organic papers
Acta Cryst.(2006). E62, o2417–o2418 doi:10.1107/S1600536806018320 Chenget al. C
14H9ClN2O
o2417
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
5-Chloro-2-(2-hydroxybenzylideneamino)-benzonitrile
Kui Cheng,aHai-Liang Zhu,a* Zhi-Bin Liband Zheng Yana
a
Department of Chemistry, Wuhan University of Science and Engineering, Wuhan 430073, People’s Republic of China, andbDepartment of Environment and Urban Construction, Wuhan University of Science and Engineering, Wuhan 430073, People’s Republic of China
Correspondence e-mail: hailiang_zhu@163.com
Key indicators
Single-crystal X-ray study
T= 292 K
Mean(C–C) = 0.005 A˚
Rfactor = 0.073
wRfactor = 0.168
Data-to-parameter ratio = 14.1
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 2 May 2006 Accepted 17 May 2006
#2006 International Union of Crystallography All rights reserved
The molecule of the title compound, C14H9ClN2O, is
essentially planar, suggesting a high degree of conjugation throughout the system. Intermolecular hydrogen bonds link adjacent molecules, forming one-dimensional chains running parallel to thebaxis.
Comment
Recently, we have reported a few Schiff base compounds (Chenget al., 2005, 2006; Zhuet al., 2005). As an extension of our work on the structural characterization of Schiff bases, the title compound, (I), is reported here.
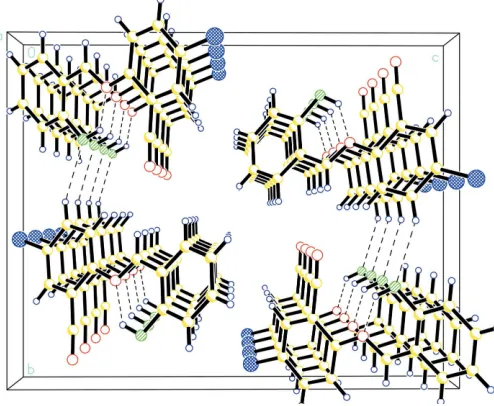
In the title compound, all bond lengths are within normal ranges (Allenet al., 1987) (Fig. 1). The C1 N1 bond length of 1.275 (4) A˚ conforms to the value for a double bond. A strong intramolecular O—H N hydrogen bond (Table 1) results in the formation of a pseudo-six-membered planar ring (C7/C6/ C1/O1/H1/N1) (Fig. 1). In the crystal packing, intermolecular C—H O interactions (Table 1) link the molecules, forming chains running parallel to thebaxis (Fig. 2).
Experimental
Salicylaldehyde and 2-cyano-4-chloroaniline were available commercially and were used without further purification. A solutiom of salicylaldehyde (2.0 mmol, 244 mg) in methanol (20 ml) was added to a solution of 2-cyano-4-chloroaniline (2.0 mmol, 304 mg) in ethanol (20 ml). The mixture was stirred for 20 min and filtered. After leaving the filtrate to stand in air for 6 d, large yellow prismatic crystals of (I) formed at the bottom of the vessel. The crystals were isolated, washed three times with methanol and dried in a vacuum desiccator using P4O10(yield 88.7%). Analysis found: C 83.8, H 5.1, N
28.3%; calculated for C17H13NO: C 84.1, H 5.0, N 28.0%.
Crystal data
C14H9ClN2O Mr= 256.68
Monoclinic,P21=c a= 4.7060 (12) A˚
b= 14.372 (4) A˚
c= 18.225 (5) A˚
= 91.228 (4)
V= 1232.4 (6) A˚3
Z= 4
Dx= 1.383 Mg m 3
MoKradiation
= 0.30 mm1
T= 292 (2) K
Data collection
Bruker SMART CCD area-detector diffractometer
!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin= 0.883,Tmax= 0.928
9840 measured reflections 2299 independent reflections 1696 reflections withI> 2(I)
Rint= 0.117
max= 25.5
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.074 wR(F2) = 0.168 S= 1.10 2299 reflections 163 parameters
H-atom parameters constrained
w= 1/[2
(Fo2) + (0.051P)2
+ 0.9611P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001 max= 0.28 e A˚
3 min=0.26 e A˚
3
Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O1—H1 N1 0.82 1.98 2.620 (4) 135 C10—H10 O1i
0.93 2.50 3.321 (4) 147
Symmetry code: (i)x;y1 2;zþ
1 2.
H atoms were positioned geometrically and constrained to ride on their parent atoms, with C—H distances of 0.93 A˚ , O—H = 0.82 A˚ and Uiso(H) = 1.2Ueq(C) or 1.5Ueq(O). The rather highRintvalue
(0.12) may result from the relatively poor quality of the crystal. Data collection:SMART(Siemens, 1996); cell refinement:SAINT (Siemens, 1996); data reduction: SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 1997a); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997a); molecular graphics: SHELXTL(Sheldrick, 1997b); software used to prepare material for publication:SHELXTL.
This project was sponsored by the Scientific Research Foundation for returned overseas Chinese scholars.
References
Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987).J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
Cheng, K., You, Z.-L., Li, Y.-G. & Zhu, H.-L. (2005).Acta Cryst.E61, o1137– o1138.
Cheng, K., Zhu, H.-L., Liu, J.-J., Gao, M. & Zeng, J.-H. (2006).Acta Cryst.E62, o1932–o1933.
Sheldrick, G. M. (1996).SADABS. University of Go¨ttingen, Germany. Sheldrick, G. M. (1997a). SHELXL97 and SHELXS97. University of
Go¨ttingen, Germany.
Sheldrick, G. M. (1997b).SHELXTL. Version 5.1. Bruker AXS Inc., Madison, Wisconsin, USA.
Siemens (1996).SMARTandSAINT. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
[image:2.610.312.566.73.204.2]Zhu, H.-L., Cheng, K., You, Z.-L. & Li, Y.-G. (2005).Acta Cryst.E61, m755– m756.
Figure 1
[image:2.610.317.564.250.453.2]The structure of (I), showing 30% probability displacement ellipsoids and the atom-numbering scheme. The dashed line indicates a hydrogen bond.
Figure 2
supporting information
sup-1 Acta Cryst. (2006). E62, o2417–o2418
supporting information
Acta Cryst. (2006). E62, o2417–o2418 [https://doi.org/10.1107/S1600536806018320]
5-Chloro-2-(2-hydroxybenzylideneamino)benzonitrile
Kui Cheng, Hai-Liang Zhu, Zhi-Bin Li and Zheng Yan
5-Chloro-2-(2-hydroxybenzylideneamino)benzonitrile
Crystal data
C14H9ClN2O Mr = 256.68
Monoclinic, P21/c Hall symbol: -P 2ybc
a = 4.7060 (12) Å
b = 14.372 (4) Å
c = 18.225 (5) Å
β = 91.228 (4)°
V = 1232.4 (6) Å3
Z = 4
F(000) = 528
Dx = 1.383 Mg m−3
Mo Kα radiation, λ = 0.71073 Å
Cell parameters from 7961 reflections
θ = 1.7–25.4°
µ = 0.30 mm−1
T = 292 K
Elongated prism, yellow 0.62 × 0.35 × 0.25 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996) Tmin = 0.883, Tmax = 0.928
9840 measured reflections 2299 independent reflections 1696 reflections with I > 2σ(I) Rint = 0.117
θmax = 25.5°, θmin = 1.8°
h = −5→5
k = −17→17
l = −22→22
Refinement
Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.074 wR(F2) = 0.168
S = 1.10
2299 reflections 163 parameters 36 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.051P)2 + 0.9611P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.28 e Å−3 Δρmin = −0.26 e Å−3
Special details
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1 0.5293 (8) 0.7436 (2) 0.36238 (18) 0.0449 (9)
C2 0.7384 (8) 0.7691 (3) 0.4127 (2) 0.0593 (11)
H2 0.8007 0.8305 0.4150 0.071*
C3 0.8541 (9) 0.7039 (3) 0.4593 (2) 0.0620 (12)
H3 0.9943 0.7214 0.4933 0.074*
C4 0.7651 (9) 0.6127 (3) 0.4564 (2) 0.0677 (12)
H4 0.8457 0.5687 0.4880 0.081*
C5 0.5568 (8) 0.5873 (3) 0.4067 (2) 0.0576 (11)
H5 0.4955 0.5258 0.4051 0.069*
C6 0.4353 (7) 0.6519 (2) 0.35857 (17) 0.0420 (9)
C7 0.2192 (8) 0.6226 (3) 0.30670 (18) 0.0401 (8)
H7 0.1644 0.5604 0.3063 0.048*
C8 −0.1121 (7) 0.6487 (2) 0.21032 (17) 0.0374 (8)
C9 −0.2116 (7) 0.5583 (2) 0.20228 (18) 0.0438 (9)
H9 −0.1416 0.5117 0.2330 0.053*
C10 −0.4130 (8) 0.5373 (2) 0.14916 (18) 0.0478 (10)
H10 −0.4744 0.4762 0.1435 0.057*
C11 −0.5252 (7) 0.6058 (2) 0.10406 (17) 0.0388 (8)
C12 −0.4333 (7) 0.6963 (2) 0.11130 (18) 0.0411 (9)
H12 −0.5091 0.7430 0.0814 0.049*
C13 −0.2253 (7) 0.7167 (2) 0.16398 (16) 0.0377 (8)
C14 −0.1226 (10) 0.8111 (3) 0.1696 (2) 0.0551 (11)
Cl1 −0.7739 (2) 0.57835 (7) 0.03661 (5) 0.0559 (4)
N1 0.0994 (6) 0.67831 (19) 0.26123 (15) 0.0401 (7)
O1 0.4206 (7) 0.81007 (18) 0.31778 (16) 0.0676 (9)
H1 0.3521 0.7858 0.2807 0.101*
N2 −0.0365 (11) 0.8850 (3) 0.1707 (2) 0.0891 (14)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C1 0.046 (2) 0.052 (2) 0.0365 (19) 0.0027 (18) −0.0014 (17) −0.0068 (17)
C2 0.055 (3) 0.065 (3) 0.058 (2) 0.002 (2) −0.007 (2) −0.019 (2)
C3 0.047 (3) 0.087 (3) 0.052 (2) 0.009 (2) −0.006 (2) −0.023 (2)
C4 0.063 (3) 0.085 (3) 0.055 (3) 0.012 (2) −0.012 (2) 0.009 (2)
C5 0.060 (3) 0.060 (3) 0.053 (2) 0.002 (2) −0.005 (2) 0.011 (2)
C6 0.042 (2) 0.051 (2) 0.0330 (19) 0.0030 (17) 0.0059 (16) −0.0006 (17)
C7 0.043 (2) 0.0438 (19) 0.0341 (19) −0.0025 (17) 0.0024 (16) −0.0017 (16)
C8 0.043 (2) 0.0384 (19) 0.0312 (17) 0.0019 (16) −0.0005 (16) −0.0056 (15)
supporting information
sup-3 Acta Cryst. (2006). E62, o2417–o2418
C10 0.058 (3) 0.037 (2) 0.047 (2) −0.0044 (18) −0.0032 (19) −0.0090 (17)
C11 0.038 (2) 0.048 (2) 0.0307 (17) −0.0019 (16) 0.0021 (15) −0.0105 (15)
C12 0.044 (2) 0.043 (2) 0.0365 (19) 0.0077 (16) −0.0010 (16) −0.0006 (15)
C13 0.045 (2) 0.0328 (18) 0.0354 (18) −0.0020 (16) 0.0032 (16) −0.0031 (15)
C14 0.072 (3) 0.040 (2) 0.053 (2) −0.004 (2) −0.014 (2) 0.0040 (18)
Cl1 0.0512 (6) 0.0660 (7) 0.0501 (6) −0.0012 (5) −0.0110 (4) −0.0148 (5)
N1 0.0461 (18) 0.0398 (16) 0.0344 (15) 0.0001 (14) 0.0005 (14) −0.0037 (13)
O1 0.094 (2) 0.0423 (15) 0.0649 (19) −0.0081 (15) −0.0241 (17) −0.0048 (14)
N2 0.126 (4) 0.043 (2) 0.096 (3) −0.016 (2) −0.036 (3) 0.008 (2)
Geometric parameters (Å, º)
C1—O1 1.348 (4) C8—C9 1.387 (3)
C1—C2 1.381 (4) C8—C13 1.390 (3)
C1—C6 1.392 (4) C8—N1 1.412 (4)
C2—C3 1.370 (4) C9—C10 1.374 (4)
C2—H2 0.9300 C9—H9 0.9300
C3—C4 1.377 (4) C10—C11 1.380 (4)
C3—H3 0.9300 C10—H10 0.9300
C4—C5 1.370 (4) C11—C12 1.376 (3)
C4—H4 0.9300 C11—Cl1 1.725 (3)
C5—C6 1.392 (4) C12—C13 1.387 (3)
C5—H5 0.9300 C12—H12 0.9300
C6—C7 1.437 (5) C13—C14 1.443 (5)
C7—N1 1.275 (4) C14—N2 1.137 (5)
C7—H7 0.9300 O1—H1 0.8200
O1—C1—C2 117.9 (3) C9—C8—C13 118.0 (3)
O1—C1—C6 121.7 (3) C9—C8—N1 125.7 (3)
C2—C1—C6 120.4 (3) C13—C8—N1 116.3 (3)
C3—C2—C1 119.9 (4) C10—C9—C8 120.4 (3)
C3—C2—H2 120.1 C10—C9—H9 119.8
C1—C2—H2 120.1 C8—C9—H9 119.8
C2—C3—C4 120.8 (4) C9—C10—C11 120.8 (3)
C2—C3—H3 119.6 C9—C10—H10 119.6
C4—C3—H3 119.6 C11—C10—H10 119.6
C5—C4—C3 119.4 (4) C12—C11—C10 120.1 (3)
C5—C4—H4 120.3 C12—C11—Cl1 119.4 (2)
C3—C4—H4 120.3 C10—C11—Cl1 120.4 (2)
C4—C5—C6 121.2 (4) C11—C12—C13 118.8 (3)
C4—C5—H5 119.4 C11—C12—H12 120.6
C6—C5—H5 119.4 C13—C12—H12 120.6
C1—C6—C5 118.3 (3) C12—C13—C8 121.8 (3)
C1—C6—C7 122.0 (3) C12—C13—C14 118.5 (3)
C5—C6—C7 119.7 (3) C8—C13—C14 119.7 (3)
N1—C7—C6 122.7 (3) N2—C14—C13 176.7 (5)
N1—C7—H7 118.6 C7—N1—C8 122.2 (3)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O1—H1···N1 0.82 1.98 2.620 (4) 135
C10—H10···O1i 0.93 2.50 3.321 (4) 147