metal-organic papers
m942
Barkeret al. [Mg(C3H7NO)6][MgCl4] doi:10.1107/S1600536806011123 Acta Cryst.(2006). E62, m942–m944
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
Hexakis(N,N-dimethylformamide-
j
O)-magnesium tetrachloromagnesate
Bobby L. Barker, David Aubry, Frank R. Fronczek,
Steven F. Watkins* and George G. Stanley
Department of Chemistry, Louisiana State University, Baton Rouge, LA 70803, USA
Correspondence e-mail: swatkins@lsu.edu
Key indicators
Single-crystal X-ray study T= 120 K
Mean(l–Mg) = 0.001 A˚ Rfactor = 0.035 wRfactor = 0.068
Data-to-parameter ratio = 11.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 14 March 2006 Accepted 27 March 2006
#2006 International Union of Crystallography All rights reserved
The crystallographically independent unit of the title compound, [Mg(C3H7NO)6][MgCl4], consists of two six-coordinate magnesium dications and two four-six-coordinate magnesium dianions. The two cations are quasi-octahedral and statistically equivalent [average Mg—O = 2.07 (3) A˚ ] and the anions are quasi-tetrahedral and statistically equivalent [average Mg—Cl = 2.334 (11) A˚ ].
Comment
The asymmetric unit of the title compound consists of two cations and two anions. Equivalent bond lengths and bond angles of the metal-bondedN,N-dimethylformamide (DMFA) ligands are statistically equal to one another and are consistent with values reported for the [(DMFA)6Mg]2+ dication by Krautscheid & Vielsack (1999). Other DMFA–Mg complexes have been reported by Hollanderet al.(1973) and Adamset al.
(2005). The bond lengths and angles in the [MgCl4] 2
dianion do not differ significantly from the values reported by Sobota
et al. (1986), Sobota & Szafert (1996), and Pavanello et al.
(1994).
Experimental
Crystals of the title compound were grown from dimethylformamide solution. Their preparation is discussed by Barker (2005).
Crystal data
[Mg(C3H7NO)6][MgCl4]
Mr= 629 Monoclinic,P21
a= 13.905 (2) A˚
b= 12.108 (3) A˚
c= 19.079 (3) A˚ = 90.717 (15)
V= 3211.9 (10) A˚3
Z= 4
Dx= 1.301 Mg m
3 MoKradiation Cell parameters from 7510
reflections = 2.5–27.5
= 0.45 mm1
Data collection
Nonius KappaCCD diffractometer with an Oxford Cryosystems Cryostream cooler !scans withoffsets
Absorption correction: multi-scan (SCALEPACK; Otwinowski & Minor 1997)
Tmin= 0.929,Tmax= 0.956
45159 measured reflections 7677 independent reflections 5986 reflections withI> 2(I)
Rint= 0.036
max= 27.5
h=18!18
k=15!15
l=24!24
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.035
wR(F2) = 0.068
S= 1.02 7677 reflections 673 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0276P)2 + 0.1249P]
whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 0.26 e A˚
3 min=0.23 e A˚
[image:2.610.314.562.67.255.2]3
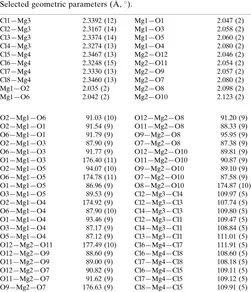
Table 1
Selected geometric parameters (A˚ ,).
Cl1—Mg3 2.3392 (12) Cl2—Mg3 2.3167 (14) Cl3—Mg3 2.3374 (14) Cl4—Mg3 2.3274 (13) Cl5—Mg4 2.3467 (13) Cl6—Mg4 2.3248 (15) Cl7—Mg4 2.3330 (13) Cl8—Mg4 2.3460 (13)
Mg1—O2 2.035 (2)
Mg1—O6 2.042 (2)
Mg1—O1 2.047 (2)
Mg1—O3 2.058 (2)
Mg1—O5 2.060 (2)
Mg1—O4 2.080 (2)
Mg2—O12 2.046 (2) Mg2—O11 2.054 (2)
Mg2—O9 2.057 (2)
Mg2—O7 2.080 (2)
Mg2—O8 2.098 (2)
Mg2—O10 2.123 (2)
O2—Mg1—O6 91.03 (10) O2—Mg1—O1 91.54 (9) O6—Mg1—O1 91.79 (9) O2—Mg1—O3 87.90 (9) O6—Mg1—O3 91.77 (9) O1—Mg1—O3 176.40 (11) O2—Mg1—O5 94.07 (10) O6—Mg1—O5 174.78 (11) O1—Mg1—O5 86.96 (9) O3—Mg1—O5 89.53 (9) O2—Mg1—O4 174.92 (9) O6—Mg1—O4 87.90 (10) O1—Mg1—O4 93.46 (9) O3—Mg1—O4 87.17 (9) O5—Mg1—O4 87.12 (9) O12—Mg2—O11 177.49 (10) O12—Mg2—O9 88.60 (9) O11—Mg2—O9 89.00 (9) O12—Mg2—O7 90.82 (9) O11—Mg2—O7 91.62 (9) O9—Mg2—O7 176.63 (9)
O12—Mg2—O8 91.20 (9) O11—Mg2—O8 88.33 (9) O9—Mg2—O8 95.95 (9) O7—Mg2—O8 87.38 (9) O12—Mg2—O10 89.81 (9) O11—Mg2—O10 90.87 (9) O9—Mg2—O10 89.10 (9) O7—Mg2—O10 87.58 (9) O8—Mg2—O10 174.87 (10) Cl2—Mg3—Cl4 109.97 (5) Cl2—Mg3—Cl3 107.74 (5) Cl4—Mg3—Cl3 109.80 (5) Cl2—Mg3—Cl1 109.47 (5) Cl4—Mg3—Cl1 108.84 (5) Cl3—Mg3—Cl1 111.01 (5) Cl6—Mg4—Cl7 111.91 (5) Cl6—Mg4—Cl8 108.60 (5) Cl7—Mg4—Cl8 108.18 (5) Cl6—Mg4—Cl5 109.11 (5) Cl7—Mg4—Cl5 109.12 (5) Cl8—Mg4—Cl5 109.91 (5)
Refinement of the Flack (1983) parameter with 6646 Friedel pairs led to a value of 0.49 (3); the crystal was thus assumed to be an inversion twin with equal components. Friedel pairs were averaged in the final refinement, and the absolute structure chosen was arbitrary. All H atoms were placed in calculated positions, with C—H distances of 0.93 A˚ andUiso= 1.2Ueqof the attached C atom, and thereafter
treated as riding. A torsional parameter was refined for each methyl group.
Data collection: COLLECT (Nonius, 2000); cell refinement: DENZO and SCALEPACK (Otwinowski & Minor, 1997); data reduction: DENZO and SCALEPACK; program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3(Farrugia, 1997); software used to prepare material for publication:SHELXL97.
The purchase of the diffractometer was made possible by grant No. LEQSF(1999–2000)-ESH-TR-13, administered by the Louisiana Board of Regents.
References
Adams, H., Rolfe, A. & Jones, S. (2005).Acta Cryst.E61, m1251–m1252. Barker, B. L. (2005). PhD dissertation, Louisiana State University, USA. URL:
http://etd.lsu.edu/docs/available/etd-04132005-131235/. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Hollander, F. J., Templeton, D. H. & Zalkin, A. (1973).Acta Cryst.B29, 1289– 1295.
Krautscheid, H. & Vielsack, F. (1999).Z. Anorg. Allg. Chem.625, 562–566. Nonius (2000).COLLECT. Nonius BV, Delft, The Netherlands.
Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
[image:2.610.45.296.288.580.2]Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
Figure 1
The atom-numbering scheme for cation–anion pair 1, with displacement ellipsoids shown at the 50% probability level. H atoms are not shown.
Figure 2
[image:2.610.313.566.296.487.2]Pavanello, L., Visona, P., Bresadola, S. & Bandoli, G. (1994).Z. Kristallogr.
209, 946–949.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Sobota, P., Pluzinski, T. & Lis, T. (1986).Bull. Pol. Acad. Sci. Chem.33, 491– 496.
Sobota, P. & Szafert, S. (1996).Inorg. Chem.35, 1778–1781.
metal-organic papers
m944
Barkeret al. [Mg(Csupporting information
Acta Cryst. (2006). E62, m942–m944 [https://doi.org/10.1107/S1600536806011123]
Hexakis(N,N-dimethylformamide-
κ
O)magnesium tetrachloromagnesate
Bobby L. Barker, David Aubry, Frank R. Fronczek, Steven F. Watkins and George G. Stanley
Hexakis(dimethylformamide-κO)magnesium tetrachloromagnesium
Crystal data
[Mg(C3H7NO)6][MgCl4] Mr = 629
Monoclinic, P21
Hall symbol: P 2ybc
a = 13.905 (2) Å
b = 12.108 (3) Å
c = 19.079 (3) Å
β = 90.717 (15)°
V = 3211.9 (10) Å3 Z = 4
F(000) = 1328
Dx = 1.301 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 7510 reflections
θ = 2.5–27.5°
µ = 0.45 mm−1 T = 120 K
Fragment, colorless 0.35 × 0.25 × 0.1 mm
Data collection
Nonius KappaCCD (with an Oxford Cryosystems Cryostream cooler) diffractometer
ω scans with κ offsets
Absorption correction: multi-scan
(SCALEPACK; Otwinowski & Minor 1997)
Tmin = 0.929, Tmax = 0.956
45159 measured reflections
7677 independent reflections 5986 reflections with I > 2σ(I)
Rint = 0.036
θmax = 27.5°, θmin = 2.6° h = −18→18
k = −15→15
l = −24→24
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.035 wR(F2) = 0.068 S = 1.02 7677 reflections 673 parameters
1 restraint
H-atom parameters constrained
w = 1/[σ2(Fo2) + (0.0276P)2 + 0.1249P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.26 e Å−3
Δρmin = −0.23 e Å−3 Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-2
Acta Cryst. (2006). E62, m942–m944
H1 0.7171 0.2214 0.3938 0.027*
C2 0.8315 (2) 0.5855 (3) 0.29553 (15) 0.0257 (7)
H2 0.8031 0.5804 0.3404 0.031*
C3 0.8060 (2) 0.4019 (3) 0.10987 (14) 0.0251 (7)
H3 0.8039 0.4785 0.1208 0.03*
C4 0.6483 (2) 0.1565 (3) 0.22322 (15) 0.0322 (8)
H4 0.6108 0.2112 0.1999 0.039*
C5 0.5867 (2) 0.4604 (3) 0.20265 (15) 0.0265 (7)
H5 0.6221 0.5111 0.1751 0.032*
C6 0.9259 (2) 0.1936 (3) 0.31337 (14) 0.0267 (7)
H6 0.8767 0.1415 0.3227 0.032*
C7 0.8651 (2) 1.4998 (3) 0.81427 (14) 0.0222 (7)
H7 0.8063 1.5388 0.8191 0.027*
C8 0.8643 (2) 1.1921 (3) 0.65451 (14) 0.0264 (7)
H8 0.8618 1.1323 0.6867 0.032*
C9 0.5895 (2) 1.1678 (3) 0.68706 (14) 0.0215 (7)
H9 0.6399 1.1253 0.6674 0.026*
C10 0.6385 (2) 1.3112 (3) 0.89857 (15) 0.0280 (7) H10 0.6395 1.2337 0.8909 0.034* C11 0.8475 (2) 1.1500 (3) 0.84557 (14) 0.0217 (7) H11 0.8808 1.2077 0.8693 0.026* C12 0.6128 (2) 1.4838 (3) 0.67797 (15) 0.0261 (7)
H12 0.5675 1.426 0.6709 0.031*
C13 0.6964 (2) 0.4241 (3) 0.50595 (15) 0.0309 (8) H13A 0.728 0.4749 0.4736 0.046* H13B 0.7313 0.4237 0.5509 0.046*
H13C 0.63 0.4482 0.5133 0.046*
C14 0.6664 (2) 0.2227 (3) 0.52163 (15) 0.0301 (8) H14A 0.6713 0.1527 0.4961 0.045* H14B 0.5997 0.2342 0.5359 0.045* H14C 0.7082 0.2202 0.5633 0.045* C15 0.8675 (3) 0.7769 (3) 0.32168 (16) 0.0372 (8) H15A 0.8372 0.7584 0.3662 0.056*
H15B 0.83 0.8344 0.2978 0.056*
H19A 0.5137 0.0866 0.1478 0.068* H19B 0.4977 −0.0212 0.1947 0.068* H19C 0.5682 −0.0258 0.1291 0.068* C20 0.6821 (4) −0.0339 (4) 0.2432 (3) 0.0737 (15) H20A 0.7411 −0.0024 0.2635 0.111* H20B 0.6988 −0.0878 0.207 0.111* H20C 0.6456 −0.0707 0.2801 0.111* C21 0.4344 (2) 0.3796 (3) 0.23331 (17) 0.0351 (8) H21A 0.4756 0.3356 0.2646 0.053* H21B 0.399 0.3304 0.2013 0.053* H21C 0.3886 0.422 0.2612 0.053* C22 0.4440 (3) 0.5263 (3) 0.14236 (16) 0.0393 (9) H22A 0.491 0.5733 0.1189 0.059* H22B 0.3974 0.5726 0.1668 0.059* H22C 0.4103 0.4806 0.1075 0.059* C23 1.0927 (3) 0.2477 (3) 0.32209 (19) 0.0449 (10) H23A 1.0657 0.3191 0.3076 0.067* H23B 1.1356 0.2202 0.2857 0.067* H23C 1.1289 0.2568 0.3661 0.067* C24 1.0394 (2) 0.0631 (3) 0.36463 (16) 0.0326 (8) H24A 0.9806 0.0202 0.372 0.049* H24B 1.0717 0.0761 0.4098 0.049* H24C 1.0823 0.022 0.3337 0.049* C25 1.0383 (2) 1.4967 (3) 0.82623 (18) 0.0353 (8) H25A 1.0538 1.4601 0.8708 0.053* H25B 1.088 1.5515 0.8158 0.053* H25C 1.0356 1.4416 0.7886 0.053* C26 0.9455 (3) 1.6619 (3) 0.86089 (17) 0.0364 (8) H26A 0.8796 1.6905 0.8611 0.055* H26B 0.9859 1.7104 0.8325 0.055* H26C 0.9709 1.6594 0.909 0.055* C27 0.9261 (3) 1.2662 (4) 0.54552 (16) 0.0446 (10) H27A 0.8965 1.2402 0.5017 0.067* H27B 0.9941 1.2832 0.5377 0.067* H27C 0.8929 1.3329 0.5614 0.067* C28 0.9673 (3) 1.0755 (3) 0.58489 (18) 0.0402 (9) H28A 0.9639 1.0281 0.6264 0.06* H28B 1.0348 1.0897 0.5738 0.06* H28C 0.9357 1.0386 0.5451 0.06* C29 0.4197 (2) 1.2044 (3) 0.69692 (16) 0.0335 (8) H29A 0.4411 1.2478 0.7376 0.05*
H29B 0.3687 1.1534 0.711 0.05*
H29C 0.3949 1.2543 0.6605 0.05* C30 0.4786 (3) 1.0575 (3) 0.61658 (16) 0.0336 (8)
H30A 0.538 1.0195 0.6036 0.05*
H30B 0.4501 1.0928 0.575 0.05*
supporting information
sup-4
Acta Cryst. (2006). E62, m942–m944
H31A 0.5802 1.1927 0.9943 0.064* H31B 0.4974 1.2777 1.0155 0.064* H31C 0.5996 1.2814 1.055 0.064* C32 0.5930 (2) 1.4634 (3) 0.97480 (16) 0.0319 (8) H32A 0.6195 1.5076 0.9366 0.048* H32B 0.6286 1.4792 1.0183 0.048* H32C 0.525 1.4819 0.9808 0.048* C33 0.9445 (2) 1.0214 (3) 0.91339 (17) 0.0357 (8) H33A 0.9753 1.0896 0.9299 0.053* H33B 0.9932 0.9723 0.8936 0.053* H33C 0.9133 0.9845 0.9528 0.053* C34 0.8256 (3) 0.9547 (3) 0.82497 (18) 0.0397 (9) H34A 0.7817 0.9188 0.8577 0.06*
H34B 0.8743 0.9015 0.81 0.06*
H34C 0.7893 0.9811 0.784 0.06*
C35 0.6597 (3) 1.6713 (3) 0.6623 (2) 0.0445 (10) H35A 0.6821 1.6748 0.7112 0.067* H35B 0.629 1.7414 0.6494 0.067* H35C 0.7146 1.6579 0.6317 0.067* C36 0.5015 (3) 1.6054 (3) 0.61609 (19) 0.0461 (10)
H36A 0.463 1.5378 0.613 0.069*
O2 0.82860 (16) 0.50255 (19) 0.25731 (10) 0.0304 (5) O3 0.78873 (16) 0.33448 (19) 0.15719 (10) 0.0317 (5) O4 0.71564 (15) 0.18888 (17) 0.26131 (10) 0.0254 (5) O5 0.63180 (16) 0.40271 (19) 0.24605 (11) 0.0311 (5) O6 0.90270 (15) 0.28161 (19) 0.28383 (10) 0.0302 (5) O7 0.86158 (15) 1.40273 (18) 0.79214 (10) 0.0243 (5) O8 0.81567 (15) 1.27627 (19) 0.66794 (10) 0.0281 (5) O9 0.61223 (15) 1.24448 (18) 0.72763 (9) 0.0250 (5) O10 0.67198 (15) 1.37135 (18) 0.85166 (10) 0.0256 (5) O11 0.78276 (15) 1.17601 (17) 0.80318 (10) 0.0258 (5) O12 0.68966 (15) 1.46176 (18) 0.70919 (10) 0.0260 (5)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-6
Acta Cryst. (2006). E62, m942–m944
C34 0.045 (2) 0.0236 (19) 0.050 (2) −0.0010 (18) −0.0105 (17) 0.0050 (16) C35 0.045 (2) 0.026 (2) 0.063 (2) −0.0001 (18) 0.0156 (18) 0.0079 (18) C36 0.048 (3) 0.048 (3) 0.042 (2) 0.016 (2) −0.0143 (18) 0.0055 (18) Cl1 0.0306 (4) 0.0239 (4) 0.0248 (4) 0.0004 (4) 0.0017 (3) −0.0036 (3) Cl2 0.0257 (5) 0.0494 (6) 0.0584 (5) −0.0019 (4) −0.0066 (4) −0.0119 (5) Cl3 0.0468 (5) 0.0226 (4) 0.0319 (4) 0.0087 (4) 0.0050 (3) −0.0003 (3) Cl4 0.0527 (6) 0.0246 (5) 0.0305 (4) −0.0026 (4) 0.0132 (4) 0.0031 (3) Cl5 0.0334 (5) 0.0309 (5) 0.0313 (4) −0.0042 (4) −0.0016 (3) 0.0002 (3) Cl6 0.0408 (5) 0.0233 (4) 0.0447 (5) 0.0050 (4) 0.0030 (4) −0.0005 (4) Cl7 0.0564 (6) 0.0444 (6) 0.0389 (5) −0.0129 (5) 0.0178 (4) 0.0080 (4) Cl8 0.0272 (4) 0.0331 (5) 0.0254 (4) 0.0037 (4) 0.0009 (3) 0.0000 (3) Mg1 0.0247 (6) 0.0239 (6) 0.0180 (5) −0.0021 (5) 0.0010 (4) 0.0024 (4) Mg2 0.0241 (6) 0.0203 (6) 0.0184 (5) 0.0015 (4) −0.0014 (4) 0.0003 (4) Mg3 0.0241 (6) 0.0194 (6) 0.0219 (5) 0.0007 (5) 0.0015 (4) −0.0009 (4) Mg4 0.0256 (6) 0.0224 (6) 0.0253 (5) −0.0003 (5) 0.0037 (4) 0.0027 (4) N1 0.0262 (15) 0.0258 (15) 0.0180 (12) 0.0021 (12) 0.0021 (10) 0.0031 (10) N2 0.0291 (15) 0.0221 (15) 0.0188 (12) −0.0003 (12) 0.0034 (10) −0.0015 (10) N3 0.0236 (15) 0.0291 (16) 0.0165 (12) −0.0011 (12) 0.0001 (9) 0.0040 (11) N4 0.0298 (17) 0.0332 (18) 0.0309 (15) −0.0117 (14) −0.0039 (12) −0.0046 (12) N5 0.0242 (15) 0.0268 (15) 0.0261 (13) 0.0024 (13) −0.0002 (10) 0.0016 (11) N6 0.0258 (15) 0.0286 (16) 0.0280 (14) −0.0021 (13) −0.0004 (11) 0.0004 (12) N7 0.0263 (15) 0.0219 (15) 0.0226 (13) −0.0003 (12) 0.0012 (11) −0.0009 (11) N8 0.0220 (15) 0.0392 (18) 0.0283 (14) −0.0009 (13) 0.0022 (11) −0.0066 (12) N9 0.0251 (16) 0.0258 (15) 0.0224 (13) −0.0027 (12) 0.0023 (10) −0.0024 (11) N10 0.0249 (15) 0.0276 (16) 0.0247 (13) −0.0016 (12) 0.0032 (10) −0.0023 (11) N11 0.0212 (15) 0.0201 (15) 0.0280 (13) 0.0005 (12) −0.0014 (10) 0.0041 (11) N12 0.0300 (16) 0.0228 (15) 0.0268 (14) 0.0017 (12) 0.0026 (11) 0.0030 (11) O1 0.0352 (13) 0.0292 (13) 0.0202 (10) −0.0051 (11) 0.0032 (9) 0.0049 (9) O2 0.0410 (14) 0.0260 (13) 0.0243 (11) −0.0062 (11) 0.0044 (9) 0.0023 (10) O3 0.0384 (14) 0.0369 (14) 0.0198 (10) −0.0086 (11) 0.0046 (9) 0.0023 (10) O4 0.0289 (13) 0.0253 (13) 0.0221 (10) −0.0042 (10) −0.0044 (9) −0.0006 (9) O5 0.0294 (13) 0.0315 (13) 0.0325 (12) 0.0030 (11) −0.0032 (9) 0.0076 (10) O6 0.0284 (13) 0.0304 (14) 0.0318 (11) 0.0007 (11) −0.0015 (9) 0.0037 (10) O7 0.0270 (13) 0.0221 (12) 0.0238 (10) −0.0005 (10) −0.0012 (9) 0.0005 (9) O8 0.0283 (12) 0.0336 (14) 0.0222 (10) 0.0038 (11) −0.0002 (9) −0.0051 (10) O9 0.0296 (13) 0.0232 (12) 0.0222 (10) −0.0011 (10) −0.0025 (9) −0.0020 (9) O10 0.0274 (12) 0.0268 (13) 0.0227 (10) 0.0001 (10) 0.0005 (9) −0.0015 (9) O11 0.0264 (12) 0.0228 (12) 0.0280 (11) 0.0046 (10) −0.0023 (9) 0.0038 (9) O12 0.0248 (12) 0.0263 (13) 0.0267 (11) 0.0027 (10) −0.0036 (9) 0.0029 (9)
Geometric parameters (Å, º)
C1—O1 1.245 (3) C23—N6 1.461 (4)
C1—N1 1.323 (3) C23—H23A 0.98
C1—H1 0.95 C23—H23B 0.98
C2—O2 1.241 (4) C23—H23C 0.98
C2—N2 1.314 (4) C24—N6 1.458 (4)
C3—O3 1.243 (4) C24—H24B 0.98
C3—N3 1.320 (4) C24—H24C 0.98
C3—H3 0.95 C25—N7 1.452 (4)
C4—O4 1.242 (4) C25—H25A 0.98
C4—N4 1.311 (4) C25—H25B 0.98
C4—H4 0.95 C25—H25C 0.98
C5—O5 1.246 (4) C26—N7 1.453 (4)
C5—N5 1.309 (4) C26—H26A 0.98
C5—H5 0.95 C26—H26B 0.98
C6—O6 1.246 (4) C26—H26C 0.98
C6—N6 1.317 (4) C27—N8 1.460 (5)
C6—H6 0.95 C27—H27A 0.98
C7—O7 1.249 (4) C27—H27B 0.98
C7—N7 1.320 (4) C27—H27C 0.98
C7—H7 0.95 C28—N8 1.465 (4)
C8—O8 1.251 (4) C28—H28A 0.98
C8—N8 1.318 (4) C28—H28B 0.98
C8—H8 0.95 C28—H28C 0.98
C9—O9 1.247 (4) C29—N9 1.456 (4)
C9—N9 1.316 (4) C29—H29A 0.98
C9—H9 0.95 C29—H29B 0.98
C10—O10 1.248 (4) C29—H29C 0.98
C10—N10 1.320 (4) C30—N9 1.468 (4)
C10—H10 0.95 C30—H30A 0.98
C11—O11 1.243 (3) C30—H30B 0.98
C11—N11 1.313 (4) C30—H30C 0.98
C11—H11 0.95 C31—N10 1.461 (4)
C12—O12 1.246 (4) C31—H31A 0.98
C12—N12 1.309 (4) C31—H31B 0.98
C12—H12 0.95 C31—H31C 0.98
C13—N1 1.455 (4) C32—N10 1.456 (4)
C13—H13A 0.98 C32—H32A 0.98
C13—H13B 0.98 C32—H32B 0.98
C13—H13C 0.98 C32—H32C 0.98
C14—N1 1.459 (4) C33—N11 1.458 (4)
C14—H14A 0.98 C33—H33A 0.98
C14—H14B 0.98 C33—H33B 0.98
C14—H14C 0.98 C33—H33C 0.98
C15—N2 1.441 (4) C34—N11 1.458 (4)
C15—H15A 0.98 C34—H34A 0.98
C15—H15B 0.98 C34—H34B 0.98
C15—H15C 0.98 C34—H34C 0.98
C16—N2 1.455 (4) C35—N12 1.445 (4)
C16—H16A 0.98 C35—H35A 0.98
C16—H16B 0.98 C35—H35B 0.98
C16—H16C 0.98 C35—H35C 0.98
C17—N3 1.465 (4) C36—N12 1.462 (4)
supporting information
sup-8
Acta Cryst. (2006). E62, m942–m944
C17—H17B 0.98 C36—H36B 0.98
C17—H17C 0.98 C36—H36C 0.98
C18—N3 1.450 (4) Cl1—Mg3 2.3392 (12)
C18—H18A 0.98 Cl2—Mg3 2.3167 (14)
C18—H18B 0.98 Cl3—Mg3 2.3374 (14)
C18—H18C 0.98 Cl4—Mg3 2.3274 (13)
C19—N4 1.453 (4) Cl5—Mg4 2.3467 (13)
C19—H19A 0.98 Cl6—Mg4 2.3248 (15)
C19—H19B 0.98 Cl7—Mg4 2.3330 (13)
C19—H19C 0.98 Cl8—Mg4 2.3460 (13)
C20—N4 1.442 (5) Mg1—O2 2.035 (2)
C20—H20A 0.98 Mg1—O6 2.042 (2)
C20—H20B 0.98 Mg1—O1 2.047 (2)
C20—H20C 0.98 Mg1—O3 2.058 (2)
C21—N5 1.458 (4) Mg1—O5 2.060 (2)
C21—H21A 0.98 Mg1—O4 2.080 (2)
C21—H21B 0.98 Mg2—O12 2.046 (2)
C21—H21C 0.98 Mg2—O11 2.054 (2)
C22—N5 1.458 (4) Mg2—O9 2.057 (2)
C22—H22A 0.98 Mg2—O7 2.080 (2)
C22—H22B 0.98 Mg2—O8 2.098 (2)
C22—H22C 0.98 Mg2—O10 2.123 (2)
O1—C1—N1 124.8 (3) N9—C29—H29C 109.5 O1—C1—H1 117.6 H29A—C29—H29C 109.5 N1—C1—H1 117.6 H29B—C29—H29C 109.5 O2—C2—N2 124.4 (3) N9—C30—H30A 109.5
O2—C2—H2 117.8 N9—C30—H30B 109.5
N2—C2—H2 117.8 H30A—C30—H30B 109.5 O3—C3—N3 123.3 (3) N9—C30—H30C 109.5 O3—C3—H3 118.4 H30A—C30—H30C 109.5 N3—C3—H3 118.4 H30B—C30—H30C 109.5 O4—C4—N4 125.4 (3) N10—C31—H31A 109.5
O4—C4—H4 117.3 N10—C31—H31B 109.5
N4—C4—H4 117.3 H31A—C31—H31B 109.5 O5—C5—N5 123.8 (3) N10—C31—H31C 109.5 O5—C5—H5 118.1 H31A—C31—H31C 109.5 N5—C5—H5 118.1 H31B—C31—H31C 109.5 O6—C6—N6 123.8 (3) N10—C32—H32A 109.5
O6—C6—H6 118.1 N10—C32—H32B 109.5
N6—C6—H6 118.1 H32A—C32—H32B 109.5 O7—C7—N7 123.9 (3) N10—C32—H32C 109.5 O7—C7—H7 118.1 H32A—C32—H32C 109.5 N7—C7—H7 118.1 H32B—C32—H32C 109.5 O8—C8—N8 124.7 (3) N11—C33—H33A 109.5
O8—C8—H8 117.7 N11—C33—H33B 109.5
supporting information
sup-10
Acta Cryst. (2006). E62, m942–m944
N8—C27—H27B 109.5 C12—N12—C36 122.4 (3) H27A—C27—H27B 109.5 C35—N12—C36 117.9 (3) N8—C27—H27C 109.5 C1—O1—Mg1 126.9 (2) H27A—C27—H27C 109.5 C2—O2—Mg1 135.7 (2) H27B—C27—H27C 109.5 C3—O3—Mg1 133.6 (2) N8—C28—H28A 109.5 C4—O4—Mg1 125.5 (2) N8—C28—H28B 109.5 C5—O5—Mg1 138.4 (2) H28A—C28—H28B 109.5 C6—O6—Mg1 130.7 (2) N8—C28—H28C 109.5 C7—O7—Mg2 125.7 (2) H28A—C28—H28C 109.5 C8—O8—Mg2 131.3 (2) H28B—C28—H28C 109.5 C9—O9—Mg2 135.6 (2) N9—C29—H29A 109.5 C10—O10—Mg2 127.6 (2) N9—C29—H29B 109.5 C11—O11—Mg2 134.9 (2) H29A—C29—H29B 109.5 C12—O12—Mg2 132.1 (2)
supporting information
sup-12
Acta Cryst. (2006). E62, m942–m944