Lactic Acidosis and Atrial Tachycardia: Unusual
Presentations of Disseminated Burkitt-Like Lymphoma
*
Hazem El-Osta1#, Abdulrahman Abdulbaki2, Prakash Peddi1, Amol Takalkar3, James Cotelingam4, Diana Veillon4, Andres Vargas5, Nuri Akkus2, Gerhard C. Hildebrandt1
1
Department of Medicine, Division of Hematology oncology, Louisiana State University Health Sciences Center, Shreveport, USA;
2
Department of Medicine, Division of Cardiology, Louisiana State University Health Sciences Center, Shreveport, USA;
3
Department of Nuclear Medicine, Louisiana State University Health Sciences Center, Shreveport, USA; 4Department of Pathology, Louisiana State University Health Sciences Center, Shreveport, USA; 5Department of Medicine, Division of Internal Medicine, Lou-isiana State University Health Sciences Center, Shreveport, USA.
Email: #helost@lsuhsc.edu
Received July 2nd,2012; revised August 7th, 2012; accepted August 18th, 2012
ABSTRACT
Lactic acidosis is a rare complication of malignancies and is seen more frequently in high grade lymphoma and leuke- mia. Although, its pathogenesis is not well understood, it remains a surrogate of poor prognosis. Herein, we present a case of Burkitt-like lymphoma presenting with metabolic abnormalities including lactic acidosis and hypoglycemia along with atrial tachycardia. We will discuss the different mechanisms involved in these metabolic disturbances and we will provide insight on novel therapeutic strategies based on our understanding of the underlying pathophysiology.
Keywords: Atrial Tachycardia; Burkitt-Like Lymphoma; B-Cell Lymphoma; Intra-Cardiac Tumor; Hypoglycemia;
Lactic Acidosis
1. Introduction
Burkitt’s lymphoma is a highly aggressive form of B-cell non Hodgkin lymphoma characterized pathologically by highly mitotic small non cleaved cells with round nuclei and multiple nucleoli giving a “starry-sky” pattern on histology. On molecular level, it is characterized by translocation of the c-myc gene leading to its dysregula- tion. It comprises 3 distinct clinical forms: endemic (Af- rican), sporadic and immunodeficiency-associated pri- marily seen in acquired immunodeficiency syndrome (AIDS) patients. Burkitt-like lymphoma is a morphologic variant of Burkitt’s lymphoma and has pathologic fea- tures intermediate between Burkitt’s lymphoma and dif- fuse large B cell lymphoma.
Patients with Burkitt’s lymphoma present with rapidly growing masses and bulky lymphadenopathy. The en- demic forms presents typically with jaw mass. On the other hand, the sporadic form presents usually with ab- dominal mass whereas the immunodeficiency-associated form often involves the lymph nodes.
It is often accompanied by oncologic emergencies secondary to compression by the tumor bulk, metabolic disturbances, or treatment-related hematological toxici-
ties. Despite its aggressive nature, response to combina- tion chemotherapy is prompt, although associated with high risk for the development of tumor lysis syndrome.
One of the rare metabolic complications of Burkitt’s lymphoma is lactic acidosis. Its underlying pathogenesis is complex and believed to be due to imbalance of lactic acid production by the tumor itself and clearance rather than hypoxia and hypoperfusion [1]. It is a surrogate of poor prognosis as described in few case reports.
2. Case Report
A 33-year-old female with no past medical history pre- sented to an outside facility for 2 months history of se- vere weight loss, malaise and dysphagia.
She underwent an esophagogastroduodenoscopy which demonstrated a large necrotic ulcerative fungating mass in the cardia, body and lesser curvature of the stomach, of which biopsies were taken. The diagnosis of B-cell lymphoma with features intermediate between diffuse large B-cell lymphoma and Burkitt’s lymphoma was made on pathology. C-myc gene rearrangement was positive on fluorescence in situ hybridization (FISH). On immunohistochemistry CD10, CD20 and leukocyte com- mon antigen (LCA) were positive and CD3, CD5 and cyclin D1 were negative.
Upon examination, she appeared cachectic. Pulse was 142/min and regular, blood pressure 104/78 mmHg, res- piratory rate 20/min, temperature 96.0 F, oxygen satura- tion 97% on room air.
Physical exam was remarkable for oral thrush and massive left abdominal mass on palpation. No lympha- denopathy was noticed.
Her laboratory studies showed the following pertinent values (reference range): sodium 125 mmol/L (136 - 145 mmol/L), potassium 4.4 mmol/L (3.5 - 5.1 mmol/L), chloride 91 mmol/L (98 - 107 mmol/L), bicarbonate 17 mmol/L (21 - 32 mmol/L), anion gap 17 mmol/L (5 - 15 mmol/L), glucose 64 mg/dl (70 - 110 mg/dL), creatinine 0.6 mg/dL (0.6 - 1.3 mg/dL), uric acid 2 mg/dL (2.6 - 6 mg/dL) , bilirubin 1.6 mg/dL (0.2 - 1 mg/dL), aspartate aminotransferase (AST) 119 U/L (15 - 37 U/L), alanine aminotransferase (ALT) 32 U/L (12 - 78 U/L), lactate 11.1 mmol/L (0.7 - 2.1 mmol/L), lactate dehydrogenase (LDH) 1884 U/L (84 - 246 U/L), HIV serology positive confirmed by western blot with 349494 RNA copies per ml. Hepatitis B and C serology negative, white blood cell count (WBC) 15.7 K/microl (3.4 - 9.2 K/microl), Hemo- globin 11.4 g/dL (11.3 - 15.4 g/dL), platelet 136 K/microl (142 - 405 K/microl), CD4 count of 83/mm3, partial thromboplastin time (PTT) 33 sec (24.7 - 35.5 sec) and international normalized ratio (INR) 1.38. Electrocardio- gram was concordant with atrial tachycardia.
She was immediately started on intravenous fluid, bi- carbonate, allopurinol for tumor lysis syndrome prophy- laxis and dexamethasone 40 mg intravenous daily for 3 days. Emtricitabine/tenofovir/efavirenz was commenced for HIV and metoprolol for tachycardia, respectively.
Further work-up by echocardiogram showed a 3 × 5 cm large right atrial mass protruding to the tricuspid valve with maintained normal ejection fraction. Positron emission tomography/computed tomography scan (PET/ CT scan) indicated extensive intensely fluorodeoxyglu- cose (FDG) avid widespread malignant disease involving the right atrium, distal esophagus and stomach, several bowel loops, left adrenal gland, right kidney, bone mar- row and possibly the intraspinal canal. Bone marrow biopsy confirmed lymphoma involvement, and spinal fluid analysis revealed presence of lymphoma cells while MRI of the brain was unremarkable.
Patient received 12 mg of methotrexate intrathecally time one. Subsequently and after written informed con- sent was obtained from the patient, dose adjusted R-EPOCH multiagent chemotherapy (rituximab, eto- poside, vincristine, cyclophosphamide, and doxorubicin) was initiated. Three days later, her blood counts dropped significantly with platelet of 67 K/microl, WBC of 0.79 K/microl and Hemoglobin of 10.2 g/dL. Her creatinine, phosphorus, potassium and uric acid remained normal, and her lactic acidosis improved with a lactate of 2.6
mmol/L. Clinically, she acutely deteriorated over the next 24 hours developing fever and severe hypotension, and was transferred to the intensive care unit. She was started on intravenous vancomycin and piperacillin/ta- zobactam and aggressive volume substitution. Microbi- ological work-up revealed positive cultures for E. coli in blood and urine, consistent with E. coli sepsis. Her clini- cal state further deteriorated over the next few hours, leading to acute respiratory failure, altered mental status and shock. Despite the short duration of events, in con- sideration of the poor overall prognosis, the family de- cided on do not resuscitate/Do not intubate (DNR/DNI), and the patient succumbed to gram negative sepsis 7 days after admission.
3. Discussion
We presented a patient with HIV-related Burkitt-like lymphoma with atrial tachycardia due to lymphomatous cardiac involvement, severe systemic extent of disease and metabolically lactic acidosis and hypoglycemia in the absence of hypoxia or hypoperfusion. Although the patient presented with tachycardia and elevated white count, the negativity of blood cultures upon admission along with the improvement of the lactic acidosis after treatment with chemotherapy are arguments that favor lymphoma as a cause of the lactic acidosis rather than an underlying sepsis. Our case underscores few particular observations. Although rarely described, lactic acidosis without evidence for hypoxia or hypoperfusion can point towards an underlying hematological malignancy and prompt diagnosis and early treatment of underlying eti-ology potentially improves outcome. The underlying pathophysiology of lactic acidosis in these patients is not yet fully understood. Several mechanisms have been postulated including 1) overproduction of lactic acid by tumor cells through an upregulation of enzymes involved in aerobic glycolysis, through a highly proliferative tu- mor in mismatch with needed tumor blood supply lead- ing to tumor cell hypoxia and increased anaerobic glycol- lysis [1-5], and it was also suggested, that lactic acidosis is simply the result of bulk of disease rather than an in- crease of lactic acid at a cellular level [6]; 2) Impaired hepatic and renal clearance of lactic acid [1,5]; 3) Mi- crovascular embolization of tumor cells causing a state of tissue hypoperfusion and therefore leading to anaerobic glycolysis [5,7]; 4) Thiamine deficiency leading to a re-duction in the pyruvate dehydrogenase activity and con-sequently to accumulation of pyruvate which will be converted to lactate [8].
Table 1. Table summarizing the characteristics and outcomes of reported cases of Burkitt and Burkitt-like lymphoma associ-ated with lactic acidosis.
Case Age Sex Presenting sign HIV
status Glycemia
Lactate peak
Lactic acidosis resolution
Treatment
received Outcome Comment
Block J. B.
et al. [2] (1966)
27 F Ascites, pleural
effusion NR Normal 3.55 Yes
Nitrogen mustard, vincristine, prednisone, ibenzmethyzin Died in 3 months Increased lactate in pleural fluid Rouzet P.
et al. [19] (1991)
8 M Small bowel
obstruction NR Normal 139 Yes LMB-84
Alive after 2.5 years LA responded quickly to thiamine Bergin C.
et al. [17] (1993)
26 M Constitutional
symptoms positive Mildly low 12.2
Slight improvement (decreased to 7.9)
Combination chemotherapy (not specified) Died in 7 days Revesz T.
et al. [16] (1995)
8 M Seizure, diabetes
insipidus NR Normal 24
Improved (decreased to 6.2)
Etoposide, steroid, XRT Died in 4 months after relapse Kidney involvement Megarbane B.
et al. [18] (2000)
77 M Fever of unknown
origin, SIRS NR Normal 27 Yes
CVP, intrathecal chemo
Died from sepsis
Glasheen J.
et al. [14] (2005)
74 M Pleural effusion,
right sided edema NR Low 15.8 NR None
Died in 13 days
Lopez- Rodriguez M.
et al. [15] (2007)
33 M Back pain Positive Low 8.41 NR None Died in
7 days
Rastogi M.
et al. [21] (2008)
11 M
Fever, edema,
rash
Positive Intractable hypoglycemia 15.2
Decreased
to 9.6 None
Death in 33 days Received glucagon for hypoglycemia Kulkarni K.
et al. [20] (2010)
12 M Abdominal
pain, weakness Negative Normal 21 Yes MCP-842
Recovery (unspecified
follow-up)
Elevated triglyceridemia
Present report 33 F
Constitutional symptoms, dysphagia, atrial
tachycardia
Positive Low 11.1 Improved to 2.6 R-EPOCH Died in 7 days
CVP: cyclophosphamide, vincristine and prednisone; LMP-84: intensive combination chemotherapy protocol containing vincristine, cyclophosphamide, pred-nisone, doxorubicin, high dose methotrexate and cytarabine; MCP-842: ifosfamide-based, moderately intensive short-duration combination chemotherapy protocol; NR: not reported; R-EPOCH: chemotherapy protocol containing rituximab, etoposide, vincristine, cyclophosphamide, doxorubicin and prednisone;
XRT: radiation therapy.
motherapy seems to exert its effect by cytoreduction of tumor cells leading to decreased production of lactic acid and in cases of liver involvement to improved lactic acid clearance. Renal dialysis can be useful in some cases [1,5,9,10]. Resolution of lactic acidosis with thiamine repletion has been described previously in lymphoma [8]; however it did not have any effect on lactic acid level in Burkitt’s lymphoma.
Amid the uncertainty in the optimal management of this condition, a better recognition of the different mechanisms involved in lactic acidosis of hematological malignancies is needed. For instance, anecdotal reports have found elevated IGF1 and TNFalpha levels in a lymphoma patient with lactic acidosis [1]. Although no causality link could be established between an increase in insulin growth factor 1 (IGF-1) and development of lac-tic acidosis, this is an interesting observation since IGF-1 induces the expression of hexokinase which results in
high rates of glycolysis and pyruvate production [3,4]. On the other hand, tumor necrosis factor alpha (TNF alpha) leads to reduced activity of pyruvate dehydro-genase and subsequently increased conversion of pyru-vate into lactate [11]. Accordingly, it can be postulated that the IGF1 pathway can work in concert with TNF alpha to increase lactic acid production. Importantly, IGF1 signaling is implicated in many types of cancers [12]. Several IGF targeting agents are in early clinical trials and have shown promising results [13]. It will be important to test whether lactic acidosis in lymphoma is a reflection of an underlying overactivated IGF-1 pathway that can drive tumor cell proliferation, and to determine whether IGF-1, TNF and maybe other candidate path-ways are valuable therapeutic targets that could change the disease’s outcome.
reported cases including our case, 8 patients had a fatal outcome, with death occurring in a matter of days to weeks from the onset of lactic acidosis. In only one case, the patient was alive after 2.5 years of diagnosis and in another case the outcome was not well specified (Table 1) [2,14-21].
Using FDG-PET/CT, generally FDG uptake is strong in myocardium and brain. In our patient, the pattern of FDG metabolic distribution demonstrated less uptake in these tissues than usually observed, whereas significant FDG uptake was seen in the lymphoma spread through- out the body. This observation eludes to an indirect vis- ual insight into one potential mechanism of hypoglyce- mia in lymphoma, as it suggests an increase in glucose uptake by tumor cells as a possible contributor of sys- temic hypoglycemia.
Our case illustrates the complementarities of multi- modality imaging in assessing cardiac involvement by Burkitt-like lymphoma. The sensitivity and specificity of FDG PET/CT scan for detection of extranodal Burkitt- like lymphoma are 100% and 94% respectively, and few
(a)
(b)
(c)
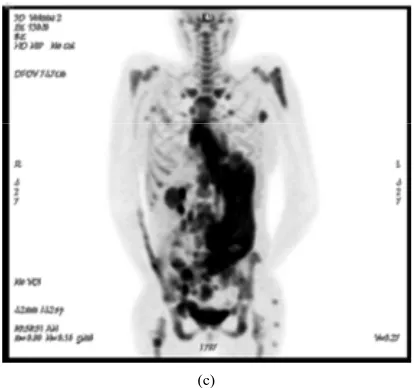
Figure 1. (a) echocardiogram demonstrating a right ventri- cular Inflow track showing the right atrial mass which extends to the tricuspid valve. (b) PET/CT scan showing an intensively FDG avid lesion in the right atrium cor- responding to the lesion seen on echocardiogram. (c) PET scan indicating diffusely intense FDG uptake consistent with extensive disease.
reports have shown its utility to detect intra-cardiac and myocardial involvement [22,23]. In our case, FDG-PET/ CT provided imaging confirmation of echocardiographic findings of a right atrial mass, and in addition its meta- bolic activity provided crucial clues on the lymphoma- tous origin versus non-metabolically active lesion such as thrombus (Figure 1).
Intra-cardiac tumors presenting as arrhythmia were re-ported in left atrial myxoma, and intracardiac follicular B cell lymphoma, the latter mostly reported as atrial flutter [24-28]. To our knowledge this is the first reported case of intracardiac metastatic Burkitt-like lymphoma presenting as atrial tachycardia, which emphasizes a meticulous work-up in patients with malignancies for cardiac in-volvement in the presence of diagnostic or clinical abnor-malities.
4. Conclusion
[image:4.595.320.527.84.278.2]therapeutic modalities.
REFERENCES
[1] E. M. Sillos, J. L. Shenep, G. A. Burghen, C. H. Pui, F. G. Behm and J. T. Sandlund, “Lactic Acidosis: A Metabolic Complication of Hematologic Malignancies: Case Report and Review of the Literature,” Cancer, Vol. 92, No. 9, 2001, pp. 2237-2246.
doi:10.1002/1097-0142(20011101)92:9<2237::AID-CNC R1569>3.0.CO;2-9
[2] J. Block, W. Bronson and W. Bell, “Metabolic Abnor- malities of Lactic Acid in Burkitt-type Lymphoma with Malignant Effusion,” Annals of Internal Medicine, Vol. 65, No. 1, 1966, pp. 101-108.
[3] S. Sebastian and U. W. Kenkare, “Insulin-Like Growth Factor 1 Induces Tumor Hexokinase RNA Expression in Cancer Cell,” Biochemical and Biophysical Research Communications, Vol. 235, No. 2, 1997, pp. 389-393. doi:10.1006/bbrc.1997.6797
[4] S. P. Mathupala, A. Rempel, P. L. Pedersen, “Aberrant Glycolytic Metabolism of Cancer Cells: A Remarkable Coordination of Genetic, Transcriptional, Post-Transla- tional, and Mutational Events that Lead to a Critical Role for Type II Hexokinase,” Journal of Bioenergetics and Biomembranes, Vol. 29, No. 4, 1997, pp. 339-343. doi:10.1023/A:1022494613613
[5] J. P. Ruiz, A. K. Singh and P. Hart, “Type B Lactic Acidosis Secondary to Malignancy: Case Report, Review of Published Cases, Insights into Pathogenesis, and Pro- spects for Therapy,” The Scientific World Journal, Vol. 11, 2011, pp. 1316-1324.doi:10.1100/tsw.2011.125 [6] S. Sariban-Sohraby, I. T. Magrath and R. S. Balaban,
“Comparison of Energy Metabolism in Human Normal and Neoplastic (Burkitt’s Lymphoma) Lymphoid Cells,”
Cancer Research, Vol. 43, No. 10, 1983, pp. 4662-4664. [7] R. G. Narins, G. G. Krishna, J. Yee, et al., “The
Meta-bolic Acidosis,” In: R. G. Narins, Ed., Maxwell & Klee-man’s Clinical Disorders of Fluid and Electrolyte Me-tabolism, 5th Edition, McGraw-Hill, Inc., New York, 1994, pp. 769-825
[8] J. Svahn, M. Schiaffino, U. Caruso, M. Calvillo, G. Min- niti and C. Dufour, “Type B Lactic Acidosis Secondary to Malignancy: Case Report, Review of Published Cases, Insights into Pathogenesis, and Prospects for Therapy,”
Journal of Pediatric Hematology/Oncology, Vol. 25, 2003, pp. 965-968.
doi:10.1097/00043426-200312000-00012
[9] S. Osorio, C. Bernis and R. de La Camara, “Lactic Aci- dosis in Non-Hodgkin’s Lymphoma and Response to Chemotherapy,” Haematologica, Vol. 87, No. 2, 2002, p. 5 [10] A. S. Friedenberg, D. E. Brandoff and F. J. Schiffman,
“Type B Lactic Acidosis as a Severe Metabolic Compli-cation in Lymphoma and Leukemia: A Case Series from a Single Institution and Literature Review,” Medicine ( Bal-timore), Vol. 86, No. 4, 2007, pp. 225-232.
doi:10.1097/MD.0b013e318125759a
[11] J. Durig, W. Fiedler, M. de Wit, M. Steffen and D. Hoss-feld, “Lactic Acidosis and Hypoglycemia in a Patient
with High-Grade Non-Hodgkin’s Lymphoma and Ele-vated Circulating TNF-a,” Annals of Hematology, Vol. 72, No. 2, 1996, pp. 97-99. doi:10.1007/BF00641317
[12] H. Werner and D. LeRoith, “The Role of the Insulin-Like Growth Factor System in Human Cancer,” Advances in Cancer Research, Vol. 68, 1996, pp. 183-223.
doi:10.1016/S0065-230X(08)60354-1
[13] T. A. Yap, D. Olmos, L. R. Molife and J. S. de Bono, “Targeting the Insulin-Like Growth Factor Signaling Pathway: Figitumumab and Other Novel Anticancer Strategies,” Expert Opinion Investigatinal Drugs, Vol. 20, No. 9, 2011, pp. 1293-1304.
doi:10.1517/13543784.2011.602630
[14] J. J. Glasheen and M. D. Sorensen, “Burkitt’s Lymphoma Presenting with Lactic Acidosis and Hypoglycemia—A Case Presentation,” Leukemia & Lymphoma, Vol. 46, No. 2, 2005, pp. 281-283. doi:10.1080/10428190400016723 [15] M. López Rodríguez, E. Vázquez Muñoz, J. Gómez
Cerezo, B. Pagán Muñoz, E. Ruiz Bravo-Burguillos, F. J. Barbado Hernández, “Lactic Acidosis, Severe Hypogly- cemia and Hepatosplenomegaly,” Revista Clínica Espa- ñola, Vol. 207, No. 10, 2007, pp. 521-522.
doi:10.1157/13111552
[16] T. Révész, K. Obeid and C. Mpofu, “Severe Lactic Aci- dosis and Renal Involvement in a Patient with Relapsed Burkitt’s Lymphoma,” Journal of Pediatric Hematol- ogy/Oncology, Vol. 12, No. 3, 1995, pp. 283-288. doi:10.3109/08880019509029570
[17] C. Bergin, R. Pilkington, C. McCreary, F. Mulcahy and V. Crowley, “Lactic Acidosis, Non-Hodgkins Lymphoma and the Acquired Immunodeficiency Syndrome,” Geni- tourinary Medicine, Vol. 70, No. 2, 1994, pp. 148-149. doi:10.1136/sti.70.2.148
[18] B. Mégarbane, F. Bruneel, V. Andrieu, M. Wolff and B. Régnier, “The Last Voyage of a Globe-Trotter,” La Revue de Médecine Interne, Vol. 21, Suppl. 3, 2000, pp. 340s- 342s. doi:10.1016/S0248-8663(00)89263-2
[19] P. Rouzet, H. Rubie, A. Robert, A. Dutour, J. P. Olives, C. Scipioni, J. Guitard and C. Régnier, “Severe Hyperlac- tacidemia in 2 Children Treated for Malignant Tumors. Role of Vitamin B1,” Archives Francaises de Pédiatrie, Vol. 48, No. 6, 1991, pp. 423-426.
[20] K. Kulkarni, S. Kaur, A. Sibal, N. Jerath and L. S. Arya, “Severe Lactic Acidosis, Hypertriglyceridemia, and Ex- tensive Axial Skeleton Involvement in a Case of Dis- seminated Burkitt’s Lymphoma,” International Journal of Hematology, Vol. 91, No. 3, 2010, pp. 546-548. doi:10.1007/s12185-010-0534-8
[21] M. V. Rastogi, N. Desai and J. B. Quintos, “Non-Islet- Cell Tumor Hypoglycemia and Lactic Acidosis in a Child with Congenital HIV and Burkitt’s Lymphoma,” Journal of Pediatric Endocrinology & Metabolism, Vol. 21, No. 8, 2008, pp. 805-810. doi:10.1515/JPEM.2008.21.8.805 [22] D. Karantanis, J. M. Durski, V. J. Lowe, M. A. Nathan, B.
Wood, “Images in Cardiovascular Medicine. Comple- mentary Role of Multimodality Imaging in the Evaluation of Intracardiac Lymphoma in an HIV-Infected Man,”
Circulation, Vol. 115, No. 12, 2007, pp. e339-e341. doi:10.1161/CIRCULATIONAHA.106.664177
[24] D. Mioulet, L. Braem, P. Heno, P. Paule, J. M. Peloni, D. Bonnet and L. Fourcade, “Cardiac Extension of a Non- Hodgkin Lymphoma Revealed by an Atrial Flutter,” An- nales de Cardiologie et d’Angéiologie, Vol. 58, No. 2, 2009, pp. 117-121. doi:10.1016/j.ancard.2008.05.007 [25] E. Abinader and S. Rauchfleisch, “Left Atrial Myoma
Presenting with Atrial Tachycardia,” Harefuah, Vol. 105, No. 9, 1983, pp. 263-264.
[26] M. Linhart, L. Lickfett, C. Hammerstingl, K. Tiemann, G.
Nickenig and T. Lewalter, “Paroxysmal Atrial Flutter Caused by Cardiac Lymphoma,” Pacing and Clinical Electrophysiology, Vol. 29, No. 6, 2006, pp. 682-684. doi:10.1111/j.1540-8159.2006.00419.x
[27] M. A. Thompson, A. Harker-Murray, A. J. Lockets and P. Chareonthaitawee, “Unusual Lymphoma Manifestations: Case 2. Myocardial Lymphoma Presenting as Atrial Flut- ter,” Journal of Clinical Oncology, Vol. 22, No. 3, 2004, pp. 558-560. doi:10.1200/JCO.2004.12.100
[28] D. Hayes Jr., D. K. M. Liles and V. L. Sorrell, “An Un-usual Cause of New-Onset Atrial Flutter: Primary Cardiac Lymphoma,” Southern Medical Journal, Vol. 96, No. 8, 2003, pp. 799-802.