after apnea, hypopnea, or a snore), techni-cian-induced, and spontaneous arousals. Arousals were
expressed as the total number of arousals per hour of sleep time (arousal index), and the respiratory arousal index (RAI) was extracted as described previously.28,29
The morning after the sleep study, a multiple sleep latency test was conducted as described previously.21
Briefly, 5 nap opportunities of 30-minute duration were allowed, every 2 hours starting at 8:00AM. Parents were
requested to stay in the room with the child, to eliminate external apprehension. Each latency test was ended after 3 successive 30-second epochs of stage 1 sleep or 1 epoch of sleep of any other stage (ie, non–rapid eye movement sleep of stage ⱖ2); therefore, this approach differs slightly from the procedures recommended by the American Academy of Sleep Medicine.30 The sleep
la-tency for each trial was calculated as the time elapsed from “lights out” to the first epoch of sleep. If no sleep occurred during a nap session, then the sleep latency for that nap was assigned a value of 30 minutes. The mean value derived from all 5 nap opportunities was defined as the mean sleep latency (MSL). A MSL ofⱕ12 minutes was considered indicative of EDS (ie, 4 SDs beyond the mean for healthy children21).
Body Mass Index
Height and weight were obtained for each child by using standard techniques. The BMI was calculated (body mass/height2) and was expressed as a BMI z score by using an online BMI z score calculator (http://apps. nccd.cdc.gov/dnpabmi/Calculator.aspx). Children with BMI z scores of ⬎1.67 were classified as fulfilling the criteria for obesity.30
Data Analysis
Data are reported as mean⫾SE unless stated otherwise. Group comparisons with dichotomous variables were made by using the 2test. After it was ascertained, by using univariate skewness and kurtosis procedures, that data were normally distributed, analyses for continuous variables were performed by using 1-way analysis of variance. Data were analyzed by using SPSS 14.0 (SPSS, Chicago, IL). Curve-fitting techniques consisting of both linear and exponential analyses were used to evaluate potential relationships between MSL, age, OAHI, BMIz
score, proportion of TST spent with arterial oxygen sat-uration (SaO2) of⬍95%, and RAI.Pvalues of .05 were considered to indicate statistical significance.
RESULTS
Fifty nonobese children of 62 potential candidates and 50 obese children of 59 potential candidates were en-rolled and completed all phases of the protocol. For the 21 children who were not enrolled, the sole reason for nonparticipation was the inability or unwillingness to stay in the sleep center for the extended period of time required for the multiple sleep latency test. There were no demographic or polysomnographic differences be-tween children who agreed to participate and those who declined.
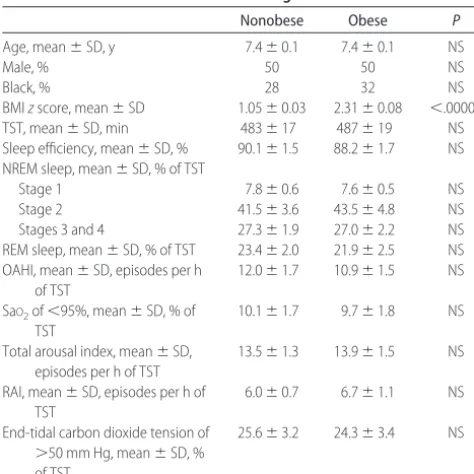
De-spite the marked differences in BMIzscores, the groups were matched with respect to age, gender, ethnicity, sleep duration, sleep stage distribution, and severity of respiratory disturbances.
Subjective perception of EDS, as reported by parents, was noted for 9 of 21 obese children with MSL ofⱕ12.0 minutes and 5 of 29 obese children with MSL of ⬎12 minutes (P⬍.04). Similarly, 3 of 5 nonobese children with MSL ofⱕ12.0 minutes and 6 of 45 nonobese chil-dren with MSL of⬎12 minutes demonstrated subjective EDS (P⬍.02). There were no differences in the reported EDS frequencies for the obese and nonobese groups.
The MSL for obese children was markedly and signif-icantly reduced, compared with that for nonobese chil-dren (12.9⫾0.8 minutes for obese subjects and 17.9⫾ 0.9 minutes for nonobese subjects; P⬍.00005). More-over, 21 obese children had MSL values ofⱕ12.0 min-utes, compared with only 5 nonobese children (odds ratio: 6.52; 95% confidence interval: 2.02-22.40; P ⬍
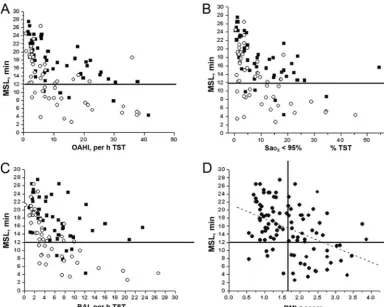
.001). Of note, significant linear correlations with the OAHI, RAI, and proportion of TST spent with SaO2of
⬍95%, but not with the degree of alveolar hypoventi-lation, were noted (Fig 1 A, B, and C). For all children, there was a significant association between BMI and MSL (r ⫽ 0.44; P ⫽ .0001) (Fig 1D). In addition, the mean OAHI required for a MSL of 12 minutes would be 33.0⫾1.2 episodes per hour of TST for nonobese chil-dren, compared with 14.0 ⫾ 0.8 episodes per hour of TST for obese subjects. Table 2 shows the coefficients of correlation, slopes, and y-intercepts for obese and nono-bese children as a function of OAHI, RAI, and proportion of TST spent with SaO2of⬍95%.
DISCUSSION
In this study, we show that prepubertal obese snoring children are at greater risk for developing EDS (objec-tively assessed with a multiple sleep latency test), com-pared with nonobese children with OSA of similar se-verity. Our findings not only confirm our previous report on the association between polysomnographic measures of respiratory disturbance and the magnitude of EDS21 but also support the concept that, although
obesity itself is not tantamount to EDS, reduced MSL is more likely to be noted for obese children even in the absence of OSA.
It has become apparent that the manifestations of sleepiness are quite protean in young children, ranging from falling asleep in school or in the home or car to labile mood, aggressiveness, hyperactivity, and inatten-tion.19–22 Parental reports of EDS are markedly
unreli-able, as evidenced by the wide range of frequencies for reported sleepiness-related complaints among different studies. Therefore, efforts have been made to evaluate sleepiness more objectively, particularly for snoring chil-dren at risk for OSA. The results of such studies sug-gested that objective EDS, assessed with the multiple sleep latency test, was relatively infrequent (ie, 13%) in a cohort of children being evaluated for snoring, in which only a minority of children were obese.21 Even
under those circumstances, however, and despite strong linear relationships between OAMI index and SaO2 na-dir, obesity seemed to be an independent contributor to the reductions in MSL.21 Our present study confirms
these preliminary assumptions and indicates that BMI plays a major role in defining the phenotype of OSA in children. At equivalent levels of OSA severity, the like-lihood of MSL ofⱕ12.0 minutes was more than sixfold greater for obese children, and a strong association emerged between BMI and sleep propensity (Fig 1). These and other differences in the clinical syndrome of OSA among nonobese and obese children prompted us to propose the existence of 2 types of clinical OSA, which may have implications not only regarding the type and extent of end-organ dysfunction induced by the recur-rent upper airway obstruction during sleep but also re-garding treatment outcomes.31–34Of note, there were no
differences in RAI between the 2 groups, and their sleep pressure scores were similar (data not shown).28 Sleep
pressure scores were developed previously by using modeling approaches in our laboratory, in an attempt to provide numerical values for sleep fragmentation asso-ciated with the repeated arousals induced by snoring or other respiratory-related events.28We then showed that
such intrapolygraphic estimates of sleep fragmentation may provide accurate markers for probability indexing of sleep propensity. In the present study, however, sleep pressure scores did not differentiate between obese and nonobese subjects with respect to their biological and physiological responses to such sleep disruption. Simi-larly, although we cannot comment with certainty on whether obese children at risk for OSA would display different electroencephalographic response characteris-tics during the respiratory cycle, compared with nono-bese subjects, we posit that this
electroencephalography-TABLE 1 Demographic and Polysomnographic Characteristics of 50
Nonobese and 50 Obese Prepubertal Children Referred for Evaluation of Habitual Snoring
Nonobese Obese P
Age, mean⫾SD, y 7.4⫾0.1 7.4⫾0.1 NS
Male, % 50 50 NS
Black, % 28 32 NS
BMIzscore, mean⫾SD 1.05⫾0.03 2.31⫾0.08 ⬍.00001
TST, mean⫾SD, min 483⫾17 487⫾19 NS
Sleep efficiency, mean⫾SD, % 90.1⫾1.5 88.2⫾1.7 NS
NREM sleep, mean⫾SD, % of TST
Stage 1 7.8⫾0.6 7.6⫾0.5 NS
Stage 2 41.5⫾3.6 43.5⫾4.8 NS
Stages 3 and 4 27.3⫾1.9 27.0⫾2.2 NS
REM sleep, mean⫾SD, % of TST 23.4⫾2.0 21.9⫾2.5 NS
OAHI, mean⫾SD, episodes per h of TST
12.0⫾1.7 10.9⫾1.5 NS
SaO2of⬍95%, mean⫾SD, % of TST
10.1⫾1.7 9.7⫾1.8 NS
Total arousal index, mean⫾SD, episodes per h of TST
13.5⫾1.3 13.9⫾1.5 NS
RAI, mean⫾SD, episodes per h of TST
6.0⫾0.7 6.7⫾1.1 NS
End-tidal carbon dioxide tension of ⬎50 mm Hg, mean⫾SD, % of TST
25.6⫾3.2 24.3⫾3.4 NS
based approach would not allow for differentiation of MSL among obese and nonobese subjects.35Despite such
considerations, the RAI in obese children emerged as accounting for the variance in MSL more prominently than either the OAHI or hypoxemia, which suggests that obese children may be more vulnerable to sleep frag-mentation and that the latter may play a significant role in inducing the biological responses that ultimately lead to increased sleepiness.
The biological mechanisms underlying the different susceptibility to EDS in obese and nonobese children remain unclear. EDS has been linked to increased levels of inflammatory mediators such as tumor necrosis factor ␣and interleukins 1and 6, as well as prostaglandins, principally prostaglandin D2.36–41Indeed, significant
im-provements in EDS occurred in adult patients with OSA after treatment with a synthetic tumor necrosis factor receptor acting as decoy.42Obesity is currently viewed as
a low-grade systemic inflammatory disorder,43–46and the
same concept applies to OSA.47–52Therefore, it is
plausi-ble that the coexistence of obesity and OSA magnifies the inflammatory response associated with each of these conditions, thereby resulting in increased release of the aforementioned sleepiness-promoting compounds or other compounds yet to be identified. These consider-ations must await future studies, however. Preliminary evidence suggesting that obesity alone, in the absence of sleep-disordered breathing, may be associated with sleepiness has been reported for both adults53and
chil-dren.54,55 Additional studies are needed to determine FIGURE 1
Individual MSL values for 50 obese prepubertal children (circles) and 50 matched nonobese children (squares) plotted against OAHI (A), proportion of TST spent with SaO2of⬍95% (B),
and RAI (C) and relationship between MSL and BMIzscore for the whole cohort of 100 children (vertical line indicates the threshold for obesity, ie, BMIzscore of 1.67) (D). For all panels, horizontal lines indicate the cutoff value for EDS (ie, MSL ofⱕ12 minutes).
TABLE 2 Linear Regression Analyses of MSL and Polysomnographic Respiratory Disturbance Measures
for 50 Obese and 50 Nonobese, Prepubertal, Snoring Children Being Evaluated for Suspected OSA
OAHI RAI Proportion of TST With SaO2
of⬍95%
A B R2 A B R2 A B R2
Obese 16.46 ⫺0.31 0.33 16.54 ⫺0.54 0.44 16.23 ⫺0.34 0.32
Nonobese 21.31 ⫺0.28 0.40 20.82 ⫺0.48 0.20 20.71 ⫺0.28 0.32
whether otherwise-healthy, nonsnoring, obese children display reduced MSL, compared with nonobese non-snoring children.
CONCLUSIONS
In the presence of OSA of similar severity, obese children are at increased risk for EDS. Furthermore, obese, habit-ually snoring children present magnified reductions in MSL (ie, increased sleep propensity) at any level of OSA severity. Therefore, the clinical presentation of habitual snoring and sleep-disordered breathing in obese children differs from that of children who are not obese, such that symptoms of EDS and difficulty remaining awake for an obese child should prompt evaluation for OSA.
ACKNOWLEDGMENTS
Dr Gozal was supported by the National Institutes of Health (grants HL65270 and SCOR 2P50HL60296-06), the Children’s Foundation Endowment for Sleep Re-search, and the Commonwealth of Kentucky Challenge for Excellence Trust Fund. Dr Kheirandish-Gozal re-ceived support from the National Space Agency (grant NNJ05HF 06G).
We thank the children and their families for their cooperation and the sleep technologists for their dedica-tion and perseverance in this project.
REFERENCES
1. Magarey AM, Daniels LA, Boulton TJ. Prevalence of over-weight and obesity in Australian children and adolescents: reassessment of 1985 and 1995 data against new standard international definitions.Med J Aust.2001;174(11):561–564 2. Lobstein T, Baur L, Uauy R; IASO International Obesity
TaskForce. Obesity in children and young people: a crisis in public health.Obes Rev.2004;5(suppl 1):4 –104
3. Malecka-Tendera E, Mazur A. Childhood obesity: a pandemic of the twenty-first century.Int J Obes (Lond). 2006;30(suppl 2):S1–S3
4. Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version.Pediatrics.2002;109(1):45– 60
5. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999 –2000.JAMA.2002;288(14):1728 –1732
6. Kaditis AG, Finder J, Alexopoulos EI, et al. Sleep-disordered breathing in 3680 Greek children.Pediatr Pulmonol.2004;37(6): 499 –509
7. Blunden S, Lushington K, Lorenzen B, Wong J, Balendran R, Kennedy D. Symptoms of sleep breathing disorders in children are underreported by parents at general practice visits.Sleep Breath.2003;7(4):167–176
8. Castronovo V, Zucconi M, Nosetti L, et al. Prevalence of habit-ual snoring and sleep-disordered breathing in preschool-aged children in an Italian community. J Pediatr. 2003;142(4): 377–382
9. O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neu-robehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/ hyperactivity disorder.Pediatrics.2003;111(3):554 –563 10. Montgomery-Downs HE, O’Brien LM, Holbrook CR, Gozal D.
Snoring and sleep-disordered breathing in young children: subjective and objective correlates.Sleep.2004;27(1):87–94
11. Eitner S, Urschitz MS, Guenther A, et al. Sleep problems and daytime somnolence in a German population-based sample of snoring school-aged children.J Sleep Res.2007;16(1):96 –101 12. Arens R, Marcus CL. Pathophysiology of upper airway
obstruction: a developmental perspective. Sleep. 2004;27(5): 997–1019
13. Bell LM, Byrne S, Thompson A, et al. Increasing body mass indexzscore is continuously associated with complications of overweight in children, even in the healthy weight range.
J Clin Endocrinol Metab.2007;92(2):517–522
14. Ievers-Landis CE, Redline S. Pediatric sleep apnea: implications of the epidemic of childhood overweight.Am J Respir Crit Care Med.2007;175(5):436 – 441
15. Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. J Pediatr. 2007;150(6): 608 – 612
16. Tauman R, Gozal D. Obesity and obstructive sleep apnea in children.Paediatr Respir Rev.2006;7(4):247–259
17. Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution.Arch Dis Child.2007;92(3):205–208
18. Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: as-sociations with obesity, race, and respiratory problems.Am J Respir Crit Care Med.1999;159(5):1527–1532
19. Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995; 108(3):610 – 618
20. Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleep-iness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep. 2006; 29(4):495–503
21. Gozal D, Wang M, Pope DW Jr. Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108(3): 693– 697
22. Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime iness and hyperactivity in children with suspected sleep-disordered breathing.Pediatrics.2004;114(3):768 –775 23. Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D.
Polysomnographic characteristics in normal preschool and early school-age children.Pediatrics.2006;117(3):741–753 24. Rechtschaffen A, Kales A.A Manual of Standardized Terminology,
Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington, DC: National Institutes of Health; 1968. Publica-tion 204
25. American Thoracic Society. Standards and indications for car-diopulmonary sleep studies in children.Am J Respir Crit Care Med.1996;153(2):866 – 878
26. American Sleep Disorders Association, Sleep Disorders Atlas Task Force. EEG arousals: scoring rules and examples.Sleep.
1992;15(2):173–184
27. Mograss MA, Ducharme FM, Brouillette RT. Movement/ arousals: description, classification, and relationship to sleep apnea in children. Am J Respir Crit Care Med. 1994;150(6): 1690 –1696
28. Tauman R, O’Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children.
Sleep.2004;27(2):274 –278
29. O’Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children.Sleep.
2004;27(2):279 –282
31. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States.Adv Data.2000;(314):1–27 32. Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood OSA: one
or two distinct disease entities? Clin Sleep Med. 2007;2(3): 433– 444
33. Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pedi-atric obstructive sleep apnea: complications, management, and long-term outcomes.Proc Am Thorac Soc.2008;5(2):274 –282 34. Mitchell RB, Kelly J. Outcome of adenotonsillectomy for
ob-structive sleep apnea in obese and normal-weight children.
Otolaryngol Head Neck Surg.2007;137(1):43– 48
35. Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstruc-tive sleep apnea syndrome in children after adenotonsillec-tomy.J Pediatr.2006;149(6):803– 808
36. Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea.Am J Respir Crit Care Med.2005;171(6):652– 658 37. Kapa´s L, Krueger JM. Tumor necrosis factor-induces sleep,
fever, and anorexia.Am J Physiol.1992;263(3):R703–R707 38. Takahashi S, Tooley DD, Kapa´s L, Fang J, Seyer JM, Krueger
JM. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep.Pflugers Arch.1995;431(2):155–160
39. El-Sheikh M, Buckhalt JA, Granger DA, Erath SA, Acebo C. The association between children’s sleep disruption and sali-vary interleukin-6.J Sleep Res.2007;16(2):188 –197
40. Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity.Sleep.2005;28(2): 177–184
41. Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats.J Neuroimmunol.2003;137(1–2):59 – 66
42. Barcelo´ A, de la Pen˜a M, Barbe´ F, Pierola J, Bosch M, Agustí AG. Prostaglandin D synthase (trace) levels in sleep apnea patients with and without sleepiness. Sleep Med. 2007;8(5): 509 –511
43. Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-␣ antago-nist.J Clin Endocrinol Metab.2004;89(9):4409 – 4413
44. Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances
in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance.J Intern Med.2003;254(1):32– 44 45. Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepi-ness and fatigue: the role of the stress system and cytokines.
Ann N Y Acad Sci.2006;1083:329 –344
46. Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression.J Clin Endocrinol Metab.2005;90(8):4510 – 4515 47. Ko¨rner A, Kratzsch J, Gausche R, Schaab M, Erbs S, Kiess W.
New predictors of the metabolic syndrome in children: role of adipocytokines.Pediatr Res.2007;61(6):640 – 645
48. Williams A, Scharf SM. Obstructive sleep apnea, cardiovascular disease, and inflammation: is NF-B the key? Sleep Breath.
2007;11(2):69 –76
49. Kheirandish-Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C-reactive protein in nonobese children with obstruc-tive sleep apnea before and after adenotonsillectomy.J Clin Sleep Med.2006;2(3):301–304
50. Tauman R, O’Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing.Sleep Breath.2007;11(2):77– 84 51. Tauman R, Ivanenko A, O’Brien LM, Gozal D. Plasma
C-reac-tive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113(6). Available at: www. pediatrics.org/cgi/content/full/113/6/e564
52. Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea.Sleep Med.2008;9(3):254 –259
53. Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheiran-dish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea.Sleep Med.
2008; In press
54. Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness.Arch Intern Med.1998;158(12):1333–1337 55. Snow A, Gozal E, Malhotra A, et al. Severe hypersomnolence
DOI: 10.1542/peds.2008-0228
2009;123;13
Pediatrics
David Gozal and Leila Kheirandish-Gozal
Obstructive Sleep Apnea
Obesity and Excessive Daytime Sleepiness in Prepubertal Children With
Services
Updated Information &
http://pediatrics.aappublications.org/content/123/1/13
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/123/1/13#BIBL
This article cites 50 articles, 6 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/obesity_new_sub
Obesity
http://www.aappublications.org/cgi/collection/endocrinology_sub
Endocrinology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2008-0228
2009;123;13
Pediatrics
David Gozal and Leila Kheirandish-Gozal
Obstructive Sleep Apnea
Obesity and Excessive Daytime Sleepiness in Prepubertal Children With
http://pediatrics.aappublications.org/content/123/1/13
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.