7255 IJSTR©2020
Effects Of Chronodisruption On The Locomotor
Activity Of Zebrafish (Danio Rerio) Larvae
Larry V. Padilla, Divine Joy A. Mauhay, Fe Corazon A. Jacinto, Eileen Z. Vitug
Abstract: Zebrafish (Danio rerio) has been used as a vertebrate model organism for various biological assays and scientific studies. It has been reared and bred because of its importance to science and the aquarium industry. Rearing and breeding conditions varies depending on the operational procedures implemented by each rearing facility. Some of the disruptive factors affecting their growth, development, and behavior in these facilities are water quality, light, temperature and feeding cycles. There may be facilities that operate beyond the reported optimal light/dark cycle of 14L/10D for zebrafishes, hence affecting their physiology including their locomotor activity. In this study, the effects of disruption of light and dark cycles on the locomotor activity of zebrafish larvae were investigated. The zebrafish larvae were exposed after acclimation to optimal light cycle (from 1dpf to 5dpf) to different hemeral light and dark cycles (from 6 to 7 dpf): 1L/23D, 7L/17D, 14L/10D, 17L/7D, and 23L/1D. Results show that higher zebrafish larvae locomotor activity in terms of swimming bouts and swimming indices were obtained for those placed under shorter light cycles or longer dark periods. Zebrafish exposed to longer dark periods probably exhibited quiescent to low locomotor activity during dark phases, which enabled them to conserve energy for eventual use at the onset of light cycle. Higher swimming bouts were recorded for those exposed at 1L/23D and 7L/17D set ups than those placed under 17L/7D and 23L/1D set ups. No definitive trends were seen for swimming velocity and routine turns although variations in the activities were recorded among the different treatments. Statistical results showed that light disruption may affect locomotor activity in terms of routine turns and swimming velocity as early as 24 hours after changing the light and dark cycles. The disruptive effect was statistically significant for swimming bouts at 8 dpf. It is possible that prolonged exposure of these zebrafishes to disruption in light and dark cycles could aggravate the effects, not just to swimming bouts and index, but also for other physiological responses. The experiment only ran until 8 dpf, however, the influence of light as a zeitgeber had already been manifested.
Index Terms: Biological clock, Chronodisruption, Danio rerio, Light/dark cycle; Locomotor activity, Swimming activity, Zebrafish
—————————— ——————————
1
INTRODUCTION
Locomotion is an essential physiological capability of zebrafishes that drives its feeding, social and defensive activities [1]. It can be an indication of the current and overall state of an organism. Locomotor activities throughout the day may vary as dictated by the chronobiology (biological rhythm) of an organism. Some environmental cues such as light and temperature are referred to as Zeitgebers (or daily synchronizers) that are considered as primary driving factors that regulate an organism’s biological rhythm [2], [3], [4] [5]. Other than locomotor activity, these zeitgebers have been shown to influence the circadian rhythm of organisms particularly their survival, development, fertility, metabolism and feeding behavior [2], [3], [6], [7], [8]. In addition to chronobiology, exposure to different substances have also been shown to alter an organism’s locomotor activity due to various mechanisms of action. Neuroactive drugs [9] and pharmacological compounds [10], pesticides [11], steroid hormones [12], microplastics and nanoplastics [13] have been studied for their effects on adult and larval zebrafish locomotor activities. Locomotor activity is crucial for optimal growth, development, behavior and survival of animals such as zebrafishes. Locomotion is deemed vital for the organisms’ feeding, predatory escape and energy conservation.
It can also be an indication of the general health condition of an individual fish. Identifying factors that influence such activity is essential for effective rearing, conservation and management. It is important to explore the influence of Light-Dark modulation cycles to further describe changes in locomotor activity of zebrafishes in response to such zeitgeber. In this study, the effects on locomotor activity of varying hemeral light cycles were investigated. The findings could be used to infer possible responses of other animals placed under similar exposure conditions. It specifically aims to describe the locomotor activity of zebrafish larvae subjected to different hemeral light and dark (LD) cycles in terms of its swimming activities, swimming velocity and swimming index. This may have an implication to zebrafish larval acclimation prior to experimentation and in the operational practice implemented by rearing and breeding facilities of zebrafishes.
2 METHODOLOGY
2.1 Rearing and breeding zebrafishes
Adult male and female zebrafishes (Danio rerio) were placed, reared and acclimated in two separate aquaria at optimal conditions (~28°C and regular ambient light) for two days prior to breeding. The fishes were fed with a combination of commercial fish feed and Artemia salina (brine shrimp) to enrich their protein diet for eventual breeding. On the third day, two male and four female zebrafishes were combined and placed in a single breeding tank containing a synthetic plant and pebbles at the bottom. The breeding tank was placed in constant darkness until the following morning. At light onset, the zebrafishes were examined for potential breeding behavior and spawning. Several minutes after spawning and fertilization by the males, the eggs that settled at the bottom and in between the pebbles were siphoned and transferred into several petridishes containing system water. The system water was decontaminated using methylene blue to prevent fungal and bacterial growth on the eggs.
___________________________________
• Larry V. Padilla, the primary and corresponding author, is a faculty member of the Biology Department, College of Science, Pamantasan ng Lungsod ng Maynila (University of the City of Manila), General Luna cor. Muralla St., Intramuros, Manila, Philippines, 1101. E-mail: lvpadilla@plm.edu.ph
2.2 Exposure to varying light cycles
Eggs were monitored for their viability until the 48th hour. Eggs that were viable hatched and were transferred to five (5) petridishes. Each petridish contained five 2-dpf larval zebrafishes. The petridishes containing the 2-dpf larvae were placed at the center of five customized boxes that allowed the researcher to regulate light cycles. These customized boxes served as light cycle chambers that were equipped at the underside of the lid with a segment of LED strip light, which allowed the larval zebra fishes to be exposed to ~100 lux of light intensity. The LED strip light was about 6 inches in length and had a wattage of 2 watts per square foot. The light source was about >20 inches from the fish larvae. The larval zebrafishes were reared in these light cycle chambers at optimal conditions and at normal light cycle of 14L/10D until the 5th day. On the 6th and 7th day, the 6 dpf larvae were exposed to the treatment of varying light cycles. This indicates that the 6dpf larvae were the acclimated group exposed to 14L/10D light cycles while the 7 dpf and 8 dpf larvae were the set up already exposed to the varying light cycles. Table 1 summarizes the light cycle introduced to the zebrafish larvae. Fifteen (15-min) minute video clips were captured ~30 minutes after the onset of light (i.e. 6:00AM) each morning to examine the effects of varying light cycles to zebrafish larvae swimming activities. The camera is capable of gathering video recordings at 8 megapixels with 1.5 focus pixels and 1080p HD at 30 fps. The camera was affixed on top of the lid of the light chamber to capture any movement in each set up. The petridishes were cleaned and the larvae were fed with ―first bite‖ commercial fish larvae feed after the videos were captured. The petridishes were also cleaned after each video recordings and feeding to maintain the optimal conditions for the fish larva.
2.3 Locomotor Activity
Locomotor activity in this study encompasses the following swimming related behaviors of zebrafish larvae: swimming activity, swimming index and swimming velocity. Swimming activity characterized as either swimming bouts and routine turns, swimming index, and swimming velocity were examined in the captured videos. Swimming bout and routine turns are operationally defined as the change in location of individual zebrafishes and change in orientation without change in location, respectively. These were counted for one minute in three separate 1-min timeframe in the 15-minute video clip. Swimming bout is expressed as number of bouts/minute
whereas routine turns is expressed as number of turns/minutes. Moreover, swimming index was also determined to compare the swimming activity between the treatment and the control groups. It was calculated using the formula below:
Swimming index= Number of swimming bouts (treatment) Number of swimming bouts (control; 14L/10D)
Values >1 indicates that the swimming activity is greater in the treatment than in the control (14L/10D) while values <1 indicates swimming activity than is higher in the control group (14L/10D). Swimming velocity (mm/s) was determined by tracking the movement of the zebrafishes in the petridishes. The video clips depicting the movement of the larval zebrafishes were screen captured in separate one-minute segments of the 15-minute video clip. The images as JPEG were imported to a geographic information system (GIS) software, manifold. The movement of the larvae were tracked as dotted points and were connected with measuring lines using the software. Numerical values generated by the software showing the distance traversed by each larva in each video recordings were recorded and converted. The diameter of the petridish in units generated by manifold was also obtained. The distance traversed by the larvae and the diameter of the petridishes in the video clips were used to extrapolate the actual distance the larvae moved in each video clip. This was calculated using the derived formula below:
Pa
Dv
Pv
Da
*
Where, Da indicates the actual distance traversed by the larvae, Dv indicates the distance traversed by the larvae in the video, Pa stands for the actual diameter of the petridish (84mm), and Pv indicates the diameter of the petridish in the video clip. The distance traversed by each larva was converted to swimming velocity (mm s-1) by dividing the swimming distance (in mm) to 60 seconds. Mean values of swimming behaviors i.e. swimming bouts, routine turns, swimming index and swimming velocity were reported in this study. Significant differences in these swimming behaviors at 6dpf, 7dpf and 8dpf zebrafish larvae were calculated using One-way ANOVA. Post-hoc analyses were done using Tukey’s HSD test. Statistical analyses were done using the SPSS software.
3 R
ESULTSA
NDD
ISCUSSIONLocomotor activity of zebrafish larvae at different stages of development from 6 dpf to 8 dpf were characterized into swimming bouts, routine turns, swimming velocity and swimming index (Fig. 1-4). The larvae were acclimated until the 5th day, hence the 6 dpf stage represent the baseline data for locomotor activity. The locomotor activity after two succeeding days, 7 dpf and 8 dpf, are considered as the treatment periods. Mortality was not recorded in any of the experimental and control set-ups. Swimming of the zebrafish larvae are characterized by beat and glide modes, which include a bout period and an interbout period [14]. Bout involves active swimming and oscillation of the tail whereas interbout is characterized by a period of coasting or stationary of the larvae. The number of swimming bouts per minute ranged from 66 to 96.3 (Fig. 1). Variability among the different
TABLE 1
Light cycles introduced the Danio rerio larva
Code Light/Dark (L/D) cycle
Light
period Dark period
A 14L/10D (control) 6:00AM-8:00PM
8:00PM-6:00AM
B 1L/23D
6:00AM-7:00AM
7:00AM-6:00AM
C 7L/17D
6:00AM-1:00PM
1:00PM-6:00AM
D 17L/7D
6:00AM-11:00PM
11:00PM-6:00AM
E 23L/1D
6:00AM-5:00AM
7257 IJSTR©2020
experimental and control set ups increased from the acclimation period (6 dpf) to the two exposure periods (7 and 8 dpf). The variations were more evident at 8 dpf than at 7 dpf. There was an overall decrease in number of swimming bouts per minute in set ups exposed to longer than normal light cycles. Number of swimming bouts per minute from 6, 7 until 8 dpf decreased from 84.2 to 74.8 to 66 at 17L/7D light/dark cycle and from 82.2 to 73.5 to 68.6 at 23L/1D cycle. In the control set up, 14L/10D, swimming bouts plateau from 6 dpf to 7 dpf (~81 bouts) before slightly increasing at 8dpf (~86 bouts). Increase in number of swimming bouts were observed at experimental set ups with shorter exposure to light, set ups 1L/23D and 7L/17D, particularly when comparing 6 dpf with 8 dpf. Light is an essential zeitgeber driving the activity of organisms, including zebrafish larvae. Although light guides an organism’s mobility, however, excessive light exposure promoting greater activity may eventually deplete the organisms’ energy. On the other hand, shorter duration of light exposure (1h light and 7h light) than the optimal cycle (14h light and 10h dark) appeared to increase the number of swimming bouts. Periods of inactivity during dark periods allowed the zebrafish larvae to conserve and reserve energy for eventual use after the onset of light cycle. This was manifested at least 30 minutes after light was turned on in the experimentation. Such behavior may possibly continue even hours after high activity in terms of swimming bouts. In the experiment conducted by Burgess and Granato [8], transient hyperactivity before transitioning to a quiescent state were observed for larval zebrafish exposed to continuing darkness. Utne-Palm and Stiansen [15] reported that light and turbulence affect swimming activities of herring larvae with low light intermediate turbulence significantly increasing prey attack rate of larger sized larvae.
Fig. 1: Number of swimming bouts exhibited by 6 dpf
(acclimation) to 8 dpf zebrafish larvae exposed to varying light/dark cycles (n=5)
A different trend was observed for routine turns per minute that ranged from 6.2 to 20.9 (Fig. 2). Sharp increases in routine turns were noted from 6 dpf to 7 dpf at longer than optimal light exposure, 17L/7D and 23L/1D. At 8 dpf, however, this drastic increase reverted to values comparable with the baseline for light/dark cycles. For optimal (14L/10D) and shorter (1L/23D and 7L/17D) light cycles, routine turns did not markedly change. Slight variations were recorded for these set ups from 6 to 8 dpf. The increase in routine turns at longer light exposure periods suggest possible disorientation of the zebrafish larvae. This may indicate higher degree of roaming or alertness for the zebrafish larvae. Longer light exposure
resulted to greater tendency of the zebrafish to scout or move about greater distance or location in the arena of the petridish. This, however, decreased and returned to values similar with the control and the other treatment as well on the 8th day after exposure. Routine turns appear to be influenced only at the initial exposure periods. Large angle turns that is different from startle responses were observed in zerbrafishes exposed to reduced illuminations, wherein individual fishes had higher tendencies to orient their body towards light source [8].
Fig. 2: Number of routine turns exhibited by 6 dpf (acclimation)
to 8 dpf zebrafish larvae exposed to varying light/dark cycles (n=5).
Swimming velocity incorporates swimming distance recorded from the zebrafish larvae over a period of observation (Fig. 3). At 6dpf, swimming velocities were already highly variable suggesting that swimming activity were dependent on an individual larva’s physiology. Changes observed at 7 dpf and 8 dpf varied among the different experimental set ups. Slight increase in swimming velocity were obtained for 14L/10D and 7L/17D light/dark cycles while reduction was noted for 1L/23D and 23L/1D cycles. No notable changes were seen in 17L/7D set up.
Fig. 3: Swimming velocity (mm s-1) exhibited by 6 dpf
(acclimation) to 8 dpf zebrafish larvae exposed to varying light/dark cycles (n=5).
Higher swimming indices (1.10-1.12) were obtained at shorter than optimal light periods (1L/23D and 7L/17D) while markedly low swimming indices were recorded at longer than optimal light periods (17L/7D and 23L/1D). These results suggest that changing the duration of light and dark exposure of zebrafish larvae influences their swimming activity, wherein shorter light promotes more frequent swimming activities. Similar trends were obtained in swimming bouts, particularly at 8 dpf larvae. Kopp et al. [16] demonstrated that if the zebrafish larva were adequately fed with high caloric diet, continuous high activity levels and significantly elevated activity levels were recorded at light cycle and at dark cycle, respectively. For adult zebrafish, total locomotor activity was found to be higher during light periods, exhibiting diurnalism at lower temperature of 20°C than at 26°C [4]. Exposure to constant dim light of 3 lux showed synchronicity to thermocycles, however, the rhythm observed was not as pronounced as those placed under LD cycle.
Fig. 4: Swimming indices in each experimental set-up.
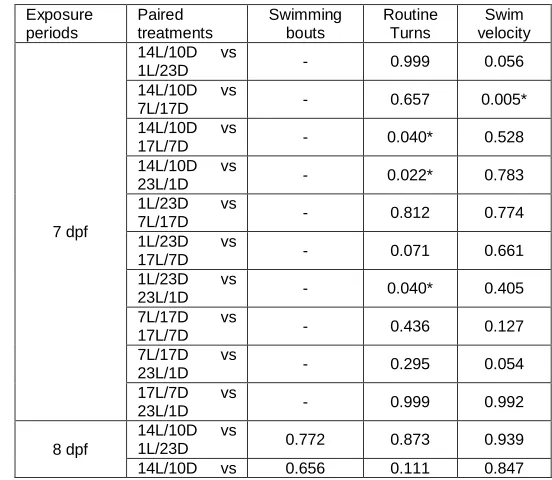
Findings of this study have shown that variation in light and dark cycles seem to influence locomotor activity of zebrafish larvae. Statistical analyses of all parameters showed no significant differences comparing the varying light and dark cycles at 6 dpf (Table 2). This suggests that the locomotor activities of the zebra fish larvae were comparable during acclimation period. However, at 7 dpf and 8 dpf, swimming velocity and routine turns were significantly different with one another. These indicate that the varying light and dark cycles affected the locomotor activity as early as one day after disruption of the cycle. For swimming bouts, no significant difference was obtained at 7 dpf but was significantly different at 8 dpf suggesting that the effect manifested one day later as compared to the two other parameters. Appendix A summarizes the post-hoc analysis for all parameters that showed significant differences in each exposure period. For swimming bouts at 8 dpf, no significant differences were obtained when all treatments were compared with the control group. However, when opposite ends of the spectra for light
and dark cycles (1L/23D vs 23L/1D and 7L/17D vs 23L/1D) were compared, significant differences were recorded. A similar trend can be noted for swimming velocity at 8 dpf. At 7dpf for this parameter, the only significant difference was obtained when the control group (14L/10D) was compared with 7L/17D. For routine turns at 7 dpf, significant differences were recorded when the control group was compared with the two longer than normal light durations (17L/7D and 23L/1D) and when the shortest and longest light exposure periods were compared. At 8dpf, significant difference was noted when routine turns at 7L/17D and 23L/1D were compared. The effects of light disruption on locomotor activity were statistically notable at 8 dpf for swimming bouts and as early as at 7 dpf for routine turns and swimming velocity. Disruption on locomotor activities were most prominent when zebrafish swimming bouts and routine turns were compared between the shortest and longest light and dark cycles. The effects were also notable when long light duration was compared with the control for the routine turn parameter. Swimming velocity were significantly different only in selected set ups. Swimming activity were found to be regulated by a couple of cells in the body. Kolmer-Agduhr cells and Rohon Beard cells were found to play significant roles in zebrafish swimming [17]. Kolmer-Agduhr was found to be involved in forward swimming while Rohon Beard cells that were equipped with light sensitive ion channels elicits C-shaped curvature of the body due to light impulse. nMLF, an assembly of neurons found in the brain, also appears to be active in all forms of swimming movements exhibited by zebrafish larvae. It also has been found that differentially driving the spinal circuits by the nMLF could modulate swimming of larval zebrafish [14]. Swimming speed of larval zebrafish seem to correspond to visual stimulus, hence may be influenced by light.
4
APPENDIX
Appendix A. Statistical results via post-hoc analysis showing p-values (α= 0.05) comparing swimming bouts, routine turns and swimming velocity among the varying light/dark cycles at 7dpf and 8 dpf only. No significant differences were obtained at 6 dpf for all parameters.
Exposure periods
Paired treatments
Swimming bouts
Routine Turns
Swim velocity
7 dpf
14L/10D vs
1L/23D - 0.999 0.056
14L/10D vs
7L/17D - 0.657 0.005*
14L/10D vs
17L/7D - 0.040* 0.528
14L/10D vs
23L/1D - 0.022* 0.783
1L/23D vs
7L/17D - 0.812 0.774
1L/23D vs
17L/7D - 0.071 0.661
1L/23D vs
23L/1D - 0.040* 0.405
7L/17D vs
17L/7D - 0.436 0.127
7L/17D vs
23L/1D - 0.295 0.054
17L/7D vs
23L/1D - 0.999 0.992
8 dpf
14L/10D vs
1L/23D 0.772 0.873 0.939
14L/10D vs 0.656 0.111 0.847
Table 2. Statistical results via one-way ANOVA showing the p-values (0.05) comparing swimming bouts, routine turns and swimming velocity among the varying light/dark cycles at each of
the three exposure periods (6dpf to 8dpf).
EXPOSURE PERIODS
SWIMMING BOUTS
ROUTINE TURNS
7259 IJSTR©2020
7L/17D 14L/10D vs
17L/7D 0.656 0.999 1.000
14L/10D vs
23L/1D 0.189 0.121 0.233
1L/23D vs
7L/17D 1.000 0.016* 0.999
1L/23D vs
17L/7D 0.124 0.958 0.934
1L/23D vs
23L/1D 0.019* 0.521 0.057
7L/17D vs
17L/7D 0.084 0.065 0.839
7L/17D vs
23L/1D 0.012* <0.001* 0.034* 17L/7D vs
23L/1D 0.884 0.196 0.240
*Significantly different p-values at 0.05 level.
5
CONCLUSION
Light may be considered as a zeitgeber for the circadian rhythm of zebrafish larvae. Disruption in light and dark cycle may have affected the locomotor activity of 7 dpf and 8 dpf zebrafish larvae. Shorter light periods than dark periods after acclimation increased swimming activity, particularly swimming bouts. This translated also to higher swimming indices. Zebrafish larvae in these treatment groups had high swimming indices, hence had higher swimming activities than the control group placed in optimal light and dark cycle. Significant differences in the disruptive effects of light and dark cycles were notable at 7dpf and at 8dpf for routine turns and swimming velocity. For swimming bouts, the influence of varying the light and dark cycles were recorded at the 8dpf set up.
6
ACKNOWLEDGMENT
The authors would like to thank Mr. Rej Winlove M. Bungabong for processing the images via Manifold GIS software.
REFERENCES
[1]. R. M. Colwill, R. Creton, ―Locomotor behaviors in zebrafish (Danio rerio) larvae‖, Behav Process, 86(2):222-229, 2011.
[2]. A.D. Santos, J.R. Lopez-Olmeda,, F.J. Sanchez-Vasquez, R. Fortes-Silva, ―Synchronization to light and mealtime of the circadian rhythm of self-feeding behavior and locomotory activity of white shrimps (Litopenaeus vannamei)‖ Comparative Biochemistry and Physiology, Part A 199: 54-61, 2016
[3]. F.G. Amaral, A. Castrucci , J. Cipolla-Neto, M.O. Poletini, N. Mendez, H.G. Richter and M.T Sellix., ―Environmental control of biological rhythms: Effects on development, fertility and metabolism‖, Journal of Neuroendocrinology, vol. 26: pp. 603-612, 2014. [4]. J.F. Lopez-Olmeda, J.A. Madrid, F.J.
Sanchez-Vasquez, ―Light and temperature cycles as Zeitgebers of zebrafish (Danio rerio) circadian activity rhythm‖, Chronobiology International, vol. 23 no. 3, pp. 537-550, 2006 (Abstract)
[5]. M.W. Hurd, J. Debruyn, M. Straum, C.G. Cahill, ―Circadian rhythms of locomotor activity in zebrafish‖ Physiology and Behavior, vol. 65 no. 3, pp :465-472, 1998
[6]. N. Villamizar, L.M. Vera, N.S. Foulkes, F.J. Sanchez-Vasquez, ―Effect of lighting conditions on zebrafish growth and development‖, Zebrafish, vol 11 no. 2, pp. 173-181, 2014
[7]. J.F. Lopez-Olmeda, F.J. Sanchez-Vasquez, ―Zebrafish temperature selection and synchronization of locomotor activity circadian rhythm to ahemeral cycles of light and temperature‖, Chronobiology International, vol. 26 no. 2, 2009 (Abstract)
[8]. H.A. Burgess, M. Granato M., ―Modulation of locomotor activity in larval zebrafish during light adaptation‖, The Journal of Experimental Biology, vol. 210, pp. 2526-2539, 2007.
[9]. L. Fei, J. Lin, X. Liu, W. Li, Y. Ding, Y. Zhang,… and Q. Li,.‖ Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs‖, Ann Transl Med, vol. 6 no. 10, pp. :1-9, 2018
[10]. P. Gupta, S..B. Hobragade, V.M. Shingatgeri, S.M. Rajaram, ―Assessment of locomotion behavior in adult zebrafish after acute exposure to different pharmacological reference compounds‖, Drug Development and Therapeutics, vol. 5 no. 2, pp. :127-133, 2014.
[11]. E. Dunn, M. Furimsky, ―Effects of carbaryl and malathion on locomotor activity and nervous system development in zebrafish‖, FASEB journal, vol. 27 no. 1, 2013. (abstract)
[12]. Y. Zhao, K. Zhang., K. Fent, ―Regulation of zebrafish locomotor behavior and circadian rhythm network by environmental steroid hormones.‖ Environmental Pollution, pp. 1-8, 2018 (abstract)
[13]. Q. Chen, M. Gundlach, S. Yang, J. Jiang, M. Velki., D. Yin… H. Henner , ―Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity‖, Science of the Total Environment, no. 584-585, pp.1022-1031, 2017. [14]. K. Severi., R. Portugues, C. Marques, D.M. O’Malley,
M.B. Orger, and F. Engert, ―Neural Control and Modulation of Swimming Speed in the Larval Zebrafish.‖ Neuron vol. 83 no. 3, pp. 692–707., 2014 [15]. A.C. Utne-Palm, J.E. Sitansen, ―Effect of larval
ontogeny, turbulence and light on prey attack rate and swimming activity in herring larvae‖ Journal of Experimental Marine Biology and Ecology, vo. 268 no. 2, pp. 147-170, 2002
[16]. R. Kopp, J. Legler, J. Legradi, ―Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors‖, Environ. Sci. Pollut. Res. Int. vol. 25 no. 5, pp. 4085-4093, 2018
[17]. H. Rosch., ―Guided by light‖. Last accessed on
November 11, 2018 at