R E S E A R C H A R T I C L E
Open Access
Effect of parathyroid hormone on early
chondrogenic differentiation from mesenchymal
stem cells
Yun Zhang, Ken Kumagai
*and Tomoyuki Saito
Abstract
Background:Treatment of articular cartilage injuries remains a difficult challenge due to the limited capacity for intrinsic repair. Mesenchymal stem cells (MSCs) can differentiate into chondrocytes under certain culture conditions. This study focused on the modulatory effects of parathyroid hormone (PTH) on chondrogenic differentiation from MSCs. Methods:MSCs were treated with various concentrations of PTH under chondrogenic pellet culture condition. RNA was isolated for real-time polymerase chain reaction (PCR) and gene expressions of collagen type IIα1 chain (Col2a1), collagen type Xα1 chain, collagen type Iα1 chain, SRY-box9 (Sox9), and type 1 PTH/PTHrP receptor (PTH1R) were examined. Chondrogenic differentiation was also evaluated by histological findings.
Results:PTH had opposite effects on chondrogenesis, depending on the concentration. A low to moderate concentration of PTH promoted chondrogenic differentiation of MSCs with increased expression of Sox9, Col2a1, and PTH1R, whereas chondrogenesis of MSCs was inhibited rather than stimulated with a higher concentration of PTH.
Conclusion:This study provides insights into the modulatory effect of PTH on chondrogenic differentiation from MSCs and the therapeutic potential for cartilage regeneration. Based on clinical experience regarding the efficacy and safety of PTH for bone metabolism, PTH may also be useful clinically for cartilage repair.
Keywords:Parathyroid hormone, Chondrogenic differentiation, Mesenchymal stem cells
Background
Treatment of articular cartilage injuries remains a difficult challenge due to the limited capacity for intrinsic repair. Tissue engineering approaches have been introduced as treatment options for cartilage repair. Treatment efficacy depends entirely on the cells in the grafted site, particu-larly the small subset of stem and progenitor cells that are capable of generating new tissue [1]. Thus, cell-based approaches are key to successful tissue engineering [2].
Mesenchymal stem cells (MSCs) are the most com-monly used cell source with a high self-renewal capacity, multilineage potential, and easy isolation from several human tissues including bone marrow [3,4]. MSCs can differentiate into chondrocytes under certain culture con-ditions [5,6] and have been used for cartilage regeneration
medicine by many researchers [7,8]. Therefore, a number of research efforts are directed to the isolation of progeni-tor cells and the understanding of the mechanisms in-volved in their chondrogenic differentiation.
Parathyroid hormone (PTH) is known as an 84-amino acid protein that regulates bone remodeling and calcium homeostasis. When PTH is administrated intermittently as a pharmacological agent, exogenous PTH has been shown to exert significant anabolic effects. Several stud-ies indicated that PTH(1–34) also affects chondrocyte. PTH(1–34) inhibits the terminal differentiation of ar-ticular chondrocytes and the progression of osteoarth-ritis (OA) [9,10]. In parallel with the suppression of chondrocyte hypertrophy, PTH(1–34) stimulates chon-drocyte proliferation and differentiation in the early stage [11-15]. However, the effects of PTH on chondro-genic differentiation of MSCs remain to be elucidated. We hypothesized that PTH promotes early chondro-genic differentiation from MSCs. Here, we show the * Correspondence:kumagai@yokohama-cu.ac.jp
Department of Orthopaedic Surgery and Muscloskeletal Science, Graduate School of Medicine, Yokohama City University, 3-9 Fukuura, Kanazawa-ku, Yokohama 236-0004, Japan
investigation of the modulatory effect of PTH on chon-drogenic differentiation from MSCs.
Materials and methods Culture of MSCs
Murine bone marrow-derived MSCs (Cyagen Biosciences, Santa Clara, CA, USA) were expanded in a monolayer culture with mesenchymal stem cell growth medium (GUXMX-90011, Cyagen Biosciences) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100μg/mL streptomycin, and glutamine at 37°C with 5% CO2 until
the cells reached 80% confluence. The cells were then trypsinized and frozen in liquid nitrogen for later use. After thawing and monolayer expansion, cells at passage 5 or 6 were harvested and subjected to pellet formation and chondrogenic differentiation.
Induction of chondrogenic differentiation and PTH administration
Pellets of 2.5 × 105MSCs were formed by centrifugation at 200 g for 5 min in 15-mL centrifuge tubes or 1.5-mL microcentrifuge tubes. After incubation at 37°C in 5% CO2 for 4 days, pellets were transferred to 96-well
U-bottomed plates. Cells were exposed to chondrogenic
medium (high-glucose DMEM with 0.1 μM
dexametha-sone, 0.17 mM ascorbic acid-2 phosphate, 5μg/mL insu-lin, 5μg/mL transferrin, 5 ng/ml selenous acid, 0.35 mM L-proline, 100 units/mL penicillin, and 100μg/mL strepto-mycin) supplemented with 10 ng/mL transforming growth factor-β3 (TGF-β3). To detect the concentration
depend-ency of PTH(1–34) treatment of TGF-β-driven
chon-drogenesis, TGF-β-enriched chondrogenic medium was
supplemented with different concentrations of PTH (0.1, 1, 10, and 100 nM). The medium was changed every 2 or 3 days. Chondrogenic pellets were harvested at 3, 7, or 21 days.
Histological analyses
After 3 weeks of culture, pellets were fixed overnight at 4°C in 4% paraformaldehyde solution, dehydrated with ethanol, washed with xylene, and embedded in paraffin. Sections at 5 μm thickness were cut from the paraffin blocks and mounted on glass slides. The sections were deparaffinized with xylene and ethanol prior to staining. To detect proteoglycan synthesis as an indicator of car-tilage production, the sections were stained with Alcian Blue according to the standard protocol. For immuno-histochemical staining of collagen type II, the sections were treated with 1 mg/ml hyaluronidase (Sigma, St. Louis, MO, USA) in PBS (pH 5.0) for 30 min at room temperature. After blocking nonspecific binding with 3% bovine serum albumin in PBS, rabbit anti-type II colla-gen antibody (Novus Biologicals, Littleton, CO, USA) was incubated overnight at 4°C. The next day, slides
were washed in PBS and incubated with biotinylated anti-rabbit IgG antibody for 45 min at room temperature. Re-action was visualized by incubation with the avidin-biotin-peroxidase reagent included in the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA) followed by
color development with 3-3′
diaminobenzidinetetrahy-drochloride (Dojindo, Kumamoto, Japan). Finally, the sec-tions were counterstained with hematoxylin and mounted with coverslips. Cartilage tissue from mouse knee joint was used for control staining of collagen type II. Normal rabbit IgG was used as an isotype control.
Western blot analysis
For total protein extraction, pellets were homogenized and incubated with lysis buffer containing 20 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM
EGTA, 1% NP-40, 0.1% sodium dodecyl sulfate, 1 mM Na3VO4, and protease inhibitor cocktail (Nacalai tesque,
Kyoto, Japan) for 30 min on ice and centrifuged at 15,000 rpm for 20 min at 4°C. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel elec-trophoresis (SDS-PAGE) and transferred onto a polyvi-nylidene fluoride (PVDF) membrane. The membranes were incubated overnight at 4°C with rabbit primary antibodies against type 1 PTH/PTHrP receptor (PTH1R) (LifeSpan Biosciences, Seattle, WA, USA), SRY-box9 (Sox9) (Millipore, Temecula, CA, USA), Runt-related transcription factor 2 (Runx2) (Novus Biologicals,
Little-ton, CO, USA) and β-actin (Novus Biologicals). The
membranes were washed and incubated with horserad-ish peroxidase labeled anti-rabbit IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) for 60 min at room temperature. After a washing step, bands were visualized by ECL Prime Western blotting detection re-agent (GE Healthcare, Piscataway, NJ, USA) and ana-lyzed using a luminescent image analyzer equipped with a cooled CCD camera (LAS 1000, Fujifilm, Tokyo, Japan).
Total RNA isolation and RT-PCR
Total RNA was isolated from homogenized pellets using Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was quantified by measuring absorbance at 260 nm, and the quality was assessed by determining the 260/280 nm absorbance ratio. First-strand cDNA synthesis was per-formed with 0.5μg or 1μg total RNA in a total volume of 20 μl using an iScript™ advanced cDNA synthesis kit (BIO-RAD, Richmond, CA, USA). Gene expressions of collagen type II α1 chain (Col2a1), collagen type X α1 chain (Col10a1), collagen type Iα1 chain (Col1a1), Sox9, and PTH1R were examined with quantitative real-time PCR. The primers used in this study were listed in Table 1. Quantitative real-time PCR was carried out
on a CFX96™ real-time PCR detection system (BIO-RAD) in a 20-μl reaction volume. Expression of gene of interest was normalized to GAPDH expression.
Statistical analysis
All experiments were repeated at least three independ-ent times. All data are presindepend-ented as the mean ± SEM. The analysis was done using SigmaStat 3.5 software (Systat Software Inc., Richmond, CA, USA). The non-parametric Kruskal-Wallis test was used to test for significant differences among the test groups. When a
significant difference was detected, Steel's post hoc test was performed to compare each of the treatments with a control. An adjusted P value < 0.05 was considered sta-tistically significant.
Results
Histological findings
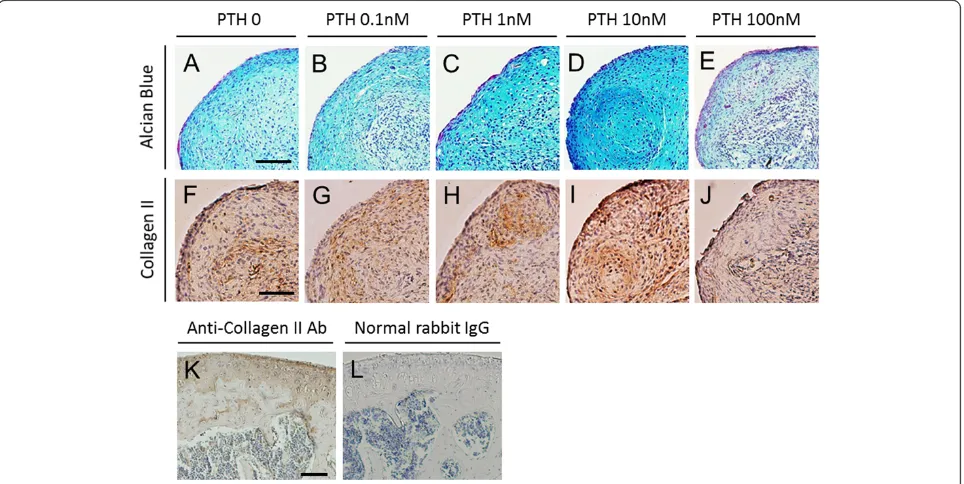
Chondrogenic differentiation was confirmed with Alcian Blue staining for proteoglycan synthesis. Positive staining with Alcian Blue was identified in all treatment groups (Figure 1A,B,C,D,E). Among the groups, stronger stain-ing was observed with 1 and 10 nM PTH, whereas less intense staining was seen with 100 nM PTH. Regarding cellular morphology, sections from cells treated with 10 nM PTH exhibited more chondrocyte-like cells with large round nuclei than cells treated with 100 nM PTH. To further address chondrogenic differentiation, we examined the deposition of type II collagen, which is a major component of the cartilage extracellular matrix (Figure 1F,G,H,I,J). Expression of type II collagen was partially localized in 0 nM PTH control. Improved expression was found in 10 nM PTH. In contrast, almost negative expression was shown in 100 nM PTH.
Effect of PTH on protein expression in chondrogenic differentiation
Protein expressions of PTH1R, Sox9, and Runx2 were de-tected by Western blotting (Figure 2). Positive expression
Table 1 Primers used for real-time RT-PCR
Gene Direction Sequences (5′–3′)
Sox9 Forward TACGACTGGACGCTGGTGCC
Sox9 Reverse CCGTTCTTCACCGACTTCCTCC
Col2a1 Forward CTGACCTGACCTGATGATACC
Col2a1 Reverse CACCAGATAGTTCCTGTCTCC
Col10a1 Forward CGAGGTATGCTTGATCTG
Col10a1 Reverse GACAGTCCAGTTCTTCAT
Col1a1 Forward TGACTGGAAGAGCGGAGAGT
Col1a1 Reverse TCTCTCCAAACCAGACGTGC
PTH1R Forward CTCCTTCTCTGCTGCCCAGT
PTH1R Reverse TGCTGTGTGCAGAACTTCCT
GAPDH Forward TGAAGCAGGCATCTGAGGG
GAPDH Reverse CGAAGGTGGAAGAGTGGGAG
of PTH receptor was confirmed in 0−10 nM PTH, whereas the intensity was remarkably reduced in 100 nM PTH. Protein expression of Sox9, a master regulator of chondrogenesis, was identified 3 weeks after chondro-genic differentiation from MSCs. Strong bands were present in 1 and 10 nM PTH and less intense one in 100 nM. Runx2, a transcription factor that promotes chondrocyte hypertrophy, was not intensely expressed in any PTH concentration.
Effect of PTH on collagen expression in early stage of chondrogenic differentiation
To determine whether PTH modulates the early stage of chondrogenic differentiation of MSCs, gene expression of type II collagen as a marker of chondrogenesis was analyzed when cells were treated with various doses of PTH (Figure 3A). Relative mRNA expression of Col2a1 was significantly reduced with 100 nM PTH at days 3, 7, and 21 (P< 0.05, vs. 0 nM PTH). In contrast, treatment with 1 and 10 nM PTH resulted in significantly in-creased expression of Col2a1 at days 7 and 21 (P< 0.05, vs. 0 nM PTH). To assess the phenotypic change of hypertrophy during chondrogenesis of MSCs, gene ex-pression of type X collagen as a marker of chondrogenic hypertrophy was also examined (Figure 3B). Relative mRNA expression of Col10a1 was not significantly chan-ged with different doses of PTH at days 3, 7, and 21. To further characterize the effect of PTH on chondrogenic differentiation of MSCs, gene expression of type I collagen as a marker of hypertrophic or osteogenic differentiation was investigated (Figure 3C). We observed no significant difference in relative mRNA expression levels of Col1a1 at days 3, 7, and 21 among the various dose groups.
Effect of PTH on activation of Sox9 and PTH receptor during chondrogenic differentiation of MSCs
To assess the effect of PTH on the key transcription factor involved in chondrogenic differentiation, gene expression
of Sox9 was analyzed (Figure 4A). Relative mRNA expres-sion of Sox9 was significantly diminished in the presence of 100 nM PTH after days 3 and 21 (P< 0.05, vs. 0 nM PTH). In contrast, significantly increased levels of Sox9 were seen with 0.1 nM PTH at day 7, and 1 and 10 nM PTH at days 7 and 21 (P< 0.05, vs. 0 nM PTH). These ex-pression patterns of Sox9 among the various doses were similar to those of Col2a1. To determine if the receptor is upregulated in response to PTH administration, gene expression of the PTH receptor was examined (Figure 4B). Relative mRNA expression of PTH1R was significantly
Figure 2Effect of PTH on protein expression in chondrogenic pellet culture for 21 days.Expression of PTH1R, Sox9, and Runx2 in various concentrations of PTH was analyzed using Western blotting.
decreased with 100 nM PTH at days 3, 7, and 21 (P< 0.05, vs. 0 nM PTH). In contrast, PTH1R was significantly in-creased with 1 and 10 nM PTH at days 7 and 21 (P< 0.05, vs. 0 nM PTH). The expression patterns for PTH1R were very similar to those for Sox9 and Col2a1.
Discussion
The present study demonstrated that chondrogenic differ-entiation of MSCs was modulated by PTH. The results revealed that PTH has opposite effects on chondrogenesis when administered at different concentrations. Namely, low to moderate concentrations of PTH promoted chon-drogenic differentiation of MSCs, whereas chondrogenesis of MSCs was inhibited and not stimulated by a higher concentration of PTH.
This study was intended to test the hypothesis that PTH has a stimulatory effect on chondrogenic differenti-ation. An effect on induction of chondrogenesis with in-creased collagen type II was previously confirmed using a single concentration of PTH in growth plate chondro-cytes [13] and MSCs from osteoarthritis patients [15]. In contrast, the inhibitory function of PTH on chondrocyte hypertrophy has been shown with reduced expression of
collagen type X under conditions that promote chondro-genic differentiation [15,16]. The constitutive expression of the PTH/PTHrP receptor in a bone morphogenetic protein-dependent differentiation system leads to a marked stimulation of chondrogenic and osteogenic development, whereas permanent application of the ligand PTH(1–34) results in opposite responses by stimulating the early and suppressing the late stages of osteo-/chondrogenic devel-opment [17]. These contrasting effects of PTH(1–34) on osteogenic and chondrocytic development seem to depend on the cellular state of differentiation. Our results were partially consistent with those previous reports. However, different from previous reports, we observed that expres-sion of both Col2a1 and PTH1R was suppressed by a higher concentration of PTH. To our knowledge, no supportive studies have been published showing that the response to PTH during chondrogenic differentiation is opposite depending on a lower or higher concentration. Therefore, the modulatory effect of PTH on chondrogenic differentiation is likely to remain controversial.
PTH and PTHrP show homology in the amino-terminal (1–34) peptide fragments with high-affinity binding to PTH1R. Biological responses elicited by either ligand through this common PTH1R are largely indistin-guishable, at least with regard to mineral ion homeosta-sis [18]. According to Weiss and colleagues [19], adding 0.1 ng/mL of PTHrP beginning on day 21 could sup-press collagen type X deposition without any negative effects on chondrogenic differentiation, whereas higher concentrations (10 or 100 ng/mL) or earlier treatment (from day 0) would lead to the suppression of chondro-genesis. These contradictory effects of PTHrP on chon-drogenic differentiation seem to be applicable to PTH on the basis of high similarity in the biological function [20]. Physicians may be interested in PTH rather than PTHrP because the former is currently available for clin-ical application. Therefore, several issues regarding the efficacy of PTH administration for successful cartilage repair need to be investigated further, including optimiza-tion of the concentraoptimiza-tion, treatment timing, and delivery method. The advantage of this study is that the investiga-tions included a concentration-response range and exam-ination of changes in expression of the exact genes.
The transcription factor Sox9 has been demonstrated to be a master regulator of the differentiation of mesenchy-mal cells into chondrocytes [21,22]. The TGF-β signal plays an essential role to induce primary chondrogenesis [4,23], which is mediated by up-regulation of Sox9 [24]. Furthermore, Sox9 is a target of PTH/PTHrP receptor sig-naling to maintain the chondrocyte phenotype and inhibit their maturation to hypertrophic chondrocytes in the growth plate [25]. Our results support the idea of a PTH/ PTHrP receptor signal-dependent increase in Sox9 ex-pression during chondrogenic differentiation from MSCs.
This study has several limitations including the concentration and timing for administration of PTH. Physiological PTH concentrations are much lower than those used in this study. However, in a number of in vitro studies examining the efficacy of PTH, the con-centration tested is usually out of therapeutic ranges [9,12,13,15,16]. The response to PTH administration increases in a concentration-dependent manner, and the minimum effective concentration is higher than physio-logical levels [13,26]. Therefore, we have chosen concen-trations that significantly altered the cellular response and that showed the expected efficacy. The ability to reflect clinical relevance with cell culture models is diffi-cult due to the lack of physiological conditions once cells are isolated from tissues and organs. The question is whether the output response is supportive of our understanding of the biology that will lead to decisions regarding translation of appropriate concentrations for assessment in human clinical testing. Furthermore, the relationship between the timing of exposure and the effi-cacy is unclear because PTH was continuously adminis-tered throughout the experimental period. For clinical use, PTH is intermittently administered when utilized for bone anabolic effects. Whether intermittent adminis-tration rather than continuous adminisadminis-tration is effective for cartilage induction remains to be determined. This point is especially important for direct administration for therapeutic use. Further studies are required to ex-trapolate the translatable efficacy and safety in humans. For current therapeutic application, indirect treatment of human organ systemsex vivo, such as treatment prior to cell implantation, seems rational.
Conclusions
This study provides insight into the modulatory effect of PTH on chondrogenic differentiation from MSCs. Ideal repair of injured cartilage involves replacement with hya-line cartilage and prevention of osteoarthritic changes. Several animal studies have shown that PTH has thera-peutic potential for cartilage regeneration and protection as well as inhibition of progression of osteoarthritis [9,10,14]. PTH(1–34) has a stimulatory effect on bone formation with intermittent administration and is cur-rently used as an anabolic drug for treatment of osteo-porosis. Based on clinical experience with the efficacy and safety of PTH for bone metabolism, PTH may also be clinically useful for cartilage repair.
Competing interests
The authors declare that they have no competing interests.
Authors’contributions
YZ, KK, and TS conceived and developed the study design. YZ and KK performed data acquisition and analysis. YZ and KK drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Kimi Ishikawa for the help with sample preparation. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (#25462347).
Received: 8 March 2014 Accepted: 18 July 2014 Published: 1 August 2014
References
1. Muschler GF, Midura RJ:Connective tissue progenitors: practical concepts for clinical applications.Clin Orthop Relat Res2002,395:66–80.
2. Muschler GF, Nakamoto C, Griffith LG:Engineering principles of clinical cell-based tissue engineering.J Bone Joint Surg Am2004,86-A:1541–1558. 3. Caplan AI:Mesenchymal stem cells.J Orthop Res1991,9:641–650. 4. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD,
Moorman MA, Simonetti DW, Craig S, Marshak DR:Multilineage potential of adult human mesenchymal stem cells.Science1999,284:143–147. 5. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU:In vitro
chondrogenesis of bone marrow-derived mesenchymal progenitor cells.
Exp Cell Res1998,238:265–272.
6. Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ:Comparison of effect of BMP-2,−4, and−6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma.Cell Tissue Res2005,320:269–276. 7. Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A:
Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering.J Cell Physiol2010,222:23–32.
8. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M:Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees.
Osteoarthritis Cartilage2002,10:199–206.
9. Chang JK, Chang LH, Hung SH, Wu SC, Lee HY, Lin YS, Chen CH, Fu YC, Wang GJ, Ho ML:Parathyroid hormone 1–34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats.Arthritis Rheum2009,60:3049–3060.
10. Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, Bukata SV, O'Keefe RJ, Awad H, Puzas JE, Rosier RN, Zuscik MJ:Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis.Sci Transl Med
2011,3:101ra193.
11. Harrington EK, Coon DJ, Kern MF, Svoboda KK:PTH stimulated growth and decreased Col-X deposition are phosphotidylinositol-3,4,5 triphosphate kinase and mitogen activating protein kinase dependent in avian sterna.
Anat Rec (Hoboken)2010,293:225–234.
12. Harrington EK, Roddy GW, West R, Svoboda KK:Parathyroid hormone/ parathyroid hormone-related peptide modulates growth of avian sternal cartilage via chondrocytic proliferation.Anat Rec (Hoboken)2007,
290:155–167.
13. Ishikawa Y, Wu LN, Genge BR, Mwale F, Wuthier RE:Effects of calcitonin and parathyroid hormone on calcification of primary cultures of chicken growth plate chondrocytes.J Bone Miner Res1997,12:356–366. 14. Kudo S, Mizuta H, Takagi K, Hiraki Y:Cartilaginous repair of full-thickness
articular cartilage defects is induced by the intermittent activation of PTH/PTHrP signaling.Osteoarthritis Cartilage2011,19:886–894. 15. Mwale F, Yao G, Ouellet JA, Petit A, Antoniou J:Effect of parathyroid
hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients.Tissue Eng Part A2010,16:3449–3455. 16. Zerega B, Cermelli S, Bianco P, Cancedda R, Cancedda FD:Parathyroid
hormone [PTH(1–34)] and parathyroid hormone-related protein [PTHrP(1–34)] promote reversion of hypertrophic chondrocytes to a prehypertrophic proliferating phenotype and prevent terminal differentiation of osteoblast-like cells.J Bone Miner Res1999,14:1281–1289.
17. Hollnagel A, Ahrens M, Gross G:Parathyroid hormone enhances early and suppresses late stages of osteogenic and chondrogenic development in a BMP-dependent mesenchymal differentiation system (C3H10T1/2).
J Bone Miner Res1997,12:1993–2004.
18. Mannstadt M, Juppner H, Gardella TJ:Receptors for PTH and PTHrP: their biological importance and functional properties.Am J Physiol1999,
277:F665–F675.
20. Zhang W, Chen J, Zhang S, Ouyang HW:Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair.Arthritis Res Ther2012,14:221.
21. Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B:The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6.Genes Dev2002,16:2813–2828.
22. Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B:Sox9 is required for cartilage formation.Nat Genet1999,22:85–89. 23. Heng BC, Cao T, Lee EH:Directing stem cell differentiation into the
chondrogenic lineage in vitro.Stem Cells2004,22:1152–1167. 24. Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H:Smad3 induces
chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment.J Biol Chem2005,280:8343–8350. 25. Huang W, Chung UI, Kronenberg HM, de Crombrugghe B:The
chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones.Proc Natl Acad Sci U S A2001,98:160–165.
26. Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, Votta B, Stroup GB, Kumar S, Nuttall ME:Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells.Bone2006,39:1361–1372.
doi:10.1186/s13018-014-0068-5
Cite this article as:Zhanget al.:Effect of parathyroid hormone on early chondrogenic differentiation from mesenchymal stem cells.Journal of Orthopaedic Surgery and Research20149:68.
Submit your next manuscript to BioMed Central and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution