organic papers
o1060
Anwar Usmanet al. C28H19NO4 DOI: 10.1107/S1600536802015350 Acta Cryst.(2002). E58, o1060±o1061 Acta Crystallographica Section EStructure Reports
Online
ISSN 1600-5368
2-Benzoyl-3-(1,2-dioxo-2-phenylethyl)-3-phenyl-2-(pyridin-2-yl)oxirane
Anwar Usman,aIbrahim Abdul
Razak,aHoong-Kun Fun,a*
Suchada Chantrapromma,a²
Yun Li,bYan Zhangband
Jian-Hua Xub
aX-ray Crystallography Unit, School of Physics,
Universiti Sains Malaysia, 11800 USM, Penang, Malaysia, andbDepartment of Chemistry,
Nanjing University, Nanjing 210093, People's Republic of China
² Permanent address: Department of Chemistry, Faculty of Science, Prince of Sonkla University, Hat-Yai, Songkla 90112, Thailand.
Correspondence e-mail: hkfun@usm.my
Key indicators Single-crystal X-ray study T= 213 K
Mean(C±C) = 0.003 AÊ Rfactor = 0.066 wRfactor = 0.155
Data-to-parameter ratio = 17.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
In the title compound, C28H19NO4, the con®gurations of the substituents attached to the oxirane ring are conditioned by the sp3 states of the oxirane C atoms. In the packing, the molecules form zigzag molecular chains along thebdirection.
Comment
Photoinduced oxygenation reactions of indolizine derivatives have been investigated intensively in our previous study (Tian
et al., 2001). In continuation of that work, we have isolated the title compound, (I), which was obtained from the photo-oxy-genation reactions of 1,3-dibenzoyl-2-phenylindolizine. We report here an X-ray crystallographic analysis at 213 K of (I), which was undertaken to establish its conformation and stereochemistry.
The bond lengths and angles observed in (I) (Fig. 1) are within normal ranges (Allenet al., 1987). The values within the oxirane (O3/C9/C16) agree with those of a related structure studied previously (Krishnakumar et al., 2002), except for a slight elongation of the C9ÐC16 bond [1.509 (3) AÊ versus
1.488 (4) AÊ (Krishnakumar et al., 2002)] due to the bulky substituents attached at atoms C9 and C16. The con®gurations
Received 1 August 2002 Accepted 27 August 2002 Online 13 September 2002
Figure 1
of the substituents are conditioned by these twosp3 atoms. Except for the bond angles within the oxirane, the average bond angles subtended at atoms C9 and C16 are 117.2 and 116.8, respectively, while the two atoms are eclipsed, as
determined by the torsion angles C8ÐC9ÐC16ÐC17 = 1.4 (3)and C10ÐC9ÐC16ÐC22 = 4.7 (3).
In the dioxophenylethylene moiety (O1/O2/C1±C9), the two carbonyl groups form O1/C6/C7/C8 and O2/C7/C8/C9 planes. These two planes are twisted out of the phenyl ring by 11.7 (1) and 38.8 (1), respectively. The O2/C7/C8/C9 plane
and the C10±C15 phenyl ring attached at atom C9 form dihedral angles of 56.1 (1) and 63.6 (2), respectivley, with the
oxirane ring plane.
The pyridine ring (N1/C17±C21) attached at atom C16 is perpendicular to the oxirane ring plane, with a dihedral angle of 88.6 (2). The carbonyl group of the benzoyl moiety (O4/
C22±C28) attached at the same atom is twisted from its aromatic ring by an angle of 20.8 (1)and the O4/C16/C22/C23
plane makes a dihedral angle of 61.8 (2)with the oxirane ring
plane.
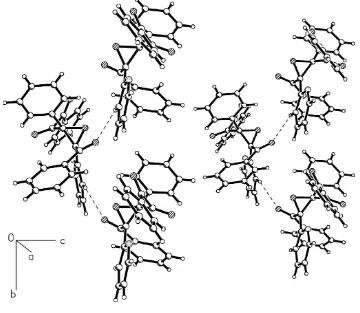
In the packing, the molecules are interconnected by C21Ð H21 O4i interactions [H21 O4i 2.56 AÊ and C21Ð H21 O4i129; symmetry code: (i) 1ÿx, yÿ1
2, 12ÿz] into zigzag molecular chains along thebdirection (Fig. 2). These interactions, along with the dipole±dipole and van der Waals interactions, stabilize the packing.
Experimental
The title compound was prepared by photoinduced oxygenation of 1,3-dibenzoyl-2-phenylindolizine in acetonitrile and was isolated by column chromatography. Single crystals for X-ray measurement were obtained by slow evaporation of the solvent from a petroleum ether± ethyl acetate (5:1v/v) solution.
Crystal data
C28H19NO4 Mr= 433.44
Monoclinic,P21/c a= 13.1513 (4) AÊ
b= 9.7358 (3) AÊ
c= 16.9960 (5) AÊ
= 95.859 (1)
V= 2164.8 (1) AÊ3 Z= 4
Dx= 1.330 Mg mÿ3
MoKradiation Cell parameters from 7367
re¯ections
= 2.6±28.3
= 0.09 mmÿ1 T= 213 (2) K Slab, colorless 0.380.340.16 mm
Data collection
Siemens SMART CCD area-detector diffractometer
!scans
12 406 measured re¯ections 5164 independent re¯ections 2808 re¯ections withI> 2(I)
Rint= 0.110
max= 28.3 h=ÿ10!17
k=ÿ12!12
l=ÿ22!22
Re®nement
Re®nement onF2 R[F2> 2(F2)] = 0.066 wR(F2) = 0.156 S= 0.79 5164 re¯ections 299 parameters
H-atom parameters constrained
w= 1/[2(F o2)]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001 max= 0.34 e AÊÿ3 min=ÿ0.28 e AÊÿ3
Extinction correction:SHELXTL
Extinction coef®cient: 0.021 (2)
Table 1
Selected geometric parameters (AÊ,).
O3ÐC9 1.434 (2) O3ÐC16 1.441 (2) O3ÐC9ÐC10 117.42 (16)
C10ÐC9ÐC16 120.90 (17) O3ÐC9ÐC8 113.57 (15) C10ÐC9ÐC8 114.73 (17) C16ÐC9ÐC8 119.33 (16)
O3ÐC16ÐC17 115.18 (15) C17ÐC16ÐC9 120.12 (17) O3ÐC16ÐC22 112.92 (16) C17ÐC16ÐC22 117.30 (17) C9ÐC16ÐC22 118.55 (16)
The H atoms were ®xed geometrically and were treated as riding on their parent C atoms, with CÐH = 0.93 AÊ and Uiso(H) =
1.2Ueq(C).
Data collection:SMART(Siemens, 1996); cell re®nement:SAINT
(Siemens, 1996); data reduction:SAINT and SADABS(Sheldrick, 1996); program(s) used to solve structure: SHELXTL (Sheldrick, 1997); program(s) used to re®ne structure: SHELXTL; molecular graphics:SHELXTL; software used to prepare material for publi-cation:SHELXTL, PARST (Nardelli, 1995) andPLATON(Spek, 1990).
The authors thank the Malaysian Government and Universiti Sains Malaysia for research grant R&D No. 305/ PFIZIK/610961. AU thanks Universiti Sains Malaysia for a Visiting Postdoctoral Fellowship.
References
Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987).J. Chem. Soc. Perkin Trans.2, pp. S1±19.
Krishnakumar, R. V., Subha Nandhini, M., Renuga, S., Natarajan, S., Selvaraj, S. & Perumal, S. (2002).Acta Cryst.E58, o504±o505.
Nardelli, M. (1995).J. Appl. Cryst.28, 659.
Sheldrick, G. M. (1996).SADABS. University of GoÈttingen, Germany. Sheldrick, G. M. (1997).SHELXTL.Version 5.1. Bruker AXS Inc., Madison,
Wisconsin, USA.
Siemens (1996).SMARTandSAINT. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Spek, A. L. (1990).Acta Cryst.A46, C-34.
Figure 2
supporting information
sup-1
Acta Cryst. (2002). E58, o1060–o1061
supporting information
Acta Cryst. (2002). E58, o1060–o1061 [doi:10.1107/S1600536802015350]
2-Benzoyl-3-(1,2-dioxo-2-phenylethyl)-3-phenyl-2-(pyridin-2-yl)oxirane
Anwar Usman, Ibrahim Abdul Razak, Hoong-Kun Fun, Suchada Chantrapromma, Yun Li, Yan
Zhang and Jian-Hua Xu
S1. Comment
Photoinduced oxygenation reactions of indolizine derivatives have been investigated intensively in our previous study
(Tian et al., 2001). In continuation of that work, we have isolated the title compound, (I), which was obtained from the
photo-oxygenation reactions of 1,3-dibenzoyl-2-phenylindolizine. We report here an X-ray crystallographic analysis at
213 K of (I), which was undertaken to establish its conformation and stereochemistry.
The bond lengths and angles observed in (I) (Fig. 1) are within normal ranges (Allen et al., 1987). The values within the
oxirane (O3/C9/C16) agree with those of a related structure studied previously (Krishnakumar et al., 2002), except for a
slight elongation of the C9—C16 bond [1.509 (3) Å versus 1.488 (4) Å (Krishnakumar et al., 2002)] due to the bulky
substituents attached at atoms C9 and C16. The configurations of the substituents are conditioned by these two Csp3
atoms. Except for the bond angles within the oxirane, the average bond angles subtended at atoms C9 and C16 are 117.2
and 116.8°, respectively, while the two atoms are eclipsed, as determined by the torsion angles C8—C9—C16—C17 =
1.4 (3)° and C10—C9—C16—C22 = 4.7 (3)°.
In the dioxophenylethylene moiety (O1/O2/C1–C9), the two carbonyl groups form O1/C6/C7/C8 and O2/C7/C8/C9
planes. These two planes are twisted out of the phenyl ring by 11.7 (1) and 38.8 (1)°, respectively. The O2/C7/C8/C9
plane and the C10–C15 phenyl ring attached at atom C9 form dihedral angles of 56.1 (1) and 63.6 (2)°, respectivley, with
the oxirane ring plane.
The pyridine ring (N1/C17–C21) attached at atom C16 is perpendicular to the oxirane ring plane, with a dihedral angle
of 88.6 (2)°. The carbonyl group of the benzoyl moiety (O4/C22–C28) attached at the same atom is twisted from its
aromatic ring by an angle of 20.8 (1)° and the O4/C16/C22/C23 plane makes a dihedral angle of 61.8 (2)° with the
oxirane ring plane.
In the packing, the molecules are interconnected by C21—H21···O4i interactions [H21···O4i 2.56 Å and C21—H21···O4i
129°; symmetry code: (i) 1 − x, y − 1/2, 1/2 − z] into zig-ag molecular chains along the b direction (Fig. 2). These
interactions, along with the dipole–dipole and van der Waals interactions, stabilize the packing.
S2. Experimental
The title compound was prepared by photoinduced oxygenation of 1,3-dibenzoyl-2-phenylindolizine in acetonitrile and
was isolated by by column chromatography. Single crystals for X-ray measurement were obtained by slow evaporation of
the solvent from a petroleum ether–ethyl acetate (5:1 v/v) solution.
S3. Refinement
The H atoms were fixed geometrically and were treated as riding on their parent C atoms, with C—H = 0.93 Å and
Figure 1
The structure of the title compound, showing 50% probability displacement ellipsoids and the atom-numbering scheme.
Figure 2
Part of packing of the title compound, viewed down the a axis, showing the zigzag molecular chains along the b
[image:4.610.126.486.349.662.2]supporting information
sup-3
Acta Cryst. (2002). E58, o1060–o1061
2-Benzoyl-3-(1,2-dioxo-2-phenylethyl)-3-phenyl-2-(pyridin-2-yl)oxirane
Crystal data
C28H19NO4 Mr = 433.44 Monoclinic, P21/c a = 13.1513 (4) Å
b = 9.7358 (3) Å
c = 16.9960 (5) Å
β = 95.859 (1)°
V = 2164.8 (1) Å3 Z = 4
F(000) = 904
Dx = 1.330 Mg m−3
Melting point: 416(1) K Mo Kα radiation, λ = 0.71073 Å Cell parameters from 7367 reflections
θ = 2.6–28.3°
µ = 0.09 mm−1 T = 213 K Slab, colorless 0.38 × 0.34 × 0.16 mm
Data collection
Siemens SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 8.33 pixels mm-1 ω scans
12406 measured reflections
5164 independent reflections 2808 reflections with I > 2σ(I)
Rint = 0.110
θmax = 28.3°, θmin = 2.6° h = −10→17
k = −12→12
l = −22→22
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.066 wR(F2) = 0.156 S = 0.79 5164 reflections 299 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F o2)]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.34 e Å−3
Δρmin = −0.28 e Å−3
Extinction correction: SHELXTL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.021 (2)
Special details
Experimental. The data collection covered over a hemisphere of reciprocal space by a combination of three sets of exposures; each set had a different φ angle (0, 88 and 180°) for the crystal and each exposure of 10 s covered 0.3° in ω. The crystal-to-detector distance was 4 cm and the detector swing angle was −35°. Crystal decay was monitored by repeating fifty initial frames at the end of data collection and analysing the intensity of duplicate reflections, and was found to be negligible.
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
O1 0.83526 (14) 0.90749 (16) 0.20400 (11) 0.0486 (5)
O2 0.92115 (12) 0.66777 (17) 0.33075 (9) 0.0361 (4)
O3 0.67590 (11) 0.71080 (14) 0.22032 (8) 0.0264 (4)
O4 0.49983 (11) 0.61336 (17) 0.27233 (10) 0.0392 (4)
N1 0.81182 (13) 0.49306 (18) 0.19663 (10) 0.0263 (4)
C1 0.9665 (2) 0.8357 (3) 0.09045 (15) 0.0440 (7)
H1 0.9237 0.9109 0.0797 0.053*
C2 1.0363 (2) 0.7997 (3) 0.03841 (17) 0.0566 (8)
H2 1.0387 0.8487 −0.0083 0.068*
C3 1.1024 (2) 0.6912 (3) 0.05578 (16) 0.0486 (7)
H3 1.1501 0.6685 0.0211 0.058*
C4 1.09795 (19) 0.6166 (3) 0.12394 (14) 0.0404 (6)
H4 1.1428 0.5438 0.1355 0.048*
C5 1.02657 (17) 0.6497 (2) 0.17546 (13) 0.0316 (5)
H5 1.0230 0.5983 0.2212 0.038*
C6 0.96051 (16) 0.7591 (2) 0.15902 (12) 0.0275 (5)
C7 0.88147 (17) 0.7990 (2) 0.21090 (13) 0.0294 (5)
C8 0.85547 (17) 0.7092 (2) 0.28065 (12) 0.0249 (5)
C9 0.74207 (15) 0.7085 (2) 0.29277 (11) 0.0210 (4)
C10 0.71388 (16) 0.7979 (2) 0.35897 (12) 0.0239 (5)
C11 0.76665 (17) 0.7884 (2) 0.43381 (12) 0.0304 (5)
H11 0.8193 0.7249 0.4434 0.037*
C12 0.7411 (2) 0.8733 (2) 0.49448 (14) 0.0377 (6)
H12 0.7761 0.8652 0.5447 0.045*
C13 0.6650 (2) 0.9688 (3) 0.48098 (15) 0.0437 (7)
H13 0.6489 1.0260 0.5218 0.052*
C14 0.6119 (2) 0.9804 (3) 0.40648 (16) 0.0464 (7)
H14 0.5600 1.0450 0.3974 0.056*
C15 0.63636 (18) 0.8950 (2) 0.34505 (14) 0.0361 (6)
H15 0.6010 0.9029 0.2950 0.043*
C16 0.67753 (15) 0.5858 (2) 0.26576 (12) 0.0216 (4)
C17 0.72551 (15) 0.4641 (2) 0.22897 (11) 0.0220 (4)
C18 0.85871 (17) 0.3884 (2) 0.16420 (13) 0.0306 (5)
H18 0.9178 0.4070 0.1403 0.037*
C19 0.82440 (17) 0.2545 (2) 0.16420 (13) 0.0324 (5)
H19 0.8609 0.1845 0.1427 0.039*
C20 0.73523 (18) 0.2268 (2) 0.19662 (13) 0.0339 (5)
H20 0.7098 0.1377 0.1968 0.041*
C21 0.68369 (17) 0.3330 (2) 0.22905 (12) 0.0281 (5)
H21 0.6224 0.3171 0.2504 0.034*
C22 0.57872 (15) 0.5610 (2) 0.30276 (12) 0.0243 (5)
C23 0.57974 (16) 0.4714 (2) 0.37314 (12) 0.0254 (5)
C24 0.66999 (18) 0.4470 (2) 0.42211 (13) 0.0328 (5)
H24 0.7316 0.4828 0.4090 0.039*
supporting information
sup-5
Acta Cryst. (2002). E58, o1060–o1061
H25 0.7271 0.3555 0.5230 0.054*
C26 0.5767 (2) 0.3136 (3) 0.50886 (15) 0.0474 (7)
H26 0.5755 0.2625 0.5550 0.057*
C27 0.4878 (2) 0.3332 (3) 0.45943 (16) 0.0441 (7)
H27 0.4272 0.2924 0.4712 0.053*
C28 0.48887 (19) 0.4135 (2) 0.39230 (14) 0.0364 (6)
H28 0.4286 0.4288 0.3599 0.044*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Geometric parameters (Å, º)
O1—C7 1.218 (3) C12—H12 0.9300
O2—C8 1.218 (3) C13—C14 1.387 (4)
O3—C9 1.434 (2) C13—H13 0.9300
O3—C16 1.441 (2) C14—C15 1.398 (3)
O4—C22 1.222 (2) C14—H14 0.9300
N1—C18 1.339 (3) C15—H15 0.9300
N1—C17 1.341 (2) C16—C17 1.507 (3)
C1—C2 1.383 (3) C16—C22 1.520 (3)
C1—C6 1.393 (3) C17—C21 1.390 (3)
C1—H1 0.9300 C18—C19 1.379 (3)
C2—C3 1.381 (4) C18—H18 0.9300
C2—H2 0.9300 C19—C20 1.372 (3)
C3—C4 1.374 (3) C19—H19 0.9300
C3—H3 0.9300 C20—C21 1.381 (3)
C4—C5 1.386 (3) C20—H20 0.9300
C4—H4 0.9300 C21—H21 0.9300
C5—C6 1.385 (3) C22—C23 1.479 (3)
C5—H5 0.9300 C23—C28 1.390 (3)
C6—C7 1.482 (3) C23—C24 1.399 (3)
C7—C8 1.539 (3) C24—C25 1.378 (3)
C8—C9 1.526 (3) C24—H24 0.9300
C9—C10 1.499 (3) C25—C26 1.379 (4)
C9—C16 1.509 (3) C25—H25 0.9300
C10—C11 1.389 (3) C26—C27 1.382 (4)
C10—C15 1.392 (3) C26—H26 0.9300
C11—C12 1.389 (3) C27—C28 1.385 (3)
C11—H11 0.9300 C27—H27 0.9300
C12—C13 1.368 (4) C28—H28 0.9300
C9—O3—C16 63.36 (12) C15—C14—H14 120.0
C18—N1—C17 116.97 (18) C10—C15—C14 119.8 (2)
C2—C1—C6 119.8 (2) C10—C15—H15 120.1
C2—C1—H1 120.1 C14—C15—H15 120.1
C6—C1—H1 120.1 O3—C16—C17 115.18 (15)
C3—C2—C1 120.1 (2) O3—C16—C9 58.09 (12)
C3—C2—H2 120.0 C17—C16—C9 120.12 (17)
C1—C2—H2 120.0 O3—C16—C22 112.92 (16)
C4—C3—C2 120.4 (2) C17—C16—C22 117.30 (17)
C4—C3—H3 119.8 C9—C16—C22 118.55 (16)
C2—C3—H3 119.8 N1—C17—C21 123.03 (19)
C3—C4—C5 120.0 (2) N1—C17—C16 114.36 (18)
C3—C4—H4 120.0 C21—C17—C16 122.60 (18)
C5—C4—H4 120.0 N1—C18—C19 123.7 (2)
C6—C5—C4 120.2 (2) N1—C18—H18 118.1
C6—C5—H5 119.9 C19—C18—H18 118.1
supporting information
sup-7
Acta Cryst. (2002). E58, o1060–o1061
C5—C6—C1 119.6 (2) C20—C19—H19 120.7
C5—C6—C7 123.0 (2) C18—C19—H19 120.7
C1—C6—C7 117.4 (2) C19—C20—C21 119.2 (2)
O1—C7—C6 122.9 (2) C19—C20—H20 120.4
O1—C7—C8 114.74 (19) C21—C20—H20 120.4
C6—C7—C8 122.26 (19) C20—C21—C17 118.5 (2)
O2—C8—C9 122.47 (18) C20—C21—H21 120.8
O2—C8—C7 121.9 (2) C17—C21—H21 120.8
C9—C8—C7 113.81 (18) O4—C22—C23 121.69 (19)
O3—C9—C10 117.42 (16) O4—C22—C16 118.90 (19)
O3—C9—C16 58.55 (12) C23—C22—C16 119.38 (17)
C10—C9—C16 120.90 (17) C28—C23—C24 119.3 (2)
O3—C9—C8 113.57 (15) C28—C23—C22 119.4 (2)
C10—C9—C8 114.73 (17) C24—C23—C22 121.21 (19)
C16—C9—C8 119.33 (16) C25—C24—C23 119.8 (2)
C11—C10—C15 119.31 (19) C25—C24—H24 120.1
C11—C10—C9 120.68 (18) C23—C24—H24 120.1
C15—C10—C9 120.0 (2) C24—C25—C26 120.6 (3)
C10—C11—C12 120.3 (2) C24—C25—H25 119.7
C10—C11—H11 119.9 C26—C25—H25 119.7
C12—C11—H11 119.9 C25—C26—C27 120.0 (2)
C13—C12—C11 120.5 (2) C25—C26—H26 120.0
C13—C12—H12 119.7 C27—C26—H26 120.0
C11—C12—H12 119.7 C26—C27—C28 120.0 (2)
C12—C13—C14 120.0 (2) C26—C27—H27 120.0
C12—C13—H13 120.0 C28—C27—H27 120.0
C14—C13—H13 120.0 C27—C28—C23 120.2 (2)
C13—C14—C15 120.1 (2) C27—C28—H28 119.9
C13—C14—H14 120.0 C23—C28—H28 119.9
C6—C1—C2—C3 −2.3 (4) C10—C9—C16—O3 105.3 (2)
C1—C2—C3—C4 1.3 (5) C8—C9—C16—O3 −101.19 (18)
C2—C3—C4—C5 0.3 (4) O3—C9—C16—C17 102.61 (18)
C3—C4—C5—C6 −0.9 (4) C10—C9—C16—C17 −152.14 (18)
C4—C5—C6—C1 −0.1 (4) C8—C9—C16—C17 1.4 (3)
C4—C5—C6—C7 179.1 (2) O3—C9—C16—C22 −100.58 (18)
C2—C1—C6—C5 1.7 (4) C10—C9—C16—C22 4.7 (3)
C2—C1—C6—C7 −177.6 (2) C8—C9—C16—C22 158.24 (18)
C5—C6—C7—O1 167.3 (2) C18—N1—C17—C21 −0.8 (3)
C1—C6—C7—O1 −13.4 (3) C18—N1—C17—C16 178.94 (18)
C5—C6—C7—C8 −9.4 (3) O3—C16—C17—N1 42.7 (2)
C1—C6—C7—C8 169.8 (2) C9—C16—C17—N1 −23.6 (3)
O1—C7—C8—O2 −125.2 (2) C22—C16—C17—N1 179.31 (17)
C6—C7—C8—O2 51.8 (3) O3—C16—C17—C21 −137.6 (2)
O1—C7—C8—C9 40.0 (3) C9—C16—C17—C21 156.13 (19)
C6—C7—C8—C9 −143.0 (2) C22—C16—C17—C21 −1.0 (3)
C16—O3—C9—C10 −111.15 (19) C17—N1—C18—C19 −1.6 (3)
O2—C8—C9—O3 −157.78 (19) C18—C19—C20—C21 −0.9 (3)
C7—C8—C9—O3 37.1 (2) C19—C20—C21—C17 −1.3 (3)
O2—C8—C9—C10 63.3 (3) N1—C17—C21—C20 2.2 (3)
C7—C8—C9—C10 −101.9 (2) C16—C17—C21—C20 −177.50 (19)
O2—C8—C9—C16 −91.9 (2) O3—C16—C22—O4 24.6 (3)
C7—C8—C9—C16 103.0 (2) C17—C16—C22—O4 −113.0 (2)
O3—C9—C10—C11 171.87 (17) C9—C16—C22—O4 89.6 (2)
C16—C9—C10—C11 103.9 (2) O3—C16—C22—C23 −157.05 (17)
C8—C9—C10—C11 −50.8 (2) C17—C16—C22—C23 65.4 (2)
O3—C9—C10—C15 −10.3 (3) C9—C16—C22—C23 −92.1 (2)
C16—C9—C10—C15 −78.3 (3) O4—C22—C23—C28 18.5 (3)
C8—C9—C10—C15 127.0 (2) C16—C22—C23—C28 −159.85 (18)
C15—C10—C11—C12 1.1 (3) O4—C22—C23—C24 −159.6 (2)
C9—C10—C11—C12 178.9 (2) C16—C22—C23—C24 22.0 (3)
C10—C11—C12—C13 −1.1 (4) C28—C23—C24—C25 −2.0 (3)
C11—C12—C13—C14 0.7 (4) C22—C23—C24—C25 176.1 (2)
C12—C13—C14—C15 −0.3 (4) C23—C24—C25—C26 1.5 (4)
C11—C10—C15—C14 −0.7 (3) C24—C25—C26—C27 0.7 (4)
C9—C10—C15—C14 −178.5 (2) C25—C26—C27—C28 −2.4 (4)
C13—C14—C15—C10 0.3 (4) C26—C27—C28—C23 1.9 (3)
C9—O3—C16—C17 −111.12 (19) C24—C23—C28—C27 0.3 (3)
C9—O3—C16—C22 110.36 (18) C22—C23—C28—C27 −177.82 (19)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C5—H5···O2 0.93 2.50 3.109 (3) 124
C11—H11···O2 0.93 2.51 3.050 (3) 117
C21—H21···O4i 0.93 2.56 3.223 (3) 129