Very Low Birth Weight Infants
WHAT’S KNOWN ON THIS SUBJECT: EOS is associated with increased risk of morbidities and mortality in VLBW infants. The effect of changes in pathogens is not well known. The risk for specific morbidities including ROP and PVL has not been established.
WHAT THIS STUDY ADDS: Here the mortality and major morbidities associated with EOS are reported in the largest population-based cohort of VLBW infants with EOS reported to date. An increased risk of ROP was found.
abstract
BACKGROUND:Early-onset sepsis (EOS) is associated with significant morbidity and mortality among infants with a very low birth weight (VLBW); however, there is a sparse amount of complete data on large cohorts.
OBJECTIVE:To evaluate the mortality and major morbidities among VLBW infants with EOS.
METHODS:This was a population-based observational study. Data were prospectively collected by the Israel Neonatal Network on all VLBW infants born in Israel from 1995 through 2005. Univariate and multivariable analyses were performed to assess the independent as-sociation of EOS on morbidity and mortality of VLBW infants.
RESULTS:The study cohort included 15 839 infants, of whom 383 (2.4%) developed EOS. EOS was associated with significantly increased odds for mortality (odds ratio [OR]: 2.57 [95% confidence interval (CI): 1.97–3.35]), severe intraventricular hemorrhage (OR: 2.24 [95% CI: 1.67–3.00]), severe retinopathy of prematurity (OR: 2.04 [95% CI: 1.32– 3.16]), and bronchopulmonary dysplasia (OR: 1.74 [95% CI: 1.24 –2.43]). EOS was associated with an increased risk of death and/or severe neurologic morbidity (OR: 2.92 [95% CI: 2.27–3.80]).
CONCLUSIONS:Although only 2.4% of VLBW infants had an episode of EOS, these infants were at an approximately threefold excess risk of death or major neurologic morbidities.Pediatrics2010;125:e736–e740
AUTHORS:Gil Klinger, MD,a,bItzhak Levy, MD,b,cLea
Sirota, MD,a,bValentina Boyko, MSc,dLiat Lerner-Geva,
MD, PhD,b,dand Brian Reichman, MBChB,b,din
collaboration with the Israel Neonatal Network
aDepartment of Neonatal Intensive Care andcInfectious Disease Unit, Schneider Children’s Medical Center of Israel, Petah Tiqva, Israel;dWomen and Children’s Health Research Unit and Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel; and bSackler School of Medicine, Tel Aviv University, Tel Aviv, Israel
KEY WORDS
early-onset sepsis, very low birth weight, outcome, mortality
ABBREVIATIONS
EOS— early-onset sepsis VLBW—very low birth weight RDS—respiratory distress syndrome BPD— bronchopulmonary dysplasia IVH— intraventricular hemorrhage PVL—periventricular leukomalacia GA— gestational age
BW— birth weight
CONS— coagulase-negative staphylococci ROP—retinopathy of prematurity OR— odds ratio
CI— confidence interval
www.pediatrics.org/cgi/doi/10.1542/peds.2009-2017
doi:10.1542/peds.2009-2017
Accepted for publication Nov 20, 2009
Address correspondence to Gil Klinger, MD, Department of Neonatology, Schneider Children’s Medical Center of Israel, 14 Kaplan St, Petah Tiqva 49202, Israel. E-mail: gilkl@post.tau.ac.il
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2010 by the American Academy of Pediatrics
Early-onset sepsis (EOS) is a serious problem among very low birth weight (VLBW) infants. The incidence of EOS among VLBW infants is 15 to 19 per 1000 live births1–3and has remained
unchanged despite routine use of an-tepartum antibiotic therapy. EOS has reportedly been associated with a more than threefold increased risk for mortality among VLBW infants.4
Fur-thermore, VLBW survivors of EOS are also at an increased risk of a wide range of neonatal morbidities such as respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), in-traventricular hemorrhage (IVH), and periventricular leukomalacia (PVL).4,5
The causative pathogens of EOS have changed over time from a predomi-nance of group B Streptococcus1,3to
that ofEscherichia coli,2,4possibly
be-cause of the influence of group B Streptococcus antibiotic prophylaxis. This trend may have an effect on mor-tality, because early mortality rates within the first 3 days of life are higher when EOS is caused by Gram-negative
organisms compared with
Gram-positive pathogens (29% vs 3%, re-spectively).4 Although VLBW infants
with EOS caused by Gram-negative or-ganisms have a higher rate of neonatal
morbidities compared with those
caused by Gram-positive organisms, this difference has not been found to be statistically significant.4
The aim of our study was to evaluate the independent effect of EOS on mor-tality and on major neonatal morbidi-ties in a large national cohort of VLBW infants.
METHODS
This population-based observational study was performed on data obtained from the Israel National VLBW Infant Database. Data were prospectively col-lected by the Israel Neonatal Network on VLBW newborn infants (birth weight [BW] ⱕ 1500 g) born in Israel from
1995 through 2005. All 28 neonatal de-partments in Israel participated in the data collection as described previous-ly.6The data collected included
demo-graphic details, antenatal and perina-tal history, postdelivery status and neonatal diagnoses, medical and sur-gical treatments, and outcome at discharge. A prestructured form was completed for each infant, checked for logic errors, and, if necessary, re-turned to the participating center for clarification. Interhospital transfers were followed by the database coordi-nator until final discharge from the hospital. All departments used an op-erating manual and standard defini-tions based on those of the Vermont Oxford Trials Network.7Data were
col-lected on all infants until death or dis-charge from the hospital.
Study Population
From 1995 through 2005, the database included records of 16 462 infants, which comprised⬎99% of all the VLBW infants born live in Israel. Excluded from analysis were 623 infants who died in the delivery room or from lethal congenital malformations. Our study
cohort comprised the remaining
15 839 infants.
Definitions
The gestational age (GA) in completed weeks was defined as the best esti-mate of GA based on last menstrual period, obstetric history and examina-tion, prenatal ultrasound, or early postnatal physical examination. The definition of infants who were small for GA was a BW⬍10th percentile for GA according to the gender-specific growth charts of Kramer et al.8
Pro-longed rupture of membranes was considered as rupture of membranes for⬎12 hours before delivery. The di-agnosis of amnionitis was based on maternal fever recorded during mem-brane rupture or within 6 hours after delivery providing no other cause for
fever was found.9 Antenatal steroid
therapy included partial or complete courses of therapy. Delivery-room resuscitation included endotracheal intubation, chest compressions, or epinephrine administration. EOS was defined clinically and required a pos-itive blood culture obtained within the first 72 hours of life.1 EOS was
not diagnosed if cultures tested positive for organisms considered to be contaminants (Corynebacterium spp., Propionibacterium, Diphtheroid spp., orMicrococcusspp.). The diagno-sis of sepdiagno-sis caused by coagulase-negative staphylococci (CONS) was determined according to the criteria of the Vermont Oxford Network Data-base6,7and required clinical signs of
sepsis, a positive blood culture re-sult, and antibiotic treatment for at least 5 days or until death. RDS was diagnosed clinically and confirmed by chest radiograph. BPD was de-fined as clinical evidence of BPD to-gether with the requirement of oxy-gen therapy at 28 days of life.10IVH
was diagnosed by ultrasound exami-nation and graded according to defi-nitions by Papile et al.11PVL was
de-termined by ultrasound examination performed 28 days or more after birth. Severe brain ultrasound ab-normalities included IVH grades 3 and 4 and/or PVL. Retinopathy of pre-maturity (ROP) was determined and graded by ophthalmologic examina-tion according to the internaexamina-tional classification of ROP.12 Severe ROP
was defined as grade 3 to 4 ROP. Ad-verse neurologic morbidity was de-fined as IVH grade 3 to 4 and/or PVL and/or ROP grade 3 to 4.
Statistical Analysis
The association between EOS and neo-natal outcomes was tested by using a
2test for categorical variables and a
2-sample t test for continuous vari-ables. Multivariable analyses were used to identify the independent
adjusted for previously reported peri-natal variables13 including GA, BW,
small for GA, gender, multiple preg-nancy, ethnicity, prolonged rupture of membranes, amnionitis, premature contractions, maternal hypertension, antenatal steroid treatment, cesar-ean delivery, and delivery-room re-suscitation. Although CONS was con-sidered to be causative of EOS in the presence of clinical signs and symp-toms compatible with sepsis,1,2 we
performed separate analyses with and without this pathogen, because some cases may represent contami-nation. Results of the multivariable analyses are presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Statistical analyses were performed by using SAS 9.1 sta-tistical software (SAS Institute, Inc, Cary, NC).
RESULTS
The study cohort comprised 15 839 in-fants, of whom 383 (2.42%) had EOS. The epidemiology and risk factors for EOS among this cohort have been de-scribed in detail.13Briefly, 55% of EOS
episodes were caused by
negative bacteria, 42% by Gram-positive bacteria, and 3% by yeasts. The most common pathogens wereE coli(26.8%), CONS (17.2%), and group BStreptococcus(9.4%). The distribu-tion of pathogens is comparable to that reported by Stoll et al,2 who
showed that EOS was caused by Gram-negative bacteria in 53% of infants and CONS in 14.7% of infants. The antenatal and perinatal characteristics of VLBW infants with and without EOS are pre-sented in Table 1. VLBW infants with EOS compared with those without it were born at an earlier mean GA (27.7⫾2.6 vs 29.1⫾3.0 weeks;P⬍ .0001) and at a lower mean BW (1005⫾ 287 vs 1102⫾283 g;P⬍.0001) and were more likely to be born after
am-nionitis or premature rupture of mem-branes (33.4% vs 6.8% and 42.0% vs 17.9%; bothP⬍.0001), to require any delivery-room resuscitation (71.0% vs 43.2%;P⬍.0001), or to require chest compressions or epinephrine admin-istration (5.5% vs 2.9%;P⬍.01).
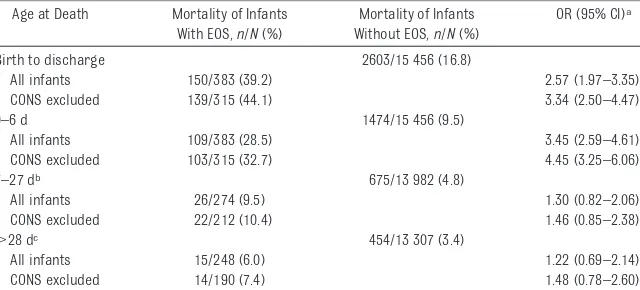
EOS sepsis in VLBW infants was associ-ated with an increased risk of death before discharge (OR: 2.57 [95% CI: 1.97–3.35]), which was mainly because of deaths occurring during the first 6
days of life (OR: 3.45 [95% CI:
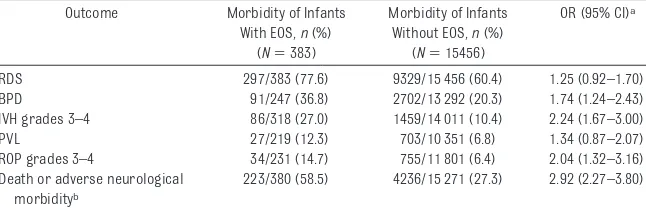
2.59 – 4.61]) (Table 2). VLBW infants with EOS were at an increased risk of major neonatal morbidities (Table 3). These morbidities included BPD (OR: 1.74 [95% CI: 1.24 –2.43]), severe IVH (OR: 2.24 [95% CI: 1.67–3.00]), and
se-vere ROP (OR: 2.04 [95% CI: 1.32–3.16]). In VLBW infants with EOS, the odds for death or discharge with severe neuro-logic morbidity was approximately threefold that of infants without EOS. A second analysis that excluded 68 in-fants with EOS caused by CONS was performed. For this group of infants with EOS (n⫽315), the odds for death within all time periods assessed was increased (Table 2), as was the risk of adverse neurologic outcome or death (OR: 3.45 [95% CI: 2.56 – 4.55]). The odds for neonatal morbidities were as follows: BPD (OR: 2.02 [95% CI: 1.38 – 2.94]), severe IVH (OR: 2.64 [95% CI: 1.92–3.62]), PVL (OR: 1.58 [95% CI: 0.96 –2.47]), and severe ROP (OR: 1.51 [95% CI: 0.86 –2.54]).
GA, wk (mean⫾SD) 27.7⫾2.6 29.1⫾3.0 ⬍.0001 BW, g (mean⫾SD) 1005⫾287 1102⫾283 ⬍.0001
Male 205 (53.5) 7843 (50.8) .28
Jewish ethnicity 202 (76.4) 11 258 (73.0) .13
Small for GA 62 (16.2) 4850 (31.4) ⬍.0001
Multiple birth 120 (31.3) 6683 (43.2) ⬍.0001 Antenatal steroid therapy (any) 251 (64.1) 9875 (65.9) .45 Maternal hypertension 43 (11.3) 3113 (20.2) ⬍.0001 Premature contractions 227 (59.6) 8709 (56.4) .22 Prolonged rupture of membranes 155 (42.0) 2710 (17.9) ⬍.0001
Amnionitis 126 (33.4) 1055 (6.8) ⬍.0001
Cesarean delivery 226 (59.0) 10 681 (69.1) ⬍.0001 Delivery-room resuscitation 272 (71.0) 6679 (43.2) ⬍.0001
TABLE 2 Mortality of VLBW Infants With and Without EOS
Age at Death Mortality of Infants With EOS,n/N(%)
Mortality of Infants Without EOS,n/N(%)
OR (95% CI)a
Birth to discharge 2603/15 456 (16.8)
All infants 150/383 (39.2) 2.57 (1.97–3.35) CONS excluded 139/315 (44.1) 3.34 (2.50–4.47)
0–6 d 1474/15 456 (9.5)
All infants 109/383 (28.5) 3.45 (2.59–4.61) CONS excluded 103/315 (32.7) 4.45 (3.25–6.06)
7–27 db 675/13 982 (4.8)
All infants 26/274 (9.5) 1.30 (0.82–2.06)
CONS excluded 22/212 (10.4) 1.46 (0.85–2.38)
⬎28 dc 454/13 307 (3.4)
All infants 15/248 (6.0) 1.22 (0.69–2.14)
CONS excluded 14/190 (7.4) 1.48 (0.78–2.60)
aAdjusted for GA, gender, ethnicity, small for GA, multiple pregnancy, antenatal steroid therapy, maternal
hyperten-sion, premature contractions, prolonged rupture of membranes, cesarean delivery, amnionitis, and delivery-room resuscitation.
DISCUSSION
Although EOS was noted in only 2.4% of the VLBW population born in Israel, it profoundly affected the outcome of these infants. VLBW infants with EOS compared with those without it had an increased risk of mortality (OR: 2.57) and an increased risk of neurologic ad-verse outcomes, including IVH (OR: 2.42) and ROP (OR: 2.04), and overall, the odds for death or discharge with severe neurologic morbidity were in-creased approximately threefold.
The excess mortality of VLBW infants was predominantly because of deaths that occurred during the first 6 days of life. Stoll et al4have similarly shown an
in-creased risk of mortality occurring dur-ing the first 3 days of life. Furthermore, we have shown that in infants with EOS, the risk of adverse neurologic outcomes including IVH grades 3 to 4 and ROP grades 3 to 4 was increased. The results of this study confirm the association be-tween EOS and IVH reported previously.4,5
In preterm infants, the association be-tween brain injury and perinatal infec-tion has been noted; however, the cause-and-effect relationship has not yet been proven.14The immature brain seems to
be vulnerable to the presence of an in-flammatory response.14
Chorioamnioni-tis, which is strongly associated with EOS, increased the risk of IVH and ad-verse long-term neurologic outcome.15–17
A number of mechanisms may explain EOS-related brain injury. Cerebral
dam-age may be caused by a direct bacterial effect, by cytokine-mediated inflamma-tory response14,18,19; alternatively, brain
injury may also reflect a poorer initial condition of infants with EOS, as evident from the increased need for delivery-room resuscitation. PVL has been asso-ciated with inflammation and infec-tion,14–16but we did not find an increased
risk for PVL in infants with EOS. In a study by Stoll et al,4an increased risk of the
combined outcome of PVL or IVH was re-ported, but this increase may have been more strongly influenced by an increase in IVH.
The association of ROP with EOS has not been reported previously, and studies evaluating the possible association be-tween ROP and late bacterial sepsis in preterm infants have been contradicto-ry.20,21The increased risk for ROP may be
due to inflammatory processes, or it may be related to the prolonged expo-sure to oxygen therapy as evident by the increased rate of BPD in infants with EOS.4 Explanations for the increase in
BPD are an inflammatory-mediated de-crease in alveolarization22,23 or an
in-creased exposure to ventilation and oxy-gen in infants with EOS. However, Lahra et al24,25 recently provided insight into
the relationship between inflammation, chronic lung disease, and RDS. The pres-ence of a fetal inflammatory response was protective for chronic lung disease, possibly because of a decreased risk of RDS. Infants who had intrauterine
in-flammation but did not have postnatal sepsis were less likely to develop chronic lung disease than infants with both intrauterine infection and postna-tal sepsis, which supports the hypoth-esis that intrauterine inflammation decreased the risk of chronic lung dis-ease. Neonatal sepsis was strongly as-sociated with an increased risk of chronic lung disease, possibly related to an increased exposure of infants with sepsis to ventilation.
The present study was based on data from a large population-based cohort of VLBW infants prospectively collected by all the NICUs in Israel using standard def-initions and with minimal exclusion cri-teria. The number of events of EOS ana-lyzed is greater than those previously reported,1,2,4which further validates the
findings of this study. However, some study limitations should be acknowl-edged. Because of the long study period, some obstetrical or neonatal policies may have changed. The definition of EOS used included CONS isolated by blood culture, which may represent contami-nation rather than a true infection in some infants. The proportion of CONS EOS in our study was, however, compa-rable to that reported by Stoll et al.2
Anal-yses with and without CONS were per-formed to overcome this limitation and the results of both analyses were similar for most outcomes. The risk for adverse outcomes related to specific groups of bacteria could not be assessed because of the small number of infants in each group. This study focused on short-term neurologic outcomes; however, the long-term effects of EOS on neurodevelop-ment should be evaluated.
CONCLUSIONS
Although only 2.4% of VLBW infants
admitted to neonatal units had
culture-proven episodes of EOS,
these infants were at a threefold ex-cess risk of death or major neuro-logic morbidities. The excess risk for TABLE 3 Morbidity of VLBW Infants With and Without EOS
Outcome Morbidity of Infants With EOS,n(%)
(N⫽383)
Morbidity of Infants Without EOS,n(%)
(N⫽15456)
OR (95% CI)a
RDS 297/383 (77.6) 9329/15 456 (60.4) 1.25 (0.92–1.70) BPD 91/247 (36.8) 2702/13 292 (20.3) 1.74 (1.24–2.43) IVH grades 3–4 86/318 (27.0) 1459/14 011 (10.4) 2.24 (1.67–3.00) PVL 27/219 (12.3) 703/10 351 (6.8) 1.34 (0.87–2.07) ROP grades 3–4 34/231 (14.7) 755/11 801 (6.4) 2.04 (1.32–3.16) Death or adverse neurological
morbidityb
223/380 (58.5) 4236/15 271 (27.3) 2.92 (2.27–3.80)
aAdjusted for GA, gender, ethnicity, small for GA, multiple pregnancy, antenatal steroid therapy, maternal hypertension,
premature contractions, prolonged rupture of membranes, cesarean delivery, amnionitis, and delivery-room resuscitation.
bDefined as death or discharge with IVH grades 3 to 4 and/or PVL and/or ROP grades 3 to 4.
future studies.
ACKNOWLEDGMENTS
The Israel National VLBW infant data-base is funded in part by the Israel Cen-ter for Disease Control and the Minis-try of Health.
The Israel Neonatal Network’s National VLBW Infant Database is coordinated by the Women and Children’s Health Research Unit, Gertner Institute. Par-ticipating centers are the Assaf
Haro-Bikur Holim Hospital, Jerusalem; Bnei Zion Medical Centre, Haifa; Carmel Medical Center, Haifa; English (Scot-tish) Hospital, Nazareth; French Hospi-tal, Nazareth; Hadassah University Hos-pital Ein-Karem, Jerusalem; Hadassah
University Hospital Har Hazofim,
Jerusalem; Haemek Medical Center, Afula; Hillel Yafe Medical Center, Hadera; Italian Hospital, Nazareth; Kaplan Hospital, Rehovot; Laniado Hos-pital, Netanya; Maayanei Hayeshua
tal, Jerusalem; Naharia Hospital, Naha-ria; Poria Hospital, Tiberias; Rambam Medical Center, Haifa; Rivka Ziv Hospi-tal, Zefat; Schneider Children’s Medical Center of Israel and Rabin Medical Cen-ter (Beilinson Campus), Petach-Tikva; Shaare-Zedek Hospital, Jerusalem; Sheba Medical Center, Tel-Hashomer; Soroka Medical Center, Beer-Sheva; Sourasky Medical Center, Tel-Aviv; Wolfson Medical Center, Holon; and Yoseftal Hospital, Eilat.
REFERENCES
1. Stoll BJ, Gordon T, Korones SB, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Insti-tute of Child Health and Human Develop-ment Neonatal Research Network.J Pedi-atr.1996;129(1):72– 80
2. Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Develop-ment Neonatal Research Network, 2002–2003. Pediatr Infect Dis J.2005;24(7):635– 639 3. Klein JO. Bacterial sepsis and meningitis. In:
Remington JS, Klein JO, eds.Infectious Dis-eases of the Fetus and Newborn Infant. 5th ed. Philadelphia, PA: WB Saunders; 2001:953–998
4. Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med.2002;347(4):240 –247
5. Lehner R, Leitich H, Jirecek S, Foldy M, Kaider A. Retrospective analysis of severe intraventricular hemorrhage and early-onset neonatal sepsis in very low birth weight infants.Eur J Clin Microbiol Infect Dis.2001;20(11):833– 834
6. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediat-rics.2002;109(1):34 –39
7. Vermont Oxford Trials Network Database Project.Manual of Operations. Release 2.0. Burlington, VT: Vermont Oxford Trials Network; 1993
8. Kramer MS, Platt RW, Wen S, et al; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A new and improved population-based Canadian reference for
birth weight for gestational age.Pediatrics. 2001;108(2). Available at: www.pediatrics.org/ cgi/content/full/108/2/e35
9. Riskin A, Riskin-Mashiah S, Lusky A, Reich-man B; Israel Neonatal Network. The rela-tionship between delivery mode and mor-tality in very low birth weight singleton vertex-presenting infants. BJOG. 2004; 111(12):1365–1371
10. Ehrenkranz RA, Walsh MC, Vohr BR, et al. Vali-dation of the National Institutes of Health con-sensus definition of bronchopulmonary dys-plasia.Pediatrics.2005;116(6):1353–1360
11. Papile LA, Burstein J, Burstein AR, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm.J Pediatr.1978;92(4):529 –534
12. International Committee for the Classifica-tion of Retinopathy of Prematurity. The In-ternational Classification of Retinopathy of Prematurity revisited. Arch Opthalmol. 2005;123(7):991–999
13. Klinger G, Levy I, Sirota L, Boyko V, Reichman B, Lerner-Geva L; Israel Neonatal Network. Epide-miology and risk factors for early onset sepsis among very low birth weight infants.Am J Ob-stet Gynecol.2009;201(1):38.e1–38.e6
14. Edwards AD, Tan S. Perinatal infections, pre-maturity and brain injury.Curr Opin Pedi-atr.2006;18(2):119 –124
15. Alexander J, Gilstrap LC, Cox S, McIntire DM, Leveno KJ. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol.1998;91(5 pt 1):725–729
16. Adams-Chapman I, Stoll BJ. Neonatal infec-tion and long-term neurodevelopmental outcome in the preterm infant.Curr Opin Infect Dis.2006;19(3):290 –297
17. Polam S, Koons A, Anwar M, Shen-Schwartz,
Hegyi T. Effect of chorioamnionitis on neuro-developmental outcome in preterm infants. Arch Pediatr Adolesc Med.2005;159(11): 1032–1035
18. Hagberg H, Mallard C. Effect of inflamma-tion on central nervous system develop-ment and vulnerability.Curr Opin Neurol. 2005;18(2):117–123
19. Babnik J, Stucin-Gantar I, Kornhauser-Cerar L, Sinkovec J, Wraber B, Derganc M. Intra-uterine inflammation and the onset of peri-intraventricular hemorrhage in premature infants.Biol Neonate.2006;90(2):113–121 20. Al-Essa M, Azad RV, Rashwan N. Threshold
stage of retinopathy of prematurity: mater-nal and neonatal risk factors.Ann Saudi Med.2000;20(2):129 –131
21. Manzoni P, Maestri A, Leonessa M, Mostert M, Farina D, Gomirato G. Fungal and bacte-rial sepsis and threshold ROP in preterm very low birth weight neonates.J Perinatol. 2006;26(1):23–30
22. Jobe AH. Antenatal factors and the develop-ment of bronchopulmonary dysplasia. Se-min Neonatol.2003;8(1):9 –17
23. Ambalavanan N, Carlo WA, D’Angio CT, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neo-natal Research Network. Cytokines associ-ated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics.2009;123(4):1132–1141
24. Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis and chronic lung disease: a 13-year hospital cohort study.Pediatrics.2009;123(5):1314 –1319
DOI: 10.1542/peds.2009-2017 originally published online March 15, 2010;
2010;125;e736
Pediatrics
Reichman and in collaboration with the Israel Neonatal Network
Gil Klinger, Itzhak Levy, Lea Sirota, Valentina Boyko, Liat Lerner-Geva, Brian
Infants
Outcome of Early-Onset Sepsis in a National Cohort of Very Low Birth Weight
Services
Updated Information &
http://pediatrics.aappublications.org/content/125/4/e736 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/125/4/e736#BIBL This article cites 22 articles, 5 of which you can access for free at:
Subspecialty Collections
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2009-2017 originally published online March 15, 2010;
2010;125;e736
Pediatrics
Reichman and in collaboration with the Israel Neonatal Network
Gil Klinger, Itzhak Levy, Lea Sirota, Valentina Boyko, Liat Lerner-Geva, Brian
http://pediatrics.aappublications.org/content/125/4/e736
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.