Interfacial Reaction in AZ91D Magnesium Alloy Matrix Composite
Reinforced with Aluminum Borate Whisker
W. G. Wang
*, K. Matsugi, H. Fukushima and G. Sasaki
Department of Mechanical System Engineering, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan
AZ91D magnesium alloy reinforced with aluminum borate whisker (Al18B4O33w, denoted by ABOw) was fabricated by squeeze-casting.
The heat treatment (T4) was carried out at 693 K in argon atmosphere for 48 and 96 hours, respectively, following water-quenched. The behaviors of interfacial reaction were studied in processes of fabrication and following heat treatment. Microstructures of interfacial reaction layers were investigated with transmission electron microscopy (TEM). Interfacial reaction layer in thickness of 5–10 nm was found in as-casted composite, and it was identified that the interfacial reaction layer was consisted of MgO. When the composite was heat-treated at 693 K for 48 hours, the surfaces of whiskers were covered with interfacial reaction layer completely and the thickness of interfacial reaction layer increased to about 20 nm. With increasing the time of heat treatment from 48 hours to 96 hours, the thickness of interfacial reaction layer on the surfaces of whiskers did not change obviously. The interfacial reaction layer was consisted of tiny MgO particles and a small quantity of MgB2particles. Although MgO particles played a good barrier to keep out of contacting between magnesium and whisker, the boundaries between MgO particles acted as a shortcut of magnesium atoms migration. Therefore magnesium could keep on reacting to whisker. The interfacial reaction could invade into the inner of whisker about 20 nm depth after the composite was heat-treated at 673 K for 96 hours. The surfaces of whiskers became wave-like because of interfacial reaction. An orientation relationship was found between MgO reaction layer and ABOw:
ð1111ÞMgO==ð320ÞABOwand½011MgO==½001ABOw. The surfaces of MgO particles were consisted of close-packed (111)MgOplane.
[doi:10.2320/matertrans.MER2007032]
(Received February 13, 2007; Accepted April 26, 2007; Published June 20, 2007)
Keywords: AZ91D magnesium alloy, Al18B4O33whisker, composites, MgO, interfacial reaction
1. Introduction
Recently, magnesium alloy matrix composites reinforced with discontinuous reinforcement (short fiber, whisker or particle) have received great attention due to low coefficient of thermal expansion, high specific strength and stiffness, and wear resistance comparing with the conventional magnesium alloy. Therefore, they have a great potential of application in aerospace, automobile and transport industries.1)
In recent years, many researchers have focused on mag-nesium alloy matrix composite reinforced with SiC whisker, because SiC whisker has good chemical stability in magne-sium alloy matrix and high Young’s modulus, high strength, low coefficient of thermal expansion and other excellent features.2–5) Nevertheless, it needs higher pressure to fab-ricate Mg/SiCw composite using squeeze-casting method.
According to the studies of Zheng,2–4) when AZ91/SiCw
composite is fabricated by squeeze-casting, the pressure of 100 MPa is needed. High cost-effective aluminum borate whisker (Al18B4O33w, denoted by ABOw) has excellent
pro-perties that can compete with those of SiC whisker. And, it is attractive that the coefficient of thermal expansion of ABOw
is only two third of that of SiC whisker. Specially, Mg/ ABOwcomposite can be fabricated with lower pressure than
that of Mg/SiCwcomposite.6)Lower pressure of fabrication
is propitious to decrease the deformation of preform. There-fore ABOw begins to attract great interest as an alternative
reinforcement of SiC whisker.6–11)
However, ABOwis thermodynamically unstable in
mag-nesium matrices unlike that in Mg/SiC composite, causing chemical reaction and reaction products.2,4,6–13) Previous researches on ABOw reinforced magnesium matrix
compo-sites have indicated that the ABOwcan react to matrix easily
in the processes of fabrication and heat treatment.14–16) The interfacial microstructure plays an important role in determining the physical and mechanical properties of metal/ ceramics composites, because reinforcement and matrix will form a system through link of interface.17–19)Especially, the
interfacial reaction in magnesium alloy matrix composite could not be neglected due to high activity of magnesium. Unfortunately, up to now, there is little information available on the interfacial reaction mechanism of magnesium alloy matrix composites reinforced with ABOw.
In this study, AZ91D magnesium alloy with good cast-ability is reinforced with ABOwby squeeze-casting. In order
to clarify the mechanism of interfacial reaction, the compo-site is heat-treated at 693 K for 48 and 96 hours, respectively. The behaviors of interfacial reaction are investigated with transmission electron microscopy (TEM), and the mecha-nism of interfacial reaction is discussed from viewpoint of microstructure.
2. Experimental
Commercial AZ91D magnesium alloy reinforced with 30%-volume fraction of aluminum borate whisker (ALBO-REX M12, produced by Shikoku Chemicals Co.) was fabricated by squeeze-casting. The sizes of whisker are
0:51:0mm in diameter and 1030mm in length, and the whisker surfaces is smooth in atomic scale. The chemical composition of AZ91D magnesium alloy is Mg–9 mass%Al– 0.7 mass%Zn–0.3 mass%Mn.
ABOwpreform was prepared by a wet forming. Whisker
was dispersed with alumina binder, stirred and filtered. And then the preform was dried at room temperature for 72 hours and 333 K for 24 hours. Subsequently, the preform was
*Graduate Student, Hiroshima University
sintered at 1373 K for 4 hours in airs to increase its strength. Table 1 lists the fabrication conditions of AZ91D composite by squeeze-casting. In order to avoid the damage of the composites because of the large thermal residual stress (TRS) between matrix and reinforcement, the composites are conserved in stove at 473 K for 2 hours after squeeze-casting. In order to clarify the mechanism of interfacial reaction, composite was heat-treated at 693 K for 48 and 96 hours, respectively. The samples for TEM observation are prepared. At first, disks of 3 mm in diameter, 200mmin thickness were punched from the composite foils for subsequent machine and argon ion milling. In order to retain the actual state of materials, these experiments were kept at room temperature. TEM observations were carried out with JEOL JEM-2010 microscope.
3. Results and Discussion
3.1 Identification of interfacial reaction products
Figure 1(a) shows the TEM micrograph of interfacial reaction layer between AZ91D magnesium alloy matrix and ABOw in as-casted composite. It illuminates clearly that
interfacial reaction product forms a thin reaction layer on the surface of ABOw in the process of squeeze-casting. Due to
the very small size of the interfacial reaction product, it is impossible to obtain electron diffraction pattern from the reaction layer by conventional selected area diffraction or micro-diffraction. In order to promote formation of a detectable amount of reaction products, the composite is heat-treated at 693 K for 96 hours, as shown in Fig. 1(b).
Selected area diffraction patterns (SADP) are taken from
interfacial reaction layer and ABOw in composite
heat-treated at 693 K for 96 hours. As shown in Fig. 2, the electron diffraction pattern of MgO (face-centered cubic), MgB2
(hexagonal) and ABOw (end-centered orthorhombic) are
demarcated, respectively. Since the electron diffraction pattern of MgB2 is incomplete shown in Fig. 2(a), the
com-plete electron diffraction pattern of MgB2 can be obtained
through turning the TEM sample, as shown in Fig. 2(b). A definite orientation relationship between MgO and ABOw
is found:ð1111ÞMgO==ð320ÞABOw and½011MgO==½001ABOw.
The good lattice matching between the two phases at the interface is the main reason for the formation of the orientation relationship, the mismatch between the (111) plane spacing of MgO (0.243 nm) and (320) plane spacing of ABOw(0.242 nm) is 0.41%.
MgO and MgB2 were found in interfacial reaction layer
between matrix and ABOwby SADP, nevertheless, it is very
difficult to distinguish MgO and MgB2because of their tiny
size and similar shapes with lower magnification images. High resolution electron microscopy (HREM) is carried out to resolve this problem. Figure 3(a) shows that interfacial reaction layer is formed on the surface of whisker in com-posite heat-treated at 693 K for 96 hours. Figure 3(b) is high magnification image of region A framed with white line in Fig. 3(a). Figure 3(b) indicates that boron acting as product of interfacial reaction reacts to Mg and forms MgB2.
As described above, it is clarified that the interfacial re-action layer is consisted of tiny particles of MgO and MgB2.
The interfacial reaction ought to obey following chemical equation:
33MgþAl18B4O33 !33MgOþ18Alþ4B ð1Þ
According to chemical eq. (1) and the fact that MgB2 is
found at interfacial reaction layer, it is suggested that the reaction produce boron would react to magnesium and form MgB2. This assumption is coherent to Mg-B binary phase
[image:2.595.48.290.85.135.2]diagram and result of HREM observation. Therefore, boron reacts to magnesium with following chemical equation: Table 1 Fabrication conditions of AZ91D composite by squeeze casting.
Temperature of molten alloy (K) 998
Temperature of perform (K) 1023
Temperature of die (K) 598
Pressure (MPa) 62.4
Fig. 1 TEM images of interfacial reaction layer in AZ91D/ABOwcomposite. (a) as-casted and (b) heat-treated at 693 K for 96 hours,
respectively.
[image:2.595.84.513.556.758.2]Mgþ2B!MgB2 ð2Þ
It is noticed that the quantity of MgB2 is very tiny.
According to eq. (1) and (2), the quantity of MgB2 is less
than one sixteenth of MgO. As a result, MgB2is very difficult
to be found. Moreover, MgB2has not been found in as-casted
composite. It is maybe because a small quantity of boron is taken away by molten magnesium alloy matrix in the process of squeeze-casting.
In addition, the AZ91D/ABOw composite could be
thought of Mg-Al-B-O system. Because the interfacial reaction lies on the oxidation-reduction of Mg-Al system mainly, the AZ91D/ABOw composite could be thought of
Mg-Al system easily. The study on thermodynamic stability of Al-Mg oxides in Al-Mg alloys20–23) shows that the formation of Al2O3, MgAl2O4 and MgO is competitive
processes. For the alloys with higher magnesium content, the formation of MgO is energetically favorable; but with
decreasing magnesium content, it becomes easy to form MgAl2O4 thermodynamically. The thermodynamic stability
diagram of Al-Mg oxides is elicited by A. D. Mcleod, as shown in Fig. 4.22)In Mg/ABOw composite, the competitive
relationship exists between MgAl2O4 and MgO due to high
magnesium content. According to our previous studies on interfacial reaction in Mg/ABOwcomposite, MgAl2O4 can
be formed acting as reaction product using the competitive relationship between MgAl2O4and MgO.10,12)
3.2 Microstructure of interfacial reaction layer
Figure 5(a) shows the TEM images of the interface between AZ91D magnesium alloy matrix and ABOw in
as-casted composite. It shows clearly that interfacial reaction products formed a thin reaction layer on the surface of ABOw. Because the time of squeeze-casting is very short and
about one minute, the interfacial reaction layer is very thin and less than 10 nm. With increasing the time of heat Fig. 3 HREM images of interfacial reaction layer taken in composite heat-treated at 693 K for 96 hours. (a) Low magnification image of
interfacial reaction layer and (b) high magnification image of region A framed with white line in Fig. 3(a).
Fig. 2 Selected area diffraction pattern (SADP) of interface in composite heat-treated at 693 K for 96 hours. (a) SADP of ABOwand MgO
[image:3.595.86.511.72.267.2] [image:3.595.85.512.323.524.2]treatment up to 48 hours, whisker is wrapped completely with interfacial reaction layer in thickness of about 20 nm, as shown in Fig. 5(b). After the time of heat treatment arrives 96 hours, the thickness of reaction layer increases rarely comparing with that in composite heat treated for 48 hours.
Surfaces of both whiskers and interfacial reaction layer become wave-like due to interfacial reaction. Moreover, the region of whisker surface is nearer to the center of MgO particle, the degree of interfacial reaction is slighter. It indicates that MgO particle plays a role of barrier to keep out of further reaction between magnesium and ABOw.
Figure 6(a) shows HREM image of interface in as-casted composite. The thickness of MgO particle is about 10 nm and the surface of ABOwis not covered completely with MgO
reaction layer. It is very difficult to get electron diffraction pattern of ten nanometer scores by conventional selected area diffraction or micro-diffraction. Fast Fourier Transform (FFT) method is adopted to resolve this problem. The FFT is analyzed with DagitalMicrograph(TM) and taken from the region framed with white line, as shown in Fig. 6(a). The image of FFT indicates that MgO forms on the surface of whisker obeying a definite orientation relationship: ð1111ÞMgO==ð320ÞABOw and½011MgO==½001ABOw. This
ori-entation relationship is same to that shown in Fig. 2(a). It indicates that MgO particles form and grow on surfaces of ABOwobeying a definite orientation relationship in process
of both fabrication and heat treatment. Figure 6(b) shows that Fig. 4 Thermodynamic stability diagram of Al-Mg oxides in Al-Mg
alloy.22)
Fig. 5 TEM images of interfacial reaction layer in AZ91D/Al18B4O33wcomposite. (a) as-casted, (b) heat-treated for 48 hours and (c)
heat-treated at 693 K for 96 hours.
[image:4.595.68.268.70.221.2] [image:4.595.89.514.354.757.2]MgO particles grow up and encounter each other in composite heat-treated for 48 hours. The surfaces of MgO particles are consisted of close-packed plane (111)MgO, and
MgO particles form triangle-like.
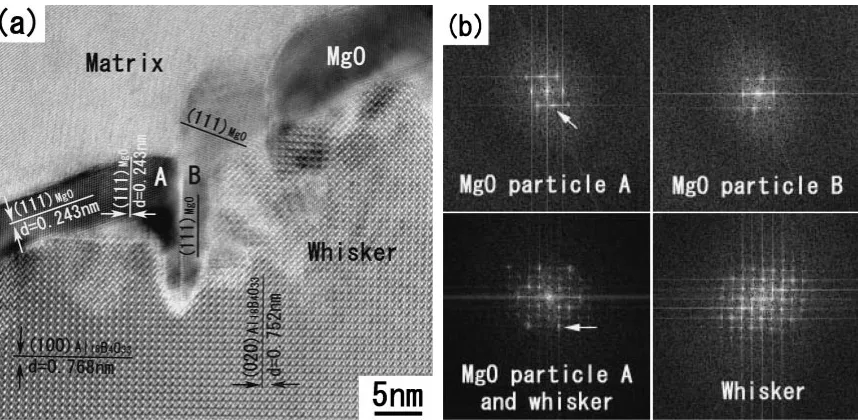
Figure 7(a) is a typical HREM image of interfacial reaction layer in AZ91D/ABOw composite heat-treated at
693 K for 96 hours. It shows clearly that the interfacial reaction invades into whisker along the boundary between two MgO particles and the depth is about 20 nm. When MgO particles grow up and encounter each other, obvious grain interface is formed between MgO particles that is marked with A and B. Although MgO particles can play a role of good barrier to keep out of contacting between magnesium and whisker, magnesium can keep on infiltrating through grain boundary of MgO particles and react to whisker. As a result, interfacial reaction invades into the inner of whisker. Figure 7(b) shows the FFT of HREM images of MgO particles A, B and whisker that are analyzed with
Dagital-Micrograph(TM). It indicates that the crystal orientations of MgO particle A and B are axisymmetric with regard to crystal plane (020) of whisker. Taking into account of the fact that MgO is face-center cubic and ABOwis end-center cubic,
according to knowledge of geometry, the orientation rela-tionship between MgO particle A/B and whisker are equivalent. It indicates that MgO and ABOwobey a definite
orientation relationship when MgO precipitate on the surfaces of whiskers.
3.3 The mechanism of interfacial reaction
Figure 8 shows the sketch map of interfacial reaction in AZ91D/ABOw composite. In this study, MgO particles
form and grow up on the surfaces of whiskers obeying definite orientation relationship:ð1111ÞMgO==ð320ÞABOw and
½011MgO==½001ABOw. Moreover, the surfaces of MgO
particles are consisted of close-packed plane (111)MgO. As
a result, MgO particles look like triangle.
Fig. 7 A typical HREM image of interface that interfacial reaction invaded into whisker in composite heat-treated at 693 K for 96 hours, and FFT images of its analyzed by DagitalMicrograph(TM).
[image:5.595.85.512.72.268.2] [image:5.595.84.513.310.520.2]Since the time of squeeze-casting is very short and about one minute, the surface of whisker is not covered completely with MgO reaction layer in thickness of about 10 nm, as shown in Fig. 8(a).
While the composite is heat-treated at 693 K, magnesium alloy matrix goes on reacting to whisker at the region where surface of whisker is not covered with MgO particles. As a result, the surfaces of whiskers become wave-like. When the time of heat treatment arrives 48 hours, the whisker is wrapped completely with interfacial reaction layer in thick-ness of 20 nm, as shown in Fig. 8(b).
As shown in Fig. 8(c), when MgO particles encounter each other, there form obvious grain interface between MgO particles. Although MgO particles play a good barrier to keep out of contacting between magnesium and whisker, the boundaries act as a shortcut of magnesium atoms’ migration and magnesium can keep on reacting to whisker. Since diffusion along the boundaries is orders of magnitude faster than that through MgO particles, these transformations are usually characterized by a relatively faster kinetics than that of the volume diffusion controlled processes, and substantial change in the bulk properties.24)Magnesium atoms migrate
along with boundaries between MgO particles and infiltrate into whisker to react to aluminum borate. The speed of interfacial reaction becomes very slowly due to the restriction of the speed of magnesium migration. And with increasing the time of heat treatment, the migration approach of magnesium atom is lengthened and the migration of magnesium atom becomes more and more difficult.
When whisker surfaces are covered with MgO reaction layer completely, interfacial reaction begins to invade into the inner of whisker and the thickness of reaction layer L1
does not increase, as shown in Fig. 8(b) and (c). It is because reaction product MgO replaces the space position of ABOw
exactly, and the volume change of interfacial reaction is listed in Table 2. In addition, interfacial reaction products aluminum and boron migrate to the surfaces of reaction layers. Aluminum dissolves into magnesium matrix, and boron reacts to magnesium to form MgB2. When the
composite is heat-treated at 693 K for 96 hours, the depth L2 that interfacial reaction invaded into whisker increases to
20 nm.
As shown in Fig. 8, MgO particles look like triangle and the surfaces of MgO particles are consisted of close-packed
plane (111)MgO. And MgO particles form and grow up on the
surfaces of whisker obeying a definite orientation relation-ship:ð1111ÞMgO==ð320ÞABOw and½011MgO==½001ABOw.
4. Conclusion
(1) Magnesium can react with aluminum borate to form MgO reaction layer between magnesium alloy matrix and aluminum borate whisker in processes of squeeze-casting and following heat treatment for AZ91D/ABOw
composite. Interfacial reaction layer in thickness of about 10 nm is found in as-casted composite. When the composite is heat-treated at 693 K for 96 hours, the thickness of interfacial reaction layer increases to about 20 nm and it is consisted of tiny MgO particles and a small quantity of MgB2particles.
(2) Although MgO particles play a good barrier to keep out of contacting between magnesium and whisker, the boundaries between MgO particles act as a shortcut of magnesium atoms migration. The interfacial reaction can invade into the inner of whisker about 20 nm depth after the composite is heat-treated at 673 K for 96 hours. (3) MgO particles form and grow up with small lattice mismatch on the surfaces of whiskers obeying a definite orientation relationship between MgO particles and ABOw: ð1111ÞMgO==ð320ÞABOw and ½011MgO==
½001ABOw.
REFERENCES
1) Y. Kojima: Mater. Trans.42(2001) 1154–1159.
2) M. Zheng, K. Wu and C. Yao: Mater. Lett.47(2001) 118–124. 3) M. Y. Zheng, K. Wu, S. Kamado and Y. Kojima: Mater. Sci. Eng., A
348(2003) 67–75.
4) M. Zheng, K. Wu, C. Yao, T. Sato, H. Tezuka, A. Kamio and D. X. Li: Mater. Lett.41(1999) 57–62.
5) M. Gu, Z. Wu, Y. Jin and M. Kocak: Mater. Sci. Eng., A272(1999) 257–263.
6) W. G. Wang, K. Matsugi, O. Yanagisawa and G. Sasaki: JSME/ASME Int. Conf. on Mater. and Proc. 2002 (The Japan Soc. of Mechan. Eng. and The American Soc. of Mechan. Eng.) Vol. 2, pp. 352–356. 7) K. Suganuma, T. Fujita, N. Suzuki and K. Niihara: J. Mater. Sci. Lett.9
(1990) 633–635.
8) L. Yao, G. Sasaki and H. Fukunaga: Mater. Sci. Eng., A225(1997) 59–68.
9) G. Sasaki, M. Yoshida, N. Fuyama and T. Fujii: J. Mater. Proc. Tech. 130(2002) 151–155.
10) G. Sasaki, M. Yoshida, J. Pan, N. Fuyama, T. Fujii and H. Fukunaga: Mater. Sci. Forum350–351(2000) 215–220.
11) M. Zheng, K. Wu, H. Liang, S. Kamado and Y. Kojima: Mater. Lett.57 (2002) 558–564.
12) W. G. Wang, Y. Hasegawa, Y. B. Choi, N. Fuyama, K. Matsugi, O. Yanagisawa and G. Sasaki: Key Engineering Materials326–328(2006) 1673–1676.
13) Y. Cai, G. J. Shen and H. Q. Su: Scripta Meterialia37(1997) 737–742. 14) X. G. Ning, J. H. Li, J. Pan and H. Q. Ye: Mater. Lett.24(1995) 113– Fig. 8 Sketch map of interfacial reaction in AZ91D/Al18B4O33w
[image:6.595.68.276.71.213.2]compo-site.
Table 2 Volume change of interfacial reaction.
Chemical equation Al18B4O33+ 33Mg!33MgO + 4B + 18Al 1 mol of Al18B4O33 33 mol of MgO
[image:6.595.305.547.84.125.2]119.
15) X. G. Ning, J. Pan, K. Y. Hu and H. Q. Ye: Mater. Lett.13(1992) 377– 381.
16) J. Pan, G. Sasaki, L. J. Yao, M. Yoshida and H. Fukunaga: Mater. Sci. Tech.15(1999) 1044–1048.
17) K. Suganuma, G. Sasaki, T. Fujita and N. Suzuki: J. Jpn. Light Met. Soc.41(1992) 297–303.
18) W. D. Fei, X. D. Jiang, C. Li and C. K. Yao: J. Mater. Sci. Lett.15 (1996) 1966–1968.
19) W. D. Fei, X. D. Jiang, C. Li and C. K. Yao: Mater. Sci. Tech.13(1997) 918–922.
20) C. G. Levi, G. J. Abbaschian and R. Mehrabian: Metall. Trans. A9A (1978) 697–711.
21) A. Munitz, M. Metzger and R. Mehrabian: Metall. Trans. A10A(1979) 1491–1497.
22) A. D. Mcleod and C. M. Gabryel: Mater. Trans. A23A(1992) 1279– 1283.
23) W. M. Zhong, G. L’Esperance and M. Suery: Metall. Mater. Trans. A 26A(1995) 2625–2635.