Original Article
MiR-153-3p suppresses atherosclerosis cells’
migration via target regulation of ROCK1
Ningning Wang1*, Xiaolu Zhang1*, Xiao Sun2, Jianfeng Du1, Yueyan Zhang3, Lin Lou4, Yong Wang5, Kaiming Chen1, Man Zhang1
1The 2nd Department of Cardiology, Central Hospital Affiliated to Shenyang Medical College, Shenyang, Liaon -ing, P. R. China; 2The Dean’s Office, Shenyang Women’s and Children’s Hospital, Shenyang, Liaoning, P. R. China; 3Department of Pathology, Liaoning Cancer Hospital & Institute, Shenyang, Liaoning, P. R. China; 4Department of
Science and Education, Central Hospital Affiliated to Shenyang Medical College, Shenyang, Liaoning, P. R. China;
5The 4th Department of Orthopaedic Surgery, Central Hospital Affiliated to Shenyang Medical College, Shenyang,
Liaoning, P. R. China. *Equal contributors and co-first authors.
Received January 21, 2017; Accepted February 23, 2017; Epub May 1, 2017; Published May 15, 2017
Abstract: The anomalous migration of vessel smooth muscle cells (VSMCs) and so-caused atherosclerosis may re-sult in a series of diseases including coronary heart disease (CHD). MicroRNAs (miRNAs) and their target genes play key roles in this complicated process. The function of microRNA-153-3p (miR-153-3p) in VSMCs migration remains unknown. In the present study, we found out that miR-153-3p was decreased in the human plasma from patients with atherosclerosis and in low and high- atherosclerosis cells that were simulated by oxidized low density
lipopro-tein (OX-LDL) stimulation. Also we verified that Rho associated coiled-coil containing prolipopro-tein kinase 1 (ROCK1), a cytoskeleton-related protein, was increased in the aforementioned samples and cells. Furthermore, we confirmed
that up-regulation of miR-153-3p could promote atherosclerosis cells migration and inhibit ROCK1 expression.
Then, through a luciferase reporter assay, we verified that miR-153-3p could target bind to ROCK1 3’ untranslated region (3’UTR). Finally, an antisense experiment was executed to ultimately elucidate that miR-153-3p could pro -mote migration via target regulating ROCK1 in atherosclerosis cells. In summary, the outcomes of the present study may reveal a new molecular in target treatment for atherosclerosis and its associated coronary artery disease (CAD).
Keywords: MiR-153-3p, ROCK1, migration, atherosclerosis
Introduction
Atherosclerosis and its associated coronary heart disease (CHD) are common but high-risk diseases in elderly patients. It is reported that CHD originating from atherosclerosis is the pri-mary cause of mortality contributing to about 27% of total deaths in males and 32% in females worldwide [1]. The gradual
accumula-tion of fibrous elements and lipids in large
arteries is the main characteristic of athero-sclerosis [2-4]. The formation of atherosclero-sis is a multifactorial, multi-mechanism and complex biological process. The anomalous migration of Vessel smooth muscle cells (VSMCs) from the media to the intima leading to thickening of arterial intimal is the main rea-son for atherosclerosis [5, 6].
MiRNAs, 22-25 nucleotides in length, are a set
with 5% CO2. Here, according to the previous research [25, 26], we used 10 µg/mL ox-LDL and 50 µg/mL oxidized low density lipoprotein
(ox-LDL; Union-Biology Co., Ltd, Beijing, China)
to stimulate HA-VSMCs for 48 h to simulate ath-erosclerosis cells and named as low athero-sclerosis cells and high atheroathero-sclerosis cells, respectively.
Reverse transcription and quantitative
real-time PCR
Total RNA was extracted by TRIzol (Invitrogen,
USA) according to the manufacturer’s instruc -tions. Synthesis of cDNA was achieved by using Reverse Transcription System Kit (Invitrogen,
Carlsbad, CA, USA). Real-time PCR was per
-formed using the standard SYBR Green PCR kit
protocol in the StepOne Plus system (Applied
Biosystems, Foster City, CA, USA). MiR-153-3p
was detected by using of Stem-Loop RT-PCR
assay as previously reported [27, 28]. The U6
small nuclear RNA and GAPDH mRNA were used as internal references. Primer sequences were synthesized as follows: ROCK1 primer:
5’-GGTACCATGTCGA CTGGGGAC AGTTT-3’ (for
-ward), 5’-CCCGGGTTAACTAGTTTTTCCAGATGTA-TT-3’ (reverse); cofilin primer: 5’-GACTGCCGCT-ATGCCCTCTATG-3’ (forward), 5’-CTTCTTCTTGAT-GGCG TCCTTG-3’ (reverse); miR-153-3p primer: 5’-ACACTCCAGCTGGGTTGCATAGTCACAAAA GT-3’ (forward), 5’-CTCAACTGGTGTCGTGGAGT-CGGCAATTCAGTTGAGGATCACTTT-3’ (reverse); GAPDH primer: 5’-CTCCTCCACCTTTGACGCTG-3’ (forward), 5’-TCCTCTTGT GCTCTTGCTGG-5’-CTCCTCCACCTTTGACGCTG-3’
(reverse). All the reactions were performed three times, independently.
Plasmids construction and oligonucleotide
transfection
Wild and mutant ROCK1 over-expression plas-mids (ROCK1-wt and pcDNA3.1-ROCK1-mut) were synthesized using the similar
method as previously reported [29]. Briefly, ROCK1-wt (i.e. ROCK1 3’UTR fragment contain -ing miR-153-3p bind-ing site) and its mutant ROCK1-mut created by using Quik Change
Site-Directed Mutagenesis kit (Agilent, USA) were amplified and cloned into the KpnI and XhoI restriction sites (Promega, Madison, WI, USA)
of pcDNA3.1 vector to generate pcDNA3.1-ROCK1-wt and pcDNA3.1-ROCK1-mut. When
cells reached 60-80% confluence, 50 nmol of
the miR-153-3p mimics, mimic negative control related protein and facilitates actomyosin
cyto-skeleton contractility [17-20]. ROCK1 plays a
key role in stress fibers and focal adhesion
complexes formation and is involved in multiple cellular processes such as cell detachment, apoptosis, cell development and migration, especially in VSMCs migration [18, 21-24]. In the present study, we measured miR-153-3p and ROCK1 expression level in human plasma with atherosclerosis and non-atherosclerosis, and in HA-VSMCs and constructed high and low atherosclerosis cells that were stimulated by different dose of oxidized low density
lipopro-tein (OX-LDL). Also, we confirmed that elevation
of miR-153-3p could promote atherosclerosis cells migration and could inhibit ROCK1
expres-sion. In addition, we testified that ROCK1 was a
target of miR-153-3p and that miR-153-3p pro-moted migration via target regulation of ROCK1
in atherosclerosis cells. The findings in the
present study may provide a new perspective in treating of atherosclerosis.
Materials and methods
Human plasma samples
Human plasma samples from 40 cases of ath-erosclerotic patients were collected from the second department of cardiology paired with human plasma samples from 40 cases of non-atherosclerotic patients were collected from the fourth department of orthopedic surgery between January 2016 and March 2016 at
Central Hospital affiliated to Shenyang
Medi-cal College. Formal informed consents were obtained from the patients whose plasma sam-ples were used in the present research. The Institute Research Medical Ethics Committee
of Central Hospital affiliated to Shenyang
Medical College granted approval of this study.
Cell culture and oxidized low density lipopro
-tein stimulation
Human aortic Vessel smooth muscle cells (HA-VSMCs) were purchased from American Type Culture Collection (ATCC, Manassas, VA,
USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Langley, OK, USA) supplemented with endothelial cell growth factors, 5% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) and 1% penicillin/strepto
used to scratch the artificial wound across the
diameter of the wells. Cells were then incubat-ed with ox-LDL and then transfectincubat-ed with ta- rget plasmids. Wound closure was observed at 0, 24 and 48 h, and imaging was performed under a microscope. Each experiment was repeated three times.
Statistical analysis
All experiments were repeated in triplicate and all data from three independent experiments were expressed as mean ± SD. GraphPad Prism
V5.0 (GraphPad Software, Inc., USA) software
and SPSS 19.0 statistical software were used for statistical analysis. Correlation between miR-153-3p and ROCK1 was analyzed by using
of two-tailed Pearson’s correlation analysis.
Differences in two groups were analyzed with
the Student’s t-test or one-way ANOVA. Differences were considered significant if P < 0.05 and highly significant if P < 0.01.
Results
Depressed miR-153-3p but increased ROCK1 were expressed in human plasma from pa
-tients with atherosclerosis and in simulated
atherosclerosis cells
MiR-153-3p expression was detected by using of real-time PCR while ROCK1 were measured by using of real-time PCR and western blot human plasma samples from 40 cases of ath-erosclerotic patients paired with human plas-ma samples from 40 cases of non-atheroscle-rotic patients. As shown in Figure 1A-C, the expression of miR-153-3p was remarkably down-regulated in human plasma from athero-sclerotic patients than that from
non-athero-sclerotic patients (P < 0.01). In the contrary,
up-regulated ROCK1 was expressed in human plasma from atherosclerotic patients than that
from non-atherosclerotic patients (P < 0.01). In
addition, we used ectogenic ox-LDL to simulate atherosclerosis cell models as ascribed before [25, 26]. The HA-VSMCs treated with 10 µg/mL ox-LDL and 50 µg/mL ox-LDL for 48 h were named as low-atherosclerosis cells and high-atherosclerosis cells, respectively. Then, we detected miR-153-3p and ROCK1 expression in HA-VSMCs, constructed low and high-ath- erosclerosis cells. As shown in Figure 1D-F, decreased miR-153-3p but increased ROCK1 were presented in cellular level detected by (mimic NC), miR-153-3p inhibitor and inhibitor
negative control (inhibitor NC) were transfected respectively into the low and high atherosclero-sis cells (simulated by ox-LDL as described above) with Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufac
-turer’s instructions. All miRNA oligonucleotides
were purchased from Genepharma (Shanghai, China).
Western blot analysis
Total cellular and tissue protein extracts were obtained by using RIPA lysis buffer (Santa Cruz,
USA). Samples were fractionated using SDS-PAGE and transferred onto polyvinylidenedifluo
-ride membrane (Millipore, Billerica, MA, USA).
Membranes were blocked for 1 h and then in-
cubated with ROCK1, cofilin, p-cofilinand Gapdh specific antibodies as follows: rabbit ROCK1 polyclonal antibody (1:1000; Abcam, USA); rab
-bit cofilin monoclonal antibody (1:1000; Cell signaling technology, USA); rabbit phospho-cofilin monoclonal antibody (1:1000; Cell sig
-naling technology, USA); rabbit anti-Gapdh anti -body (0.5 µg/ml; Abcam, UK).
Bioinformatics prediction and dual luciferase
reporter assay
The potential miR-153-3p-binding sites in
ROCK1 3’UTR were predicted with online pre -diction software TargetScan (http://www.tar-getscan.org). Construction of ROCK1 luciferase reporter vector was according to the methods
as previously described [30]. Briefly, the full length ROCK1 3’-UTR was PCR amplified and
cloned at the SacI and XhoI sites into pmirGLO
vector (Promega, Madison, WI, USA). The mu-tant construct of ROCK1 3’UTR was generated
by a Quick Change mutagenesis kit (Stratagene, Germany). HA-VSMCs were pretreated with ox-LDL as ascribed above and seeded at 24-well plate and then co-transfected with reporter vectors and miR-153-3p mimics or negative control using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufac
-turer’s instructions. 48 h later, luciferase activ -ity was measured by a dual luciferase reporter
assay system according to the manufacturer’s instructions (Promega, Madison, WI, USA).
Wound-healing assay
Cells were seeded in 6-well plates and allowed
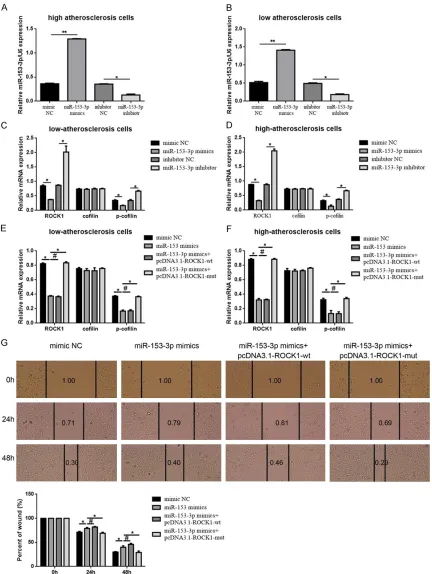
protein and was participated in multiple cells migration including VSMCs [23, 24]. Hence, we also measured ROCK1 expression in miR-153-3p intervened low and high-atherosclerosis cells. Just as shown in Figure 2E and 2F, the expression of ROCK1 was down-regulated
when miR-153-3p was increased (P < 0.01).
ROCK1 is a target of miR-153-3p
It is generally accepted that miRNAs can
regu-late its target genes by binding to their 3’ untranslated region (3’UTR). The outcomes
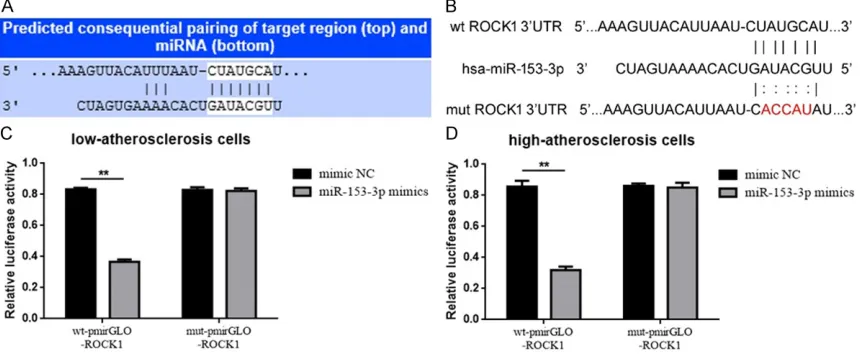
above showed us that miR-153-3p could pro-mote VSMCs migration and inhibit ROCK1 expression, now we wondered miR-153-3p could target ROCK1. First of all, the online pre-dictive software provided us delightful results. As shown in Figure 3A, there is a theoretical binding site for miR-153-3p in the ROCK1
3’UTR predicted by Targetscan (http://www.tar -getscan.org). Accordingly, we constructed lucif-erase reporter plasmids pmirGLO-ROCK1-wt and pmirGLO-ROCK1-mut that respectively
containing wild type and mutant ROCK1 3’UTR
(Figure 3B). Then the dual luciferase assays were executed to verify the target binding effect
between miR-153-3p and ROCK1 3’UTR. As the
results shown in Figure 3C, compared to mimic NC, co-transfection of wt-pmirGLO-ROCK1 and
miR-153-3p mimics led to a statistically signifi -means of retime PCR and western blot.
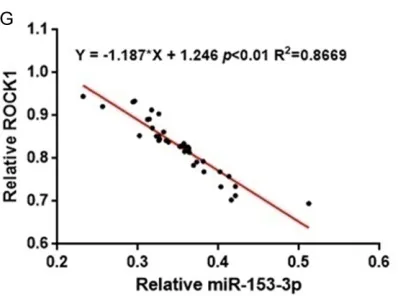
Furthermore, we analyzed the relationship between miR-153-3p and ROCK1 expression in human plasma samples from 40 cases of ath-erosclerotic patients by using of two tailed
Pearson’s correlation analysis. As shown in
Figure 1, there was an obvious negative
corre-lation between miR-153-3p and ROCK1 (P <
0.01).
Up-regulation of miR-153-3p inhibited athero
-sclerosis cells migration and ROCK1 expres -sion
It is well known that VSMCs migration from the media to the intima resulting in intimal thicken-ing plays a key role in the development of arte-riosclerosis [5, 31]. So we wondered whether miR-153-3p was involved in VSMCs migration. We up-regulated miR-153-3p expression in low and high-atherosclerosis cells by transfection
of miR-153-3p mimics (verified by real-time
PCR, compared to mimic NC, Figure 2A and 2B), then wound healing assay were executed to detect the migration ability changes in miR-153-3p intervened low and high-atherosclero-sis cells. As presented in Figure 2C and 2D, the migration ability was weakened in miR-153-3p mimics transfection group than that in mimic
NC transfection group (P < 0.01). As reported
[image:5.612.94.293.70.218.2]before, ROCK1 was a cytoskeleton associated
Figure 1. Depressed miR-153-3p but increased ROCK1 were expressed in clinical human plasma and in simulated atherosclerosis cells. A. MiR-153-3p expression in 40 cases of clinical human plasma with atherosclerosis was
lower than that in plasma with atherosclerosis as measured by using of real-time PCR. **P < 0.01 vs non-atherosclerosis. B, C. Elevated ROCK1 was expressed in the samples as of A measured by using of real-time PCR and western blot. **P < 0.01 vs non-atherosclerosis. D. Decreased miR-153-3p was expressed in low and high atherosclerosis cells than that in VSMCs evaluated by using of real-time PCR. *P < 0.05 and **P < 0.01 vs
HA-VSMCs. E, F. Elevated ROCK1 was expressed in low and high atherosclerosis cells than that in HA-VSMCs detected
by using real-time PCR and western blot. *P < 0.05 and **P < 0.01 vs HA-VSMCs. G. Two tailed Pearson’s correla -tion analysis for the expression of miR-153-3p and ROCK1 mRNA in clinical human plasma with atherosclerosis. r
reconfirmed that up and down-regulation of
miR-153-3p could decrease and increase
ROCK1 and the phosphorylation level of
cofilin-a symbolic downstrecofilin-am of ROCK1 pcofilin-athwcofilin-ay (Figure 4A-D). Secondly, an antisense experi-ment was executed. We constructed ROCK1 over-expression plasmid which containing wild type and mutant miR-153-3p binding site-wt and pcDNA3.1-ROCK1-mut. And then, both miR-153-3p mimics and pcDNA3.1-ROCK1-wt/pcDNA3.1-ROCK1-mut were transfected into atherosclerosis cells. As shown in Figure 4E and 4F, ROCK1 and p-cofilin
level were inhibited by miR-153-3p mimics, but
cant decrease in fluorescence. But the phe -nomenon vanished when co-transfection of mut-pmirGLO-ROCK1 and miR-153-3p mimics.
The findings above indicated that ROCK1 was a
target of miR-153-3p.
MiR-153-3p inhibited migration via target regulation of ROCK1 in atherosclerosis cells
In the previous parts, we verified that
[image:7.612.97.480.73.198.2]miR-153-3p could target ROCK1 and promoted athero-sclerosis cells migration. We then tried to eluci-date whether the facilitative effect was achieved via ROCK1 pathway. First of all, we
Figure 2. Up-regulation of miR-153-3p inhibited atherosclerosis cells migration and inhibited ROCK1 expression. A, B. MiR-153-3p mRNA was remarkably elevated by transfection of miR-153-3p mimics in low and high atherosclero
-sis detected by using of real-time PCR. **P < 0.01 vs mimic NC. C, D. Up-regulation of miR-153-3p by transfection of miR-153-3p mimics significantly crippled migration ability in low and high atherosclerosis cells evaluated by using of wound healing assay. **P < 0.01 vs mimic NC. E-H. ROCK1 protein and mRNA were significantly decreased by
transfection of miR-153-3p mimics in low and high atherosclerosis measured by using of real-time PCR and western
blot. **P < 0.01 vs mimic NC.
Figure 3. ROCK1 is a target of miR-153-3p. A. ROCK1 was a target gene of miR-153-3p predicted by Targetscan.
B. Diagram of the luciferase reporter plasmids and over-expression vector plasmids with the wild-type or mutant ROCK1 3’UTR. C, D. The relative luciferase activity was determined after co-transfection of miR-153-3p mimics
and pmirGLO-ROCK1-wt/pmirGLO-ROCK1-mut. Three independent experiments were performed in duplicate. All the
[image:7.612.91.521.301.480.2]Figure 4. MiR-153-3p inhibited migration via target regulation of ROCK1 in atherosclerosis cells. A-D. Up and
down-regulation of miR-153-3p could negatively affect ROCK1 and its downstream expression measured by using of
real-time PCR. **P < 0.01 vs mimic NC, *P < 0.05 vs mimic NC, *P < 0.05 vs inhibitor NC. E, F. The suppressive effect miR-153-3p played on ROCK1 and its downstream could be reversed by mutant ROCK1 3’UTR (pcDNA3.1-ROCK1-mut) confirmed by using of real-time PCR. *P < 0.05 vs miR-153-3p mimics, #P > 0.05 vs miR-153-3p mimics. G.
The suppressive effect miR-153-3p played on migration in high atherosclerosis was reversed by mutant ROCK1
nuclear translocation of ERK in PDGF-BB-stimulated platelet-derived growth factor-BB (PDGF-BB) treated VSMCs models [24]. Cui Y and workmates also reported that PDGF-BB
induced matrix metalloproteinase-2 expression (MMP-2) expression and facilitated VSMCs migration via ROCK/ERK/p38 MAPK pathways in rats [47]. In the present study, we observed the expression and function both in tissue and
cellular level. We confirmed that ROCK1 was
elevated in plasma form patients with athero-sclerosis and in low and high atheroathero-sclerosis cells and that up-regulation of miR-153-3p inhibited VSMCs migration and ROCK1
expres-sion respectively. Meanwhile, we verified that
up-regulation of mutant ROCK1 by pcNDA3.1-ROCK1-mut could reverse the inhibitory effect of miR-153-3p mimics on migration in
athero-sclerosis cells. The findings indicated that
ROCK1 functioned as the inverse role of miR-153-3p and as a facilitative factor in athero-genesis. Furthermore, we predicted ROCK1 had a theoretical binding site for miR-153-3p, and through the luciferase reporter assay, we
clarified that ROCK1 was a target of miR-153-3p. At last, we verified that, miR-153-3p could
inhibit atherosclerosis migration via targeting ROCK1.
Atherogenesis is very intricately, multifactorial and multi-mechanism-involved biological pro-cess. MiR-153-3p and its targeting ROCK1 is only a small branch among the anfractuous
network. Our findings provided a new insight
into molecular regulation of atherosclerosis.
Acknowledgements
This work was supported by the National Na- tural Science Foundation of China (No. 815- 02333), Technological innovation fund of Shenyang Technology Division (No. F12-277-1-22 and F13-F12-277-1-220-9-14) and Natural science foundation of Liaoning Province of China (No. 20102220).
Disclosure of conflict of interest
None.
Address correspondence to: Man Zhang, The 2nd
Department of Cardiology, Central Hospital Affiliat-ed to Shenyang MAffiliat-edical College, 5# South Seven
West Road, Tiexi District, Shenyang 110024, Liaoning, P. R. China. Tel: +86-24-85715321; Fax: the inhibitory effect was reversed by pcDNA-
3.1-ROCK1-mut but not pcDNA3.1-ROCK1-wt.
Theses outcomes further confirmed that
RO-CK1 was the target of miR-153-3p. Moreover, the wound healing assay was re-executed in high atherosclerosis cells to evaluate the migra-tion ability changes. As presented in Figure 4G, compared to mimic NC, transfection of
miR-153-3p mimics resulted in significantly
str-engthens of migration ability in high
atheroscle-rosis cells. But the strengthen effect couldn’t
be reversed by pcDNA3.1-ROCK1-wt (co-trans-fection of miR-153-3p and
pcDNA3.1-ROCK1-wt). Convincingly, when the ROCK1 3’UTR was
mutated (co-transfection of miR-153-3p and pcDNA3.1-ROCK1-mut), the strengthen effect was reversed. Taking all, the outcomes de-
scribed above confirmed that miR-153-3p
could inhibit migration via target down-regula-tion of ROCK1 in atherosclerosis cells.
Discussion
As a class of non-coding RNAs, miRNAs play multifunctional roles in various ageing and age-ing-related human diseases like cancer, auto-immune diseases, neurodegenerative diseas-es, also, in cardiovascular diseases [32-36]. Mir-153 is located at chromosome 2q36.3, and is widely involved in numerous biological behav-iors like metastasis, apoptosis, proliferation, epithelial-to-mesenchymal transition (EMT), autophagy and migration/invasion [13, 14, 37-40]. In the present study, we focused the effect miR-153-3p on VSMCs migration. We
confirmed that miR-153-3p was down-regulat -ed in plasma form patients with atherosclero-sis and in low and high atheroscleroatherosclero-sis cells. Also, through the wound healing assay we
veri-fied that elevated miR-153-3p suppressed
migration in atherosclerosis cells. It is well known that faster proliferation and the follow-ing anomalous migration of VSMCs, from the tunicaare the key steps in thickening of arterial
wall [41]. The findings in the present study indi -cated that miR-153-3p might function as a pro-tective factor in atherogenesis.
ROCK1 is a serine/threonine kinases and is the downstream target of the small GTPasesRhoA,
RhoB, and RhoC [42]. Related reports about
apoptosis induced by ox-LDL in endothelial cells. Int J Mol Sci 2013; 14: 22708-22720. [12] Li W, Zhai L, Zhao C and Lv S. MiR-153 inhibits
epithelial-mesenchymal transition by targeting
metadherin in human breast cancer. Breast
Cancer Res Treat 2015; 150: 501-509. [13] Shan N, Shen L, Wang J, He D and Duan C.
MiR-153 inhibits migration and invasion of human non-small-cell lung cancer by target-
ing ADAM19. Biochem Biophys Res Commun
2015; 456: 385-391.
[14] Wang Z and Liu C. MiR-153 regulates metasta-ses of gastric cancer through snail. Tumour
Biol 2015; [Epub ahead of print].
[15] Zuo J, Wang D, Shen H, Liu F, Han J and Zhang X. MicroRNA-153 inhibits tumor progression in esophageal squamous cell carcinoma by
tar-geting SNAI1. Tumour Biol 2016; [Epub ahead of print].
[16] Song L, Duan P, Guo P, Li D, Li S, Xu Y, Zhou Q. Downregulation of miR-223 and miR-153 me-diates mechanical stretch-stimulated prolifera-tion of venous smooth muscle cells via activa-tion of the insulin-like growth factor-1 receptor.
Archives Biochem Biophys 2012; 528:
204-211.
[17] Lin SC, Gou GH, Hsia CW, Ho CW, Huang KL, Wu YF, Lee SY, Chen YH. Simulated micrograv-ity disrupts cytoskeleton organization and in-creases apoptosis of rat neural crest stem cells via upregulating CXCR4 expression and RhoA-ROCK1-p38 MAPK-p53 signaling. Stem Cells Dev 2016; 25: 1172-1193.
[18] Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, Kapur R, Wei L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis 2013; 4: e483.
[19] Wei L, Surma M, Gough G, Shi S, Lambert-Cheatham N, Chang J, Shi J. Dissecting the mechanisms of doxorubicin and oxidative stress-induced cytotoxicity: the involvement of actin cytoskeleton and ROCK1. PLoS One 2015; 10: e0131763.
[20] Schackmann RC, van Amersfoort M, Haarhuis JH, Vlug EJ, Halim VA, Roodhart JM, Vermaat JS, Voest EE, van der Groep P, van Diest PJ, Jonkers J, Derksen PW. Cytosolic p120-catenin regulates growth of metastatic lobular carci-noma through Rock1-mediated anoikis resis-tance. J Clin Invest 2011; 121: 3176-3188. [21] Julian L and Olson MF. Rho-associated
coiled-coil containing kinases (ROCK): structure, reg-ulation, and functions. Small GTPases 2014; 5: e29846.
[22] Surma M, Handy C, Chang J, Kapur R, Wei L
and Shi J. ROCK1 deficiency enhances protec -tive effects of antioxidants against apoptosis and cell detachment. PLoS One 2014; 9: e90758.
+86-24-85715321; E-mail: zhangmcar2@163.com; Yong Wang, The 4th Department of Orthopaedic
Surgery, Central Hospital Affiliated to Shenyang Medical College, 5# South Seven West Road, Tiexi
District, Shenyang 110024, Liaoning, P. R. China. Tel: +86-24-85715382; Fax: +86-24-85715382; E-mail: WY_landy1116@163.com
References
[1] Yu X and Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclero-sis (review). Int J Mol Med 2014; 34: 923-933. [2] Bennett BJ, Davis RC, Civelek M, Orozco L, Wu
J, Qi H, Pan C, Packard RR, Eskin E, Yan M, Kirchgessner T, Wang Z, Li X, Gregory JC, Ha-zen SL, Gargalovic PS, Lusis AJ. Genetic archi-tecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genet 2015; 11: e1005711.
[3] Aherrahrou Z and Schunkert H. Genetics of
atherosclerosis and vascular calcification go
hand-in-hand. Atherosclerosis 2013; 228: 325-326.
[4] Spence JD. Genetics of atherosclerosis: the power of plaque burden and progression:
in-vited commentary on Dong C, Beecham A, Wang L, Blanton SH, Rundek T, Sacco RL. Fol
-low-Up association study of linkage regions re -veals multiple candidate genes for carotid plaque in Dominicans atherosclerosis 223 (1) (2012) 177-183. Atherosclerosis 2012; 223: 98-101.
[5] Fong GH. Potential contributions of intimal and plaque hypoxia to atherosclerosis. Curr Atheroscler Rep 2015; 17: 510.
[6] Jiang D, Li D and Wu W. Inhibitory effects and mechanisms of luteolin on proliferation and migration of vascular smooth muscle cells. Nu-trients 2013; 5: 1648-1659.
[7] Jakob P and Landmesser U. Role of microRNAs
in stem/progenitor cells and cardiovascular re-pair. Cardiovasc Res 2012; 93: 614-622. [8] Loyer X, Mallat Z, Boulanger CM and Tedgui A.
MicroRNAs as therapeutic targets in athero-sclerosis. Expert Opin Ther Targets 2015; 19: 489-496.
[9] Takaya T, Nishi H, Horie T, Ono K and Hasega-wa K. Roles of microRNAs and myocardial cell
differentiation. Prog Mol Biol Transl Sci 2012;
111: 139-152.
[10] Liang X, Xu Z, Yuan M, Zhang Y, Zhao B, Wang
J, Zhang A, Li G. MicroRNA-16 suppresses the
activation of inflammatory macrophages in
atherosclerosis by targeting PDCD4. Int J Mol Med 2016; 37: 967-975.
[36] Romaine SP, Tomaszewski M, Condorelli G and Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart 2015; 101: 921-928.
[37] Liu JY, Lu JB and Xu Y. MicroRNA-153 inhibits
the proliferation and invasion of human laryn-geal squamous cell carcinoma by targeting KLF5. Exp Ther Med 2016; 11: 2503-2508. [38] Wu X, Li L, Li Y and Liu Z. MiR-153 promotes
breast cancer cell apoptosis by targeting HECTD3. Am J Cancer Res 2016; 6: 1563-1571.
[39] Xia W, Ma X, Li X, Dong H, Yi J, Zeng W, Yang Z. miR-153 inhibits epithelial-to-mesenchymal transition in hepatocellular carcinoma by tar-geting Snail. Oncol Rep 2015; 34: 655-662. [40] Zou Y, Liu W, Zhang J and Xiang D. miR-153
regulates apoptosis and autophagy of cardio-myocytes by targeting Mcl-1. Mol Med Rep 2016; 14: 1033-1039.
[41] Johnson JL, Dwivedi A, Somerville M, George SJ and Newby AC. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc
Biol 2011; 31: e35-44.
[42] Hartmann S, Ridley AJ and Lutz S. The function of rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol 2015; 6: 276.
[43] Noma K, Oyama N and Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 2006; 290: C661-668.
[44] Rigassi L, Barchiesi Bozzolo F, Lucchinetti E, Zaugg M, Fingerle J, Rosselli M, Imthurn B,
Jackson EK, Dubey RK. 2-Methoxyestradiol blocks the RhoA/ROCK1 pathway in human aortic smooth muscle cells. Am J Physiol Endo-crinol Metab 2015; 309: E995-1007.
[45] Shen M, Zhou S, Li Y, Pan P, Zhang L and Hou T. Discovery and optimization of triazine derivatives as ROCK1 inhibitors: molecular docking, molecular dynamics simulations and
free energy calculations. Mol Biosyst 2013; 9:
361-374.
[46] Shimokawa H, Sunamura S and Satoh K. RhoA/Rho-kinase in the cardiovascular sys-tem. Circ Res 2016; 118: 352-366.
[47] Cui Y, Sun YW, Lin HS, Su WM, Fang Y, Zhao Y, Wei XQ, Qin YH, Kohama K, Gao Y.
Platelet-derived growth factor-BB induces matrix
me-talloproteinase-2 expression and rat vascular smooth muscle cell migration via ROCK and
ERK/p38 MAPK pathways. Mol Cell Biochem
2014; 393: 255-263. [23] Zhao Y, Lv M, Lin H, Cui Y, Wei X, Qin Y, Kohama
K, Gao Y. Rho-associated protein kinase iso-forms stimulate proliferation of vascular smooth muscle cells through ERK and
induc-tion of cyclin D1 and PCNA. Biochem Biophys
Res Commun 2013; 432: 488-493.
[24] Zhao Y, Lv M, Lin H, Hong Y, Yang F, Sun Y, Guo Y, Cui Y, Li S, Gao Y. ROCK1 induces ERK
nucle-ar translocation in PDGF-BB-stimulated migra -tion of rat vascular smooth muscle cells.
IUBMB Life 2012; 64: 194-202.
[25] Geng H, Wang A, Rong G, Zhu B, Deng Y, Chen
J, Zhong R. The effects of ox-LDL in human ath-erosclerosis may be mediated in part via the
toll-like receptor 4 pathway. Mol Cell Biochem
2010; 342: 201-206.
[26] Ma X, Qiu R, Dang J, Li J, Hu Q, Shan S, Xin Q,
Pan W, Bian X, Yuan Q, Long F, Liu N, Li Y, Gao
F, Zou C, Gong Y, Liu Q. ORMDL3 contributes to the risk of atherosclerosis in Chinese Han pop-ulation and mediates oxidized low-density lipo-protein-induced autophagy in endothelial cells. Sci Rep 2015; 5: 17194.
[27] Lin Q, Mao W, Shu Y, Lin F, Liu S, Shen H, Gao
W, Li S, Shen D. A cluster of specified microR -NAs in peripheral blood as biomarkers for met-astatic non-small-cell lung cancer by stem-loop RT-PCR. J Cancer Res Clin Oncol 2012; 138: 85-93.
[28] Varkonyi-Gasic E and Hellens RP. Quantitative stem-loop RT-PCR for detection of microRNAs.
Methods Mol Biol 2011; 744: 145-157.
[29] Liu ZJ, Gao X, Cai Y, Yang X, Fu XL, Chen J,
Zhang XB, Xin HM. Construction of a full-length
iASPP expression plasmid pcDNA3.1+/iASPP and its biological activity. Plasmid 2009; 62: 10-15.
[30] Wang Y, Zhao W and Fu Q. miR-335 suppress-es migration and invasion by targeting ROCK1
in osteosarcoma cells. Mol Cellular Biochem
2013; 384: 105-111.
[31] Lacolley P, Regnault V, Nicoletti A, Li Z and
Mi-chel JB. The vascular smooth muscle cell in
arterial pathology: a cell that can take on mul-tiple roles. Cardiovasc Res 2012; 95: 194-204.
[32] Deng X, Su Y, Wu H, Wu R, Zhang P, Dai Y, Chan TM, Zhao M, Lu Q. The role of microRNAs in autoimmune diseases with skin involvement. Scand J Immunol 2015; 81: 153-165.
[33] Heyns M and Kovalchuk O. Non-coding RNAs including miRNAs, piRNAs, and tRNAs in hu-man cancer. Oncotarget 2015; 6: 23055-23057.
[34] Jung HJ and Suh Y. Circulating miRNAs in age-ing and ageage-ing-related diseases. J Genet Ge-nomics 2014; 41: 465-472.
[35] Qiu L, Tan EK and Zeng L. MicroRNAs and
neu-rodegenerative diseases. Adv Exp Med Biol