Neurologic Status of Newborns With Congenital Heart Defects Before
Open Heart Surgery
Catherine Limperopoulos, OT, MSc*; Annette Majnemer, OT, PhD*‡; Michael I. Shevell, MD, CM, FRCP(C)‡\; Bernard Rosenblatt, MD, CM, FRCP(C)*‡\; Charles Rohlicek, MD, CD, PhD, FRCP(C)\; and Christo Tchervenkov, MD, CM, FRCS(C)§
ABSTRACT. Controversy exists regarding the integrity of the nervous system in the newborn with a congenital heart defect who must undergo corrective or palliative open heart surgery. Neurodevelopmental sequelae have been primarily attributed to surgical procedures without standardized evaluation of the preoperative neurologic status.
Objective. To determine whether newborns with con-genital heart defects demonstrate abnormalities in neu-robehavioral status before surgery.
Study Design. In this prospective study, a standard-ized neonatal neurobehavioral assessment and a neuro-logic examination were conducted independently in a consecutive series of 56 neonates referred to our hospital for investigation of open heart surgery.
Results. Neurobehavioral and neurologic abnormali-ties were documented in greater than half of the cohort and included hypotonia, hypertonia, jitteriness, motor asymmetries, and absent suck. Poor state regulation (62%) and feeding difficulties (34%) also were commonly observed. Furthermore, 3 subjects had seizures, 35.7% were microcephalic, and 12.5% were macrocephalic. The overall likelihood of neurobehavioral abnormalities was not enhanced by indicators of cardiorespiratory compro-mise. Interestingly, newborns with acyanotic congenital heart defects were more likely to demonstrate neurologic compromise than were those with cyanotic defects.
Conclusions. Findings suggest that the prevalence of neurobehavioral abnormalities before surgery in new-borns with congenital heart defects has been underap-preciated and would indicate that factors other than in-traoperative procedures should be considered in the genesis of brain injury in this population. Pediatrics
1999;103:402– 408;congenital heart defects, neurologic ex-amination, newborn.
ABBREVIATIONS. CHD, congenital heart defects; CPB, cardio-pulmonary bypass; CNS, central nervous system; ENNAS, Ein-stein Neonatal Neurobehavioral Assessment Scale; NICU, neona-tal intensive care unit; OR, odds ratio; SD standard deviation.
A
congenital heart defect (CHD) is defined as a gross structural abnormality of the heart or of the major thoracic vessels.1The incidence ofCHD is estimated to be between 5 and 8 per 1000 live births, and many will require surgical interven-tions.2,3 Recent improvements in diagnostic testing
and surgical techniques have enabled early correc-tion or palliacorrec-tion of most complex CHD in infancy, resulting in dramatic reduction in mortality.4 – 6
Sur-gical intervention early in life often is necessary in infants with CHD because of persistent cardiac fail-ure or hypoxemia.7,8Cardiopulmonary bypass (CPB)
and deep hypothermia coupled with total circulatory arrest (DHCA) are two surgical techniques that allow accurate repair of the heart in a bloodless, motionless operative field.7,9 Considerable controversy exists
with respect to neurologic morbidity, particularly with reference to intraoperative events such as CPB versus deep hypothermia coupled with total circula-tory arrest.5,9 –13
The integrity of the developing nervous system is influenced by a complex interaction between preop-erative, intraoppreop-erative, and postoperative factors in children with CHD. Few studies to date have for-mally evaluated children with CHD preoperatively. One study14 reported that 10 of 12 subjects had
ab-normal neurologic status before surgery. In a second study describing preoperative neurologic status on 36 subjects, 5 children had microcephaly, 4 were hemiparetic, and 1 was hypotonic. Similarly, Brun-berg and associates7found that only 6 of 21 subjects
assessed were normal neurologically. Psychomotor delay was present in 62%, hypotonia in 43%, and seizures and motor asymmetry in 1 patient. New-burger and colleagues9 reported suspect or definite
neurologic abnormalities in 60% before surgery. Miller and colleagues15reported on the perioperative
neurologic findings of 91 infants and noted reduced levels of alertness (19%), seizures (15%), and severe hypotonia (11%) before surgery.
Only one study has described the neurobehavioral status of newborns with CHD. Gillon16summarized
her observations in the context of her nursing care of 82 newborns before surgery. Respiratory difficulties and poor coordination of suck, swallow, and breath-ing were often documented. Neurologic findbreath-ings in-cluded tone abnormalities, abnormal posturing and activity level, weak cry, and poor auditory and visual orienting.
From the *School of Physical and Occupational Therapy, and the Depart-ments of ‡Neurology and Neurosurgery, §Surgery, and\Pediatrics, McGill University, Montreal Children’s Hospital, Quebec, Canada.
Received for publication Nov 26, 1997; accepted Aug 13, 1998.
Neuroimaging and neuropathologic studies have provided evidence that central nervous system (CNS) injury or malformation already may be appar-ent preoperatively. Congenital malformations of the CNS may co-exist in a subset of individuals with CHD.17,18In magnetic resonance imaging studies of
children with CHD, malformations such as callosal agenesis, abnormal neuronal migration, temporal lo-bar hypoplasia, and Chiari type I malformation have been reported.19,20 Two recent studies reported on
ultrasounds in term newborns with CHD, and found that 55% to 59% had findings including cerebral at-rophy and linear echodensities in the basal ganglia and thalamus.15,21 Human neuropathologic studies
have demonstrated acquired CNS injury before sur-gery. Brain injury primarily involving the white mat-ter may be of intraumat-terine or perinatal origin, or a genetic aberration.22–24 These findings in part may
account for an increased frequency of microcephaly.25
Postoperative neurologic complications continue to be reported in the pediatric literature.15,26
Devel-opmental sequelae include cerebral palsy; seizure disorders; and cognitive, language, behavioral, and social problems.27–32 These neurodevelopmental
se-quelae may be caused not only by intraoperative events, but by preoperative brain abnormalities as a result of cardiac-related stresses17 (eg, chronic
hyp-oxia, congestive heart failure, cardiac arrest, failure to thrive) and diagnostic or therapeutic procedures (eg, cardiac catheterization, balloon-atrial septos-tomy).
There is increasing evidence to suggest that new-borns with CHD are at high risk for neurologic ab-normalities; however, there is a paucity of studies that have described the baseline neurologic status of newborns with CHD using objective clinical assess-ments of CNS integrity. The primary aim of our study was to examine in a standardized and system-atic manner the neurologic status of newborns with CHD before open heart surgery. Therefore, we hy-pothesized that a proportion of newborns with CHD will manifest neurologic abnormalities before sur-gery.
METHODOLOGY
This study is the first phase of an ongoing prospective study that is examining the neurodevelopmental status of young chil-dren with CHD before and after open heart surgery.33This project was scientifically and ethically approved by the hospital’s Scien-tific Review Committee and the Institutional Review Board. A consecutive series of newborns referred to the Division of Cardi-ology at the Montreal Children’s Hospital for investigation of CHD were recruited (fall 1994 to spring 1997). Neurologic, neu-robehavioral, and cardiorespiratory assessments were performed within the first month of life before cardiac surgery.
Subjects
Inclusion criteria were 1) gestational age.36 weeks, and 2) a diagnosis of a CHD requiring palliative or corrective open heart surgery. All subjects had CPB6circulatory arrest during surgery. Subjects were excluded because of 1) hypoplastic left heart syn-drome (because of the higher prevalence of neurologic morbidity in this subgroup than in others);342) closed surgical procedures; 3) a language barrier (ie, parents who did not speak either English or French); 4) known extracardiac anomalies involving the CNS (eg, Down syndrome); or 5) known insult to the CNS (eg, perinatal asphyxia) not directly attributed to the heart defect. Criteria for
perinatal asphyxia included criteria established by the American Academy of Obstetrics and Gynecology and the American Acad-emy of Pediatrics35and included 1) profound metabolic or mixed acidosis (pH,7.10); 2) Apgar scores of#3 at 5 or more minutes; 3) clinical evidence of neonatal encephalopathy; and 4) evidence of multiorgan dysfunction in the immediate neonatal period. It should be noted that CNS insults or malformations were limited to those recognized clinically (by neonatology or cardiology attend-ing physicians) by physical examination and by neuroimagattend-ing. Furthermore, patients with clinically recognized syndromes that are associated with developmental disability (and confirmed by genetic testing) were excluded. Therefore the population of inter-est was newborns with CHD who were not known to have any disorder or impairment of the CNS attributable to causes other than complications of the CHD at the time of testing.
Procedures
Once informed parental consent was obtained, the subjects were assessed within the newborn period (ie, first month of life). The Einstein Neonatal Neurobehavioral Assessment Scale (EN-NAS) was performed by an occupational therapist. Data from 37 healthy control subjects obtained for a previous study36were used to compare performance between subjects with CHD and full-term, healthy newborns. The control group was recruited at a well-baby nursery and were term neonates (.36 weeks’ gesta-tional age) with an Apgar score of 8 to 10 at 5 minutes, a birth weight appropriate for age, and no history of prenatal or perinatal complications.
One of two pediatric neurologists examined the subjects, and a pediatric cardiologist reviewed the medical charts to document cardiorespiratory status at the time of assessment. All inpatients were assessed in the neonatal intensive care unit (NICU). All subjects were evaluated clinically by a neurologist and/or an occupational therapist who were blinded to the cardiac diagnosis and to each other’s clinical findings.
Neurobehavioral Assessment
The ENNAS is a formal neurologic assessment that evaluates neurologic status and behavioral organization of the newborn infant. Standardized procedures and methods are used in per-forming each individual item; however, the order of presentation of each item is such that state is optimized. The areas evaluated include muscle tone, passive and active movements, primitive reflexes, and responses to visual and auditory stimuli.37 The ENNAS comprises 20 individual items and 4 summary items and takes 20 to 30 minutes to administer. Interrater reliability has been determined to be 0.97 by Kurtzberg and co-workers.38Each item is scored on an ordinal scale sequenced from minimum to maximum response. Cut-offs for normal/abnormal for each item is based on established criteria with the suspect or “mildly abnormal” rating recently being added (C. McCarton, personal communication, 1994). A deviant score (ie, number of items failed) between 0 and 2 is considered normal, whereas a deviant score between 3 and 6 is a suspect examination and greater than 6 is considered abnor-mal. Neonates with “suspect” findings demonstrate subtle or mild abnormalities (eg, mildly hypotonic, poor orienting responses) and would be considered clinically abnormal. For the purpose of analysis, suspect or abnormal examinations were clustered as abnormal. This test discriminates well among groups of healthy and high-risk newborns.36Two prospective studies have exam-ined the predictive value of the ENNAS in preterm and full-term high-risk newborns. The ENNAS had very good negative predic-tive value and sensitivity (83% to 100%) for cognipredic-tive and motor performance at school entry.39 In particular, the auditory and visual composite scores correlated with intelligence quotients at 6 years.40
Neurologic Examination
A pediatric neurologist examined all subjects based on criteria outlined by Volpe.41The examination took;15 minutes and in-cluded assessment of head circumference, muscle bulk and tone, cranial nerves, deep tendon reflexes, and activity level and the presence of any abnormal movement patterns such as posturing or tremor. Subjects were categorized as normal or abnormal.
Cardiorespiratory Status
A pediatric cardiologist reviewed the medical chart of each subject on the day of assessment and documented the transcuta-neously measured arterial oxygen saturation, respiratory rate, whether newborns were intubated, medications in use, and the presence of cyanotic/acyanotic lesions and/or congestive heart failure. The cardiologist also recorded whether the subjects were evaluated as in- or outpatients because, presumably, outpatients would be more stable from a cardiorespiratory viewpoint. These parameters were used as indicators of cardiorespiratory compro-mise.
Statistical Analysis
Descriptive statistics were used to characterize the sample with respect to perinatal and cardiorespiratory factors. Proportions were used to describe the presence and type of neurologic abnor-malities. The performance on the ENNAS was compared between subjects and healthy control subjects usingx2Fisher exact test (for items) and the Mann–Whitney test (distribution of items).x2 (Fish-er exact test) analysis also was used to compare neurobehavioral abnormalities with cardiorespiratory factors. A multiple regres-sion model was applied with deviant score and classification of normal/abnormal on the ENNAS and neurologic examination as the outcomes. Microcephaly and cyanotic/acyanotic lesion were the primary independent variables, adjusting for use of prosta-glandins, congestive heart failure, ventilation, oxygen saturation.
RESULTS Group Characteristics
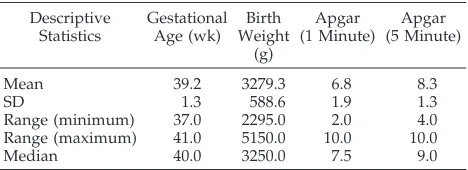
Of 135 newborns who presented in the neonatal period to Cardiology and required cardiovascular surgery for a CHD, 57 were excluded because of prematurity (35); “closed” surgical procedures not requiring CPB (9); language barrier (5); CNS anom-alies (3); hypoplastic left heart syndrome (3); and syndromes (2). Of the remaining 78 newborns, 60 families were approached for consent and 56 (93.3%) agreed to participate in the study. Eighteen families were not approached for consent because of lack of availability of the caregivers or investigators. Of the 56 subjects recruited, 33 (58.9%) were males. All sub-jects were full-term, and birth weights were appro-priate for gestational age in all subjects. Apgar scores at 5 minutes ranged from 7 to 10 in all but 1 subject, whose score was 4 (this subject’s 10-minute Apgar score was 7) (Table 1). Nine of 56 (16.1%) subjects were evaluated as outpatients, whereas the remain-der were assessed in the NICU. Subjects fell into one of the following diagnostic categories of CHD: trans-position of the great arteries (12), complex (6),
tetral-ogy of fallot (6), tetraltetral-ogy of fallot with pulmonary atresia (5), coarctation of the aorta (with aortic arch hypoplasia) (5), ventricular septal defect (3), aortic stenosis (3), interrupted aortic arch (3), double outlet right ventricle with subaortic ventricular septal de-fect (3), double outlet right ventricle with subpulmo-nary ventricular septal defect (3), atrioventricular septal defect (2), truncus arteriosus (2), pulmonary atresia with intact ventricular septum (2), and total anomalous pulmonary venous drainage (1).
Cardiorespiratory Characteristics
Cardiorespiratory status was documented by the cardiologist in all 56 newborns at the time of neu-robehavioral assessment. Twelve (21.4%) subjects were intubated and ventilated, 2 (3.6%) subjects were not intubated but were receiving supplemental in-spired oxygen, and the remainder (75.0%) were breathing spontaneously in room air. Thirty-nine newborns (69.6%) had cyanotic heart defects and 17 (30.4%) had acyanotic heart lesions. Twenty-two (39.3%) had arterial oxygen saturations ,85%, 17 (30.4%) were tachypneic (ie, respiratory rate $55 breaths per minute), and 13 (23.2%) had clinical signs of congestive heart failure. Twelve subjects were not receiving any medications, whereas 33 were receiv-ing prostaglandin E2, 15 furosemide, 5 digoxin, 2
hydrochlorothiazide, 1 spironolactone, 1 ethacrymic acid, 1 dobutamine and dopamine, and 4 fentanyl/ midazolam.
Description of Test Performance of Newborns With CHD
Neurologic Examination
Of 56 subjects recruited, the neurologist was avail-able to examine 50 before surgery at a mean age of 13.9611.8 days (range, 1 to 44 days), with outpatient evaluations conducted somewhat later (mean, 25.4 days). In 1 subject, although recruited before 30 days of life, medical complications delayed surgery and therefore testing was conducted at 44 days. All other subjects were younger than 1 month of age. Fifty-six percent (28 of 50) demonstrated one or more abnor-mal neurologic findings including diffuse hypotonia (20), hypertonia (6), jitteriness (4), no suck (3), motor asymmetry (2), decreased muscle power in the upper and lower extremities (5), and cranial nerve abnor-malities (2). Altered states of consciousness such as lethargy or stupor also were documented (7), as well as restlessness and agitation (4). Preoperative sei-zures were clinically reported in 4 subjects. In addi-tion, microcephaly (ie, head circumference at or be-low the third percentile) was documented in 35.7% (20) of subjects (Fig 1). Two thirds of these neonates with microcephaly had abnormal neurologic exami-nations. There was a negative effect (odds ratio [OR]: 1.72; confidence interval: 0.42–7.12) of microcephaly on neurologic status; however, this did not reach significance. Results were comparable with the ENNAS as the outcome variable (odds ratio: 1.86).
ENNAS
Of the 56 subjects in our sample, all but 6 were evaluated by an occupational therapist using the TABLE 1. Group Characteristics
Descriptive Statistics
Gestational Age (wk)
Birth Weight
(g)
Apgar (1 Minute)
Apgar (5 Minute)
Mean 39.2 3279.3 6.8 8.3
SD 1.3 588.6 1.9 1.3
Range (minimum) 37.0 2295.0 2.0 4.0
Range (maximum) 41.0 5150.0 10.0 10.0
ENNAS, at a mean age of 12.6611.5 days (range, 1 to 44 days). Twenty-one (42%) subjects had a normal examination (deviant score, 0 to 2); 19 neonates (38%) had a deviant score between 3 and 6, indicating a suspect examination; and 10 (20%) had an abnormal ENNAS (.6). Abnormalities observed and recorded on suspect or abnormal ENNAS examinations in-cluded hypotonia (22), hypertonia (5), jitteriness (4), absent suck (4), and motor asymmetry (2). Further-more, poor state regulation was documented in 31 (62%) subjects. This was characterized by lethargy defined as an inability to arouse or maintain an op-timal state of arousal (14), and/or excessive irritabil-ity and abrupt swings between sleep and crying states with no quiet alert periods (17). Poor state modulation limited the ability to evaluate orienting responses in some subjects. Nonetheless, in those assessed, poor orienting responses were observed often, which consisted of poor visual fixation and tracking (15/30) as well as poor auditory alerting (29/44). It should be considered that clinical assess-ment of the very ill neonates was limited at times and incomplete because of the presence of central or pe-ripheral lines, ventilators, etc, restricting the amount of handling and position changes. As a result, the prevalence of neurobehavioral abnormalities may be underestimated because some items could not be completed.
Feeding
Of 56 subjects, 26 were fed orally (breast or bottle); however, 5 required nasogastric supplementation, and 30 were on total parenteral feedings. Thirteen (26%) subjects had a weak suck, and 4 had no suck as documented on the ENNAS (item 4). All newborns with a weak nonnutritive suck also were found to be hypotonic on neurobehavioral assessment. Feeding efficiency was documented informally on subjects who were fed orally, based on information provided by the primary caregiver (eg, primary nurse or mother) regarding the infant’s feeding behaviors. Decreased feeding efficiency was noted in 34% and was characterized by a longer feeding time and more
frequent feedings to ensure adequate oral intake and sufficient weight gain.
Comparison of Performance on the ENNAS Between Subjects and Healthy Controls
The overall deviant score was significantly differ-ent between newborns with CHD (mean, 3.86; stan-dard deviation [SD], 2.4; median, 4.0) and healthy controls (mean, 0.5; SD, 0.7; median, 0). There also were significant differences in test performance on several subtests, including orienting responses (both visual and auditory subtests) and passive and active movements (eg, head extension, head lag, muscle tone) (Table 2).
Significant differences in the distribution of scores were documented in the majority of items, with the exception of the following: lateral head preference (which evaluated head position in supine at rest), popliteal angle (which determines the degree of flex-ion at the knees), asymmetric tonic neck reflex, pos-trotatory nystagmus, and the presence of tremor (Ta-ble 2).
Relationship Between Neurobehavioral Abnormalities and Cardiorespiratory Status
x2 analyses (Fisher’s exact test) demonstrated a
significant association (P , .05) between neurobe-havioral status (normal/abnormal) and cyanotic ver-sus acyanotic CHDs, in which newborns with acya-notic CHDs were more likely to be neurologically abnormal (78.6%) than those with cyanotic defects (47.2%) (Fisher exact P 5 .06). Multiple logistic re-gression demonstrated further that acyanotic lesions were associated with an abnormal neurologic exam-ination (odds ratio, 4.10; CI, 0.98 –17.2). However, there was no significant association (P . .05) be-tween neurobehavioral performance and any other indicators of cardiorespiratory compromise includ-ing the presence of congestive heart failure, tachy-pnea, oxygen saturation,85%, use of prostaglandin E2, mechanical ventilation, or inpatient versus out-patient status. Furthermore, an unpaired t test showed no significant difference (P5.19) in oxygen saturation in those subjects with normal ENNAS scores (mean oxygen saturation, 88.2% 6 8.4) and those with abnormal ENNAS (mean oxygen satura-tion, 91.0%68.0).
In addition, specific medical factors that may in-fluence neurologic status were reviewed in selected patients. Hypertension, a complication that may be associated with neurologic abnormalities, was not a feature in our newborns with coarctation of the aorta, because all were normotensive before surgery. Sim-ilarly, hypocalcemia may be associated with jitteri-ness and seizures41; however, calcium levels were
DISCUSSION
The results of this study demonstrated that neu-robehavioral abnormalities on standardized testing were common in newborns with CHD before open heart surgery. There was excellent agreement (crude agreement, 96.9%;kstatistic5.94) between the two independent evaluations of overall neurologic status conducted by the neurologist and occupational ther-apist.42 This reinforces the validity of the findings,
because the two approaches in neurologic evaluation of these newborns yielded very similar results. Neu-robehavioral findings were characterized by sei-zures, muscle tone abnormalities, motor asymme-tries, jitteriness, and poor orienting responses. These findings support Gillon’s16clinical observations.
More than one third of newborns with CHD also were microcephalic despite birth weights appropri-ate for gestational age, suggesting possible disrup-tion in antenatal brain development, when the brain is undergoing the most rapid rate of cell division.43
Moreover, 66.7% of newborns with microcephaly in this sample also had abnormal neurologic examina-tions. This subgroup of children may be at increased risk for later developmental disability. Although there was a nonsignificant association between mi-crocephaly and abnormal neurologic status, a larger sample would be needed to confirm this finding.
Neurobehavioral state organization is defined as an infant’s ability to demonstrate well defined states, and to make smooth and organized transitions be-tween states (eg, sleep to arousal, to alert, to crying). The infant who can not be aroused, who is exces-sively irritable, or who swings abruptly between states with no alert periods may be demonstrating CNS immaturity or a pathologic condition.44 Thirty
one (62%) newborns with CHD demonstrated “poor-ly modulated” behavioral state organization profiles. Als and associates45 proposed that poor state
orga-nization in preterm infants may serve as a necessary protective reflex for shutting out excessive stimula-tion in an attempt to maintain physiologic homeosta-sis. However, this may have a secondary conse-quence of sensory and interactional deprivation. Poor state regulation in our subjects may serve as an intrinsic protective mechanism in an attempt to oblit-erate additional physiologic instability exacerbated by the environment. One also could postulate that the poor state regulation may be attributable to acute hypoxic–ischemic injury in a subset of subjects. It should be noted that poor state regulation alone (or poor orienting responses) did not constitute an ab-normal neurobehavioral assessment, but occurred in concert with other neuromotor abnormalities. Al-though poor state control may affect orienting re-sponses, it could not explain the neuromotor abnor-malities noted in our cohort.
Feeding difficulties also were noted frequently. Thirteen (26%) subjects had a weak nonnutritive suck, 4 had no suck, and 17 (34%) presented with decreased feeding efficiency. Moreover, all new-borns with a weak suck also were found to be hypo-tonic. Therefore, poor oral–motor skills did not ap-pear to be related exclusively to decreased endurance, but possible CNS injury or transient per-turbation may have impacted on feeding patterns. More standardized measures of feeding performance are needed to validate these findings.
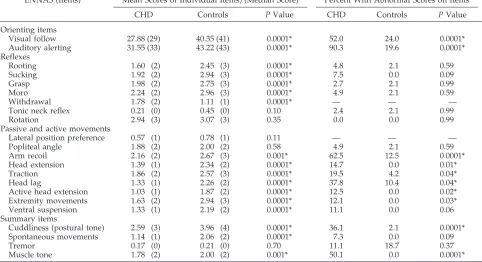
Comparison of performance on the ENNAS be-tween subjects with CHD and healthy term control subjects demonstrated significant differences in test TABLE 2. Comparison of Performance on the ENNAS
ENNAS (Items) Mean Scores of Individual Items/(Median Score) Percent With Abnormal Scores on Items
CHD Controls PValue CHD Controls PValue
Orienting items
Visual follow 27.88 (29) 40.35 (41) 0.0001* 52.0 24.0 0.0001*
Auditory alerting 31.55 (33) 43.22 (43) 0.0001* 90.3 19.6 0.0001*
Reflexes
Rooting 1.60 (2) 2.45 (3) 0.0001* 4.8 2.1 0.59
Sucking 1.92 (2) 2.94 (3) 0.0001* 7.5 0.0 0.09
Grasp 1.98 (2) 2.75 (3) 0.0001* 2.7 2.1 0.99
Moro 2.24 (2) 2.96 (3) 0.0001* 4.9 2.1 0.59
Withdrawal 1.78 (2) 1.11 (1) 0.0001* — — —
Tonic neck reflex 0.21 (0) 0.45 (0) 0.10 2.4 2.1 0.99
Rotation 2.94 (3) 3.07 (3) 0.35 0.0 0.0 0.99
Passive and active movements
Lateral position preference 0.57 (1) 0.78 (1) 0.11 — — —
Popliteal angle 1.88 (2) 2.00 (2) 0.58 4.9 2.1 0.59
Arm recoil 2.16 (2) 2.67 (3) 0.001* 62.5 12.5 0.0001*
Head extension 1.39 (1) 2.34 (2) 0.0001* 14.7 0.0 0.01*
Traction 1.86 (2) 2.57 (3) 0.0001* 19.5 4.2 0.04*
Head lag 1.33 (1) 2.26 (2) 0.0001* 37.8 10.4 0.04*
Active head extension 1.03 (1) 1.87 (2) 0.0001* 12.5 0.0 0.02*
Extremity movements 1.63 (2) 2.94 (3) 0.0001* 12.1 0.0 0.03*
Ventral suspension 1.33 (1) 2.19 (2) 0.0001* 11.1 0.0 0.06
Summary items
Cuddliness (postural tone) 2.59 (3) 3.96 (4) 0.0001* 36.1 2.1 0.0001*
Spontaneous movements 1.14 (1) 2.06 (2) 0.0001* 7.3 0.0 0.09
Tremor 0.17 (0) 0.21 (0) 0.70 11.1 18.7 0.37
Muscle tone 1.78 (2) 2.00 (2) 0.001* 50.1 0.0 0.0001*
performance between the two groups, particularly in the distribution of scores. Poor visual and auditory responses were characteristic of the vast majority of newborns with CHD; however, performance may have been influenced by poor state regulation, which was a hallmark feature in this cohort. Newborns with CHD also manifested motor deficits such as de-creased limb traction and recoil, poor antigravity movements, limited spontaneous movements, as well as tone abnormalities. The high prevalence of acute neurobehavioral abnormalities is comparable with the findings of other NICU populations and would suggest that careful surveillance of develop-mental progress may be justified. The literature suggests that many of these neonates with neuro-behavioral abnormalities may be at greater risk for long-term neurodevelopmental sequelae.39,46
The relationship between specific cardiorespira-tory factors and neurobehavioral performance was examined to determine whether neurobehavioral sta-tus was influenced by the acuity of illness. Analysis revealed no significant association between indi-vidual medical parameters examined (ie, oxygen saturation, tachypnea, congestive heart failure, in-tubation, administration of prostaglandins) and an increased risk for neurologic findings with the excep-tion of cyanotic versus acyanotic heart defects. This perhaps may be attributed to decreased systemic blood flow and potentially cerebral blood flow in those patients with acyanotic heart defects exhibiting congestive heart failure. Additionally, a number of acyanotic heart defects result in impaired blood flow in the ascending aorta and thus may limit cerebral perfusion. This compromise in cardiovascular phys-iology may have an antenatal origin. Brain injury may ensue after alterations in cerebral perfusion, possibly resulting in impaired autoregulation of ce-rebral blood flow.7Conversely, newborns with
cya-notic CHD may demonstrate sufficient adaptability to chronic hypoxemia. Metabolic needs may be re-duced or blood flow may be redistributed to organ systems with the greatest oxygen requirements to accommodate for the oxygen insufficiency in the blood. These hypotheses would need to be substan-tiated by future experimental studies. Interestingly, van Houten and co-workers21 reported that
new-borns with coarctation of the aorta and ventricular septal defects (acyanotic lesions) had a higher inci-dence (71%) of ultrasound abnormalities such as in-traventricular hemorrhage, cerebral atrophy, and echodensities in the basal ganglia and thalamus be-fore surgery. The association between acyanotic heart lesions and a greater risk for neonatal neuro-logic abnormalities was borderline significant and would therefore need to be validated on a larger sample.
As mortality rates after open heart surgery con-tinue to decline, the morbidity of survivors has come under increasing scrutiny. Our findings suggest that the prevalence of neurobehavioral abnormalities in the infant cardiac population before surgery has been underappreciated, and that these infants may require regular developmental screening. Moreover, preop-erative neurologic dysfunction in turn may render
infants more susceptible to additional neurologic in-jury during cardiac surgery. Studies are needed to assess the determinants of brain injury so that neu-rologic sequelae of CHD may be reduced. Given the multiple needs of this population, interdisciplinary efforts among cardiovascular surgeons, cardiolo-gists, pediatricians, neurolocardiolo-gists, neonatalocardiolo-gists, re-habilitation specialists, and nutritionist experts are critical to best meet the needs of this high-risk group. Such collaborative efforts are essential to prevent or minimize neurodevelopmental morbidity through early identification of infants at risk and permit the initiation of early intervention programs to enhance the outcome of survivors.
ACKNOWLEDGMENTS
This study was funded by the National Health Research and Development Program (Health Canada), and the Montreal Chil-dren’s Hospital—Research Institute.
We thank the attending staff of the Division of Newborn Med-icine, Dr Marie Beland and Dr Luc Jutras from the Division of Cardiology, and Johanne Therrien for their assistance in recruit-ment of subjects. Special thanks to Joseph Falworth for coordina-tion of the project, data entry, and manuscript preparacoordina-tion, as well as to Dr Michel Abrahamowicz and the Biostatistical Consultating Service at the Montreal Children’s Hospital for statistical consul-tation. We are indebted to the families who participated in the study.
REFERENCES
1. Nuutinen M, Koivu M, Rantakallio P. Long-term outcome for children with congenital heart defects.Arctic Med Res. 1989;48:175–184 2. Perry LW, Neill CA, Ferencz C, et al. Infants with congenital heart
disease: the cases. In: Ferencz C, Rubin JD, Loffredo CA, Magee CA, eds. Epidemiology of Congenital Heart Disease: The Baltimore–Washington Infant Heart Study 1981–1989. Mount Kisco, NY: Furuta; 1993:33– 61 3. Clark EB, ed. Epidemiology of congenital cardiovascular
malforma-tions. In: Emmanouilides GC, Riemenschdeider TA, Allen HD, Gutesell HP, eds.Heart Disease in Infants, Children and Adolescents. 5th ed. 1995; 1:60 –70
4. Clare MD. Home care of infants and children with cardiac disease.Heart Lung. 1985;14:218 –222
5. Ferry PC. Neurologic sequelae of open-heart surgery in children.Am J Dis Child. 1987;141:309 –312
6. Stuart AG. Encephalopathy following cardiac surgery and heart trans-plantation.Clin Paediatr. 1994;2:149 –172
7. Brunberg JA, Reilly D, Doty DB. Central nervous system consequences in infants of cardiac surgery using deep hypothermia and circulatory arrest.Circulation. 1974;49(suppl 11):60 – 68
8. Ferry PC. Neurologic sequelae of cardiac surgery in children.Am J Dis Child. 1990;144:369 –373
9. Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest ver-sus low-flow cardiopulmonary bypass in infant heart surgery.N Engl J Med. 1993;329:54 –59
10. Mayer JE. Development, growth and cardiac surgery.Am J Dis Child. 1991;145:33–34
11. Royston D. Interventions to reduce cerebral injury during cardiac sur-gery—the effect of physical and pharmacological agents.Perfusion. 1989; 4:153–161
12. Sanderson JM, Wright G, Sims FW. Brain damage in dogs immediately following pulsatile and non-pulsatile blood flows in extracorporeal circulation.Thorax. 1972;27:275–278
13. Taylor R, Burrows F, Bissonette B. Cerebral autoregulation during cardiopulmonary bypass in temperature dependent infants. Anesthesi-ology. 1990;73:71
14. Clarkson PM, MacArthur BA, Barratt-Boyes BG, Whitlock RM, Neutze JM. Developmental progress after cardiac surgery in infancy using profound hypothermia and circulatory arrest. Circulation. 1980;62: 855– 861
16. Gillon JE. Behavior of newborns with cardiac distress. Am J Nurs. 1973;73:254 –257
17. Newburger JW. Central nervous system sequelae of congenital heart disease. In: Flyer DC, ed.Nadas Pediatric Cardiology. Philadelphia, PA: Hanley & Belfus; 1992:77– 82
18. Burn J. Aetiology of congenital heart disease. In: Anderson RH, Macart-ney FJ, Shinebourne EA, Tynan M, eds.Pediatric Cardiology. Edinburgh, Scotland: Churchill Livingstone; 1987:2–14
19. Miller G, Mamourian AC, Tesman JR, Baylen BG, Myers JL. Long-term MRI changes in brain after pediatric open heart surgery.J Child Neurol. 1994;9:390 –397
20. Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neu-rologic status of children after heart surgery with hypothermic circula-tory arrest or low-flow cardiopulmonary bypass.N Engl J Med. 1995; 332:549 –555
21. Van Houten JP, Rothman A, Bejar R. High incidence of cranial ultra-sound abnormalities in full-term infants with congenital heart disease. Am J Perinatol. 1996;13:47–53
22. Schilr M, Brierley J. Brain damage complicating open heart surgery: a neuropathologic study.Proc R Soc Med. 1984;60:858 – 859
23. Cohen M. The central nervous system in congenital heart disease. Neurology. 1990;10:452– 456
24. Terplan KL. Brain changes in newborns, infants and children with congenital heart disease in association with cardiac surgery. Additional observations.J Neurol. 1976;212:225–236
25. Gilles FH, Leviton A, Jammes J. Age-dependent changes in white matter in congenital heart disease.J Neuropathol Exp Neurol. 1973;32:179. Ab-stract
26. Gilman S. Neurological complications of open heart surgery.Ann Neu-rol. 1990;28:475– 476
27. Bellinger DC, Wernovsky G, Rappaport LA, Mayer JE, Castaneda AR, Farell DM. Cognitive development of children following early repair of transposition of the great arteries using deep hypothermic circulatory arrest.Pediatrics. 1991;87:701–707
28. Haka-Ikse K, Blackwood MJ. Psychomotor development of infants and children after profound hypothermia during surgery for congenital heart defects.Dev Med Child Neurol. 1978;20:67–70
29. Hesz N, Clark EB. Cognitive development in transposition of the great vessels.Arch Dis Child. 1988;63:198 –200
30. Messmer B, Gattiker R, Senning A. Psychomotor and intellectual devel-opment after deep hypothermia and circulatory arrest in early infancy. J Thorac Cardiovasc Surg. 1976;72:495–502
31. Veelken N, Gravinghoff L, Keck EN, Freiting HJ. Improved neurological outcome following early anatomical correction of transposition of the great arteries.Clin Cardiol. 1992;15:275–279
32. Wells FC, Coghill S, Caplan HL, Lincoln C. Duration of circulatory arrest does influence the psychological development of children after
cardiac operation in early lifeJ Thorac Cardiovasc Surg. 1983;86:823– 831 33. Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status prior to and following open heart surgery in newborns and infant. Circulation. 1997;96(suppl 1):130. Section I
34. Rogers BT, Msall ME, Buck GM, et al. Neurodevelopmental outcome of infants with hypoplastic left heart syndrome.J Pediatr. 1995;126: 496 – 498
35. American Academy of Pediatrics/American College of Obstetricians and Gynecologists. Relationship between perinatal factors and neuro-logic outcome. In: Poland RC, Freeman RK, eds.Guidelines for Perinatal Care. 3rd ed. Elk Grove Village, IL: American Academy of Pediatrics; 1992:221–224
36. Majnemer A, Rosenblatt B, Riley P. The influence of gestational age, birthweight and asphyxia on neonatal neurobehavioral performance. Pediatr Neurol. 1993;9:181–186
37. Daum C, Grellong B, Kurtzberg D, Vaughan HG. The Albert Einstein Neonatal Neurobehavioral Scale. 1977. Unpublished manual 38. Kurtzberg D, Vaughan HJ, Daum D, Crellong BA, Albin S, Rotkin L.
Neurobehavioral performance of low-birth infants at 40 weeks concep-tional age: comparison with normal full term infants.Dev Med Child Neurol. 1979;21:590 – 607
39. Majnemer A, Rosenblatt B. Prediction of outcome at school entry in the neonatal intensive care unit survivors with use of clinical and electro-physiologic techniques.J Pediatr. 1995;127:823–30
40. Wallace IF, Rose SA, McCarton DM, Kurtzberg D, Vaughan HG. Rela-tions between infant neurobehavioral performance and cognitive out-come in very low birth weight preterm infants.Dev Behav Pediatr. 1995;16:309 –317
41. Volpe JJ, ed.Neurology of the Newborn. 3rd ed. Philadelphia, PA: WB Saunders; 1995
42. Limperopoulos C, Majnemer A, Rosenblatt B, Shevell M, Rohlicek C, Tchervenkov C. Agreement between the neonatal neurological exami-nation and a standardized assessment of neurobehavioral performance in a group of high-risk newborns.Pediatr Rehab. 1997;1:9 –14 43. Winick M, Ross P. Head circumference and cellular growth of the brain
in normal and marasmic children.J Pediatr. 1969;74:181–184 44. Hunter JG. The neonatal intensive care unit. In: Case-Smith J, Allen AS,
Pratt PN, eds.Occupational Therapy for Children. St Louis, MO: Mosby; 1996:583– 647
45. Als H, Lester B, Brazelton TB. Dynamics of the behavioral organization of the premature infant: a theoretical perspective. In: Field TM, ed. Infants Born at Risk. New York, NY: Spectrum Publications; 1979: 173–192
DOI: 10.1542/peds.103.2.402
1999;103;402
Pediatrics
Charles Rohlicek and Christo Tchervenkov
Catherine Limperopoulos, Annette Majnemer, Michael I. Shevell, Bernard Rosenblatt,
Heart Surgery
Neurologic Status of Newborns With Congenital Heart Defects Before Open
Services
Updated Information &
http://pediatrics.aappublications.org/content/103/2/402 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/103/2/402#BIBL This article cites 36 articles, 5 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/cardiology_sub Cardiology
http://www.aappublications.org/cgi/collection/surgery_sub Surgery
http://www.aappublications.org/cgi/collection/neurology_sub Neurology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.103.2.402
1999;103;402
Pediatrics
Charles Rohlicek and Christo Tchervenkov
Catherine Limperopoulos, Annette Majnemer, Michael I. Shevell, Bernard Rosenblatt,
Heart Surgery
Neurologic Status of Newborns With Congenital Heart Defects Before Open
http://pediatrics.aappublications.org/content/103/2/402
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.