Effects of Oseltamivir on Influenza-Related Complications
in Children With Chronic Medical Conditions
WHAT’S KNOWN ON THIS SUBJECT: Neuraminidase inhibitors can reduce the severity and duration of influenza symptoms in children. There are, however, limited data on the efficacy of oseltamivir among patients who may be at higher risk of influenza complications as a result of chronic disorders.
WHAT THIS STUDY ADDS: The results provide the first evidence that oseltamivir, when prescribed at the presentation of clinically diagnosed influenza, may be of benefit to children who are at higher risk of morbidity and secondary infections after influenza because of chronic medical conditions.
abstract
OBJECTIVE:This study investigated the influence of oseltamivir on in-fluenza-related complications and hospitalizations for children and ad-olescents, 1 to 17 years of age, with chronic medical conditions or neurologic or neuromuscular disease.
METHODS:In a retrospective study, outcomes for patients who were given oseltamivir within 1 day after influenza diagnosis were compared with those for patients who received no antiviral therapy. Anonymous data from MarketScan databases (Thomson Reuters, Cambridge, MA) were used to identify patients from 6 influenza seasons between 2000 and 2006. The study outcomes were frequencies of pneumonia, respiratory illnesses other than pneumonia, otitis media, and hospitalization.
RESULTS:Oseltamivir was prescribed for 1634 patients according to the study criteria, and 3721 patients received no antiviral therapy for their influenza. After adjustment for demographic and medical history variables, oseltamivir was associated with significant reductions in the risks of respiratory illnesses other than pneumonia, otitis media and its complications, and all-cause hospitalization in the 14 days after influenza diagnosis. Analyses for 30 days after influenza diagnosis also showed significant risk reductions for respiratory illnesses other than pneumonia, otitis media and its complications, and all-cause hospital-ization with oseltamivir.
CONCLUSION:When it was prescribed at influenza diagnosis, oselta-mivir was associated with reduced risks of influenza-related complica-tions and hospitalizacomplica-tions for children and adolescents at high risk of influenza complications.Pediatrics2009;124:170–178
CONTRIBUTORS:Pedro A. Piedra, MD,a,bKathy L. Schulman, MA,c
and William A. Blumentals, PhDd
Departments ofaMolecular Virology and Microbiology and bPediatrics, Baylor College of Medicine, Houston, Texas; cHealthcare Division, Thomson Reuters, Cambridge,
Massachusetts; anddRoche, Nutley, New Jersey
KEY WORDS
antiviral treatment, influenza, oseltamivir, pediatric, complications
ABBREVIATIONS
ICD-9-CM—International Classification of Diseases, Ninth Revision, Clinical Modification
HR— hazard ratio CI— confidence interval
www.pediatrics.org/cgi/doi/10.1542/peds.2008-0977
doi:10.1542/peds.2008-0977
Accepted for publication Nov 14, 2008
Address correspondence to Pedro A. Piedra, MD, Department of Molecular Virology and Microbiology, Baylor College of Medicine, One Baylor Plaza, MS-280, Houston, TX 77030. E-mail:
ppiedra@bcm.tmc.edu
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2009 by the American Academy of Pediatrics
Children with conditions such as asthma or other respiratory diseases, cardiac conditions, immunosuppres-sive disorders (eg, chemotherapy or HIV infection), renal disease, or diabe-tes mellitus are considered to be at particularly high risk of influenza-related complications and hospitaliza-tions.1–4Neurologic and
neuromuscu-lar diseases that can compromise respiratory function also yield an in-creased risk of respiratory failure in influenza in children.1,3,5,6
Beginning in the 2008 –2009 influenza season, the Centers for Disease Con-trol and Prevention Advisory Commit-tee on Immunization Practices recom-mended annual influenza vaccination for all children 6 months to 18 years of age. Vaccination rates in the high-risk pediatric population remain low,1
how-ever, and many children remain at risk from influenza.
Treatment with neuraminidase inhibi-tors has been found to be effective in reducing the severity and duration of influenza symptoms.7,8 For children 1
to 12 years of age, the neuraminidase inhibitor oseltamivir reduced the du-ration of illness, symptoms, and new diagnoses of otitis media.9A
retrospec-tive claims database study with children showed that oseltamivir reduced the risks of pneumonia diagnoses and anti-biotic use after influenza.10
There are few studies and limited data, however, on the efficacy of oseltamivir among individuals at high risk of influ-enza complications because of chronic disorders.11–14Two studies in children
with asthma reported no impact of oseltamivir on influenza duration.13,14
Both of those studies were under-powered; however, the secondary effi-cacy analyses in one of the studies showed significantly fewer exacerba-tions of asthma and greater improve-ments in forced expiratory volume with oseltamivir treatment.13
There-fore, we conducted a retrospective
in-surance claims database study to eval-uate the impact of oseltamivir treatment for influenza on complications and hos-pitalizations among children and adoles-cents with chronic medical conditions.
METHODS
Study Design and Data Source This retrospective analysis used anony-mous, patient-level, health insurance claims data for children and adolescents 1 to 17 years of age who were considered to be at high risk of influenza complica-tions. Data were included for patients di-agnosed as having influenza during 6 in-fluenza seasons (October 1 to March 31), from the 2000 –2001 season to the 2005– 2006 season. The period from October 1 to March 31 was selected to ensure a consistent period covering all or most of each season.
Medical and pharmacy benefits data were obtained from the Thomson Re-uters MarketScan Commercial Claims and Encounters and Medicare Supple-mental and Coordination of Benefits databases (Thomson Reuters, Cam-bridge, MA), in which claims files are linked with outpatient prescription drug data through unique encrypted identifiers. These databases are derived from employer- and government-funded health care insurance plans and in-cludes data on⬃25 million individuals of all ages who are covered under a va-riety of fee-for-service or capitated pro-vider reimbursement schemes. It was used previously in an influenza out-comes study10 and to provide cost/
utilization data for an influenza study.15
In compliance with the Health Insur-ance Portability and Accountability Act, patient data were deidentified. This project was therefore exempt from the requirement for institutional review board approval.
Study Cohort
Children and adolescents were de-fined as being at high risk if they had a
primary or secondary diagnosis ofⱖ1 of the following chronic medical condi-tions during the 12 months preceding influenza diagnosis: history of trans-plant; diagnosis of HIV/AIDS; cancer treated withⱖ1 administration of che-motherapy ⱕ6 months before influ-enza diagnosis; chronic renal dysfunc-tion, before or after onset of dialysis treatment; diabetes treated with an-tidiabetic therapies; serious chronic lung disease (such as asthma or chronic obstructive pulmonary dis-ease) treated with bronchodilators, mast cell stabilizers, corticosteroids, or other antiasthmatic medications; or a history of neurologic/neuromuscu-lar diseases. Conditions were identi-fied on the basis ofInternational Clas-sification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), Cur-rent Procedural Terminology, Health-care Common Procedure Coding Sys-tem, and revenue codes.
Patients with an outpatient influenza diagnosis claim (primary or second-ary diagnosis; ICD-9-CM code 487.xx) were included. Patients were included only once per influenza season but could be included again if they had in-fluenza in different seasons. The index date for each patient was the date of influenza diagnosis.
osel-tamivir (amantadine, rimantadine, or zanamivir) during the same influenza season also were excluded.
Patients meeting all criteria were in-cluded in the oseltamivir group if they had an outpatient claim for oseltamivir within 1 day after the influenza diag-nosis; they were included in the no-antiviral group if they had no claims for antiviral medication during that in-fluenza season. The 1-day period was selected to ensure the exclusion of pa-tients who did not initiate oseltamivir treatment within the recommended 48 hours after symptom onset.16
Outcomes Evaluated
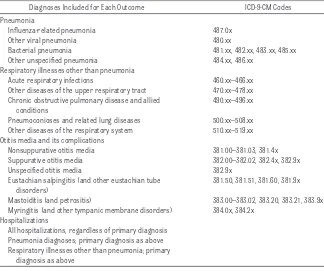
Outpatient diagnoses of pneumonia, respiratory illnesses other than pneu-monia, and otitis media and its compli-cations were identified on the basis of claims evidence (Table 1). Hospital-izations also were assessed. Admis-sions attributable to pneumonia or other respiratory illnesses were iden-tified on the basis of claims evidence of inpatient primary discharge
diag-noses (Table 1); other hospitalizations were identified from primary diagnoses.
Outcomes were evaluated for 14 and 30 days after influenza diagnosis. The 14-day period enabled capture of acute secondary complications most likely attributable to influenza, and the 30-day period allowed evaluation of po-tential, extended, treatment-related protection.
Statistical Analyses
Demographic data and medical histo-ries over the 12 months before influ-enza diagnosis were compared be-tween the 2 patient groups by using Student’s t test. Although propensity matching can reduce bias in nonran-domized studies and was used in a pre-vious study with healthy children,10
this method is inappropriate when the patient population is small, as in this study, or when there are large numbers of events per confounder.17
Multivariate modeling enabled some reduction of the effects of residual
variations in characteristics between the 2 groups.
The proportions of patients in the 2 groups who were experiencing each outcome were compared by using multivariate regression models with Cox proportional-hazards regression to estimate hazard ratios (HRs). A range of variable characteristics, in-cluding age, gender, population den-sity, geographic region, health care expenditures, vaccination, season of influenza diagnosis, and comorbidities or high-risk conditions (as listed previ-ously), were included in the models. Differences were considered statisti-cally significant (2-tailed test,P⬍.05) if 95% confidence intervals (CIs) for HRs excluded 1.0.
RESULTS
Patient Characteristics
Figure 1 shows the distributions ac-cording to week for the 5355 pa-tients included in this analysis from October 1 to March 31, 2000 –2001 through 2005–2006, and for labora-tory test–positive influenza isolates from week 40, 2000, to week 25, 2006, as reported by the Centers for Dis-ease Control and Prevention.18Of the
eligible patients, 1634 received osel-tamivir and 3721 received no antivi-ral treatment (Fig 2). There was a small but significant difference in the mean ages of the 2 groups, with that for the no-antiviral group being
⬃9 months lower (Table 2). The geo-graphic distributions also differed; almost three fourths of the patients in the oseltamivir group but less than one half of the patients in the no-antiviral group resided in the southern United States (Table 2). The distributions of patients across the 6 influenza seasons also differed, with larger proportions of patients in the no-antiviral group in the 2000 –2001 to 2003–2004 seasons
TABLE 1 Respiratory, Otitis Media, and Hospitalization Outcomes
Diagnoses Included for Each Outcome ICD-9-CM Codes
Pneumonia
Influenza-related pneumonia 487.0x
Other viral pneumonia 480.xx
Bacterial pneumonia 481.xx, 482.xx, 483.xx, 485.xx
Other unspecified pneumonia 484.xx, 486.xx
Respiratory illnesses other than pneumonia
Acute respiratory infections 460.xx–466.xx
Other diseases of the upper respiratory tract 470.xx–478.xx
Chronic obstructive pulmonary disease and allied conditions
490.xx–496.xx
Pneumoconioses and related lung diseases 500.xx–508.xx
Other diseases of the respiratory system 510.xx–519.xx
Otitis media and its complications
Nonsuppurative otitis media 381.00–381.03, 381.4x
Suppurative otitis media 382.00–382.02, 382.4x, 382.9x
Unspecified otitis media 382.9x
Eustachian salpingitis (and other eustachian tube disorders)
381.50, 381.51, 381.60, 381.9x
Mastoiditis (and petrositis) 383.00–383.02, 383.20, 383.21, 383.9x
Myringitis (and other tympanic membrane disorders) 384.0x, 384.2x Hospitalizations
All hospitalizations, regardless of primary diagnosis Pneumonia diagnoses; primary diagnosis as above Respiratory illnesses other than pneumonia; primary
and in the oseltamivir group in the 2004 –2005 and 2005–2006 seasons (Table 2).
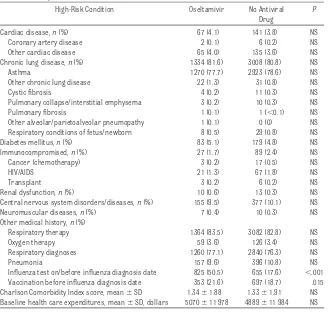
There were few significant differences in the preinfluenza medical histories for the 2 groups (Table 3). There was a slightly higher rate of influenza vacci-nation in the oseltamivir group (21.6% vs 18.7%). Vaccination status was fac-tored into the multivariate models, however. Also, more patients given os-eltamivir had been tested for influenza at or before diagnosis.
Clinical Outcomes
In the 14 days after influenza diagno-sis, patients given oseltamivir had significant reductions in the risks of respiratory illnesses other than pneu-monia (risk reduction: 26%; HR: 0.74 [95% CI: 0.63– 0.87]) and otitis media and its complications (risk reduction: 31%; HR: 0.69 [95% CI: 0.48 – 0.99]), compared with the no-antiviral group (Table 4 and Fig 3). When data were stratified according to age, the risk of respiratory illnesses other than pneu-monia was reduced by 37% for 1- to 2-year-old children (HR: 0.63 [95% CI: 0.44 – 0.91]) and by 27% for 6- to 12-year-old children (HR: 0.73 [95% CI: 0.59 – 0.91]) with oseltamivir. For 3- to 5-year-old children, the risk of otitis media and its complications was re-duced by 60% (adjusted HR: 0.40 [95% CI: 0.20 – 0.82]).
Among patients with a history of asthma, substantial proportions of the respiratory illnesses other than pneu-monia were asthma events (oseltami-vir group: 42.4% of these diagnoses; no-antiviral group: 46.1% [no history of asthma: oseltamivir group: 2.4%; no-antiviral group: 3.0%]). Other common diagnoses within this category in-cluded allergic rhinitis and acute phar-yngitis for patients with a history of asthma and acute bronchitis, bron-chiolitis, and acute sinusitis for other patients.
00/2001 01/2002 02/2003 03/2004 04/2005 05/2006 0
1000 2000 3000 4000 5000 6000
No. of diagnoses (all)
0 50 100 150 200 250 300 350 400 450 500
No. of diagnoses
(high-risk pediatric patients)
All patients
High-risk pediatric patients 0
1000 2000 3000 4000
T
o
ta
l p
o
s
itiv
e
is
o
la
te
s
40/2000 40/2001 40/2002 40/2003 40/2004 40/2005
Week/year
Study database sample period, week/year A
B
FIGURE 1
Weekly distributions of influenza-positive isolates reported to the Centers for Disease Control and Prevention and patients included in the analysis. A, The number of laboratory test–positive influenza isolates from week (Wk) 40, 2000, to week 25, 2006, as reported by the Centers for Disease Control and Prevention.18B, The same time period and shows the numbers of all high-risk pediatric patients with
an influenza diagnosis included in the present study and all eligible patients from the study database with an influenza diagnosis for the periods of October 1 to March 31, 2000 –2001, through 2005–2006.
FIGURE 2
The risk of hospitalization for any rea-son was reduced by 67% for patients given oseltamivir (HR: 0.33 [95% CI: 0.13– 0.83]) (Table 4). However, the numbers of hospitalizations attribut-able to pneumonia or other respira-tory illnesses were small, with no sig-nificant differences (Table 4). This was also the case for age-stratified hospitalization rates. Aside from pneu-monia or other respiratory illnesses, the most common reasons for hospi-talizations in the 14 days after influ-enza were hypovolemia (30%), other manifestations of influenza (18%), gastrointestinal tract-related causes (15%), and infections (9%).
In the 30 days after influenza, patients given oseltamivir had reduced risks of respiratory illnesses other than pneu-monia (risk reduction: 13%; HR: 0.87 [95% CI: 0.77– 0.97]) and otitis media and its complications (risk reduction: 30%; HR: 0.70 [95% CI: 0.53– 0.92]) (Table 4). The risk of hospitalization also was reduced (risk reduction: 51%; HR: 0.49 [95% CI: 0.27– 0.89]), al-though the numbers of patients hos-pitalized because of pneumonia or other respiratory illnesses were small. When data were stratified according to age, patients 6 to 12 years of age who were given oseltamivir had a 22% lower risk of a respiratory condition other than pneumonia (HR: 0.78 [95% CI: 0.65– 0.94]), whereas patients 13 to 17 years of age had a 74% lower risk of otitis media or its complications (HR: 0.26 [95% CI: 0.08 – 0.87]). There were no significant differences between the oseltamivir and no-antiviral groups in outcomes for patientsⱕ5 years of age or in age-stratified, all-cause hospital-ization rates. Comparable risk reduc-tions after 14 and 30 days suggested that the beneficial effects of oseltami-vir were maintained throughout the 30-day period after influenza diagno-sis, particularly for otitis media and its
TABLE 2 Demographic Characteristics of Children and Adolescents in the Oseltamivir and No-Antiviral Groups
Oseltamivir (N⫽1634)
No Antiviral Drug (N⫽3721)
P
Gender,n(%)
Male 944 (57.8) 2197 (59.0) NS
Female 690 (42.2) 1524 (41.0) NS
Age group,n(%)
1–2 y 248 (15.2) 668 (18.0) .013
3–5 y 278 (17.0) 781 (21.0) .001
6–12 y 629 (38.5) 1398 (37.6) NS
13–17 y 479 (29.3) 874 (23.5) ⬍.001
Age, mean⫾SD, y 8.69⫾4.98 7.90⫾4.96 ⬍.001
Geographic region,n(%)
Northeast 68 (4.2) 483 (13.0) ⬍.001
North central 293 (17.9) 1023 (27.5) ⬍.001
South 1203 (73.6) 1674 (45.0) ⬍.001
West 68 (4.2) 506 (13.6) ⬍.001
Unknown 3 (0.2) 35 (0.9) .002
Influenza season,n(%)
2000–2001 43 (2.6) 297 (8.0)
2001–2002 48 (2.9) 494 (13.3)
2002–2003 173 (10.7) 655 (17.6)
2003–2004 272 (16.6) 813 (21.8)
2004–2005 501 (30.7) 801 (21.5)
2005–2006 597 (36.5) 661 (17.8)
Commercial payer,n(%) 1634 (100.0) 3721 (100.0)
Pvalues of⬍.05 were considered statistically significant. NS indicates not significant.
TABLE 3 Baseline Medical Histories of Children and Adolescents in the Oseltamivir and No-Antiviral Groups
High-Risk Condition Oseltamivir No Antiviral
Drug
P
Cardiac disease,n(%) 67 (4.1) 141 (3.8) NS
Coronary artery disease 2 (0.1) 6 (0.2) NS
Other cardiac disease 65 (4.0) 135 (3.6) NS
Chronic lung disease,n(%) 1334 (81.6) 3008 (80.8) NS
Asthma 1270 (77.7) 2923 (78.6) NS
Other chronic lung disease 22 (1.3) 31 (0.8) NS
Cystic fibrosis 4 (0.2) 11 (0.3) NS
Pulmonary collapse/interstitial emphysema 3 (0.2) 10 (0.3) NS
Pulmonary fibrosis 1 (0.1) 1 (⬍0.1) NS
Other alveolar/parietoalveolar pneumopathy 1 (0.1) 0 (0) NS
Respiratory conditions of fetus/newborn 8 (0.5) 29 (0.8) NS
Diabetes mellitus,n(%) 83 (5.1) 179 (4.8) NS
Immunocompromised,n(%) 27 (1.7) 89 (2.4) NS
Cancer (chemotherapy) 3 (0.2) 17 (0.5) NS
HIV/AIDS 21 (1.3) 67 (1.8) NS
Transplant 3 (0.2) 6 (0.2) NS
Renal dysfunction,n(%) 10 (0.6) 13 (0.3) NS
Central nervous system disorders/diseases,n(%) 155 (9.5) 377 (10.1) NS
Neuromuscular diseases,n(%) 7 (0.4) 10 (0.3) NS
Other medical history,n(%)
Respiratory therapy 1364 (83.5) 3082 (82.8) NS
Oxygen therapy 59 (3.6) 126 (3.4) NS
Respiratory diagnoses 1260 (77.1) 2840 (76.3) NS
Pneumonia 157 (9.6) 396 (10.8) NS
Influenza test on/before influenza diagnosis date 825 (50.5) 655 (17.6) ⬍.001
Vaccination before influenza diagnosis date 353 (21.6) 697 (18.7) .015
Charlson Comorbidity Index score, mean⫾SD 1.34⫾1.88 1.33⫾1.91 NS
Baseline health care expenditures, mean⫾SD, dollars 5070⫾11 978 4889⫾11 984 NS
complications (eg, 31% reduction at 14 days and 30% at 30 days) (Table 4).
DISCUSSION
This retrospective study of data from 6 influenza seasons in the United States provides evidence from the clinical practice setting that, when it is pre-scribed for the treatment of influenza, oseltamivir reduces the risk of compli-cations in children and adolescents with chronic medical conditions. The relative risks of respiratory illnesses other than pneumonia, otitis media and its complications, and all-cause hospitalization were all significantly reduced for patients given oseltamivir. Significant risk reductions were ap-parent 14 and 30 days after influenza diagnosis.
All-cause hospitalization was selected as an outcome for this study because several chronic medical conditions (eg, cardiovascular disease19) may be
exacerbated by influenza, resulting in increased rates of hospitalizations that may not be recorded through
cod-HR
FIGURE 3
Clinical outcomes for children at high risk with an influenza diagnosis who were given or were not given oseltamivir. Relative risks (as HRs) of pneumonia, respiratory illnesses other than pneumonia (other respiratory diagnosis), otitis media or its complications, and hospitalization for any reason during the 14 days after the influenza diagnosis for patients given oseltamivir versus no antiviral therapy are presented; results for all children and adolescents were stratified according to age. Dashed horizontal lines indicate upper 95% confidence limits of⬎2.0.
TABLE 4 Relative Risks (as HRs) of Pneumonia, Other Respiratory Illnesses, Otitis Media, and Hospitalization in the 14- and 30-Day Periods After Influenza Diagnosis, With and Without Oseltamivir
n(%) HR (95% CI)
Oseltamivir (N⫽1634)
No Antiviral Drug (N⫽3721)
Unadjusted Adjusted
Outcomes in 14 d after influenza diagnosis
Pneumonia 17 (1.0) 71 (1.9) 0.54 (0.32–0.92) 0.55 (0.29–1.03)
Respiratory illnesses other than pneumonia 324 (19.8) 885 (23.8) 0.81 (0.71–0.92) 0.74 (0.63–0.87)b
Otitis media/complications 46 (2.8) 184 (4.9) 0.56 (0.41–0.78) 0.69 (0.48–0.99)b
All-cause hospitalizationsa 10 (0.6) 48 (1.3) 0.47 (0.24–0.93) 0.33 (0.13–0.83)b
Pneumonia-related hospitalizations 2 (0.1) 13 (0.3) 0.35 (0.08–1.55) 0.49 (0.09–2.49)
Hospitalizations related to respiratory illnesses other than pneumonia
1 (0.1) 9 (0.2) 0.25 (0.03–2.00) 0.23 (0.03–2.09)
Outcomes in 30 d after influenza diagnosis
Pneumonia 26 (1.6) 91 (2.4) 0.65 (0.42–1.00) 0.67 (0.42–1.07)
Respiratory illnesses other than pneumonia 498 (30.5) 1201 (32.3) 0.91 (0.82–1.01) 0.87 (0.77–0.97)b
Otitis media/complications 75 (4.6) 276 (7.4) 0.61 (0.47–0.79) 0.70 (0.53–0.92)b
All-cause hospitalizationsa 15 (0.9) 61 (1.6) 0.56 (0.32–0.98) 0.49 (0.27–0.89)b
Pneumonia-related hospitalizations 4 (0.2) 16 (0.4) 0.57 (0.19–1.70) 0.56 (0.17–1.83)
Hospitalizations related to respiratory illnesses other than pneumonia
3 (0.2) 14 (0.4) 0.49 (0.14–1.70) 0.34 (0.09–1.20)
aOther than pneumonia and other respiratory illnesses, causes of hospitalizations included hypovolemia, other manifestations of influenza, gastrointestinal tract-related causes, and
infections.
ing as being attributable to influenza. Inclusion of this outcome also reduced the risk of hospitalizations being over-looked because of coding errors. Eval-uations of hospitalizations attribut-able to pneumonia or respiratory illnesses other than pneumonia and of age-stratified hospitalizations were constrained by the small numbers of patients in these categories.
Vaccination status was included as a factor in the multivariate models. How-ever, the finding that more children and adolescents who were given osel-tamivir in this study had received influ-enza vaccine and had been tested for influenza suggests that those patients were more likely to have used health care services than were patients who were not given antiviral medications. In addition, it has been noted that a beneficial effect is not always ob-served for patients who receive inacti-vated influenza vaccine where there are differences in health care utiliza-tion between study groups.20Risk
re-ductions were observed for children and adolescents given oseltamivir, however. Finally, the fact that more pa-tients in the oseltamivir group were tested for influenza might indicate simply that physicians are more likely to test for influenza if they are consid-ering prescribing oseltamivir.
This study included children and ado-lescents who were considered to be at high risk of influenza complications because they had an active chronic medical condition within the 12 months preceding influenza diagnosis. In healthy pediatric patients, influenza can cause significant morbidity and particularly virulent strains can be fa-tal.3,21,22For patients with chronic
med-ical conditions, the risk of complica-tions has been shown to be substantial and higher. Hospitalization rates for acute respiratory disease among 5- to 17-year-old patients with chronic health conditions have been reported
to be 10- to 20-fold higher than those among healthy children.23In one study,
influenza accounted for up to 19 per 1000 hospitalizations attributable to cardiopulmonary disease among chil-dren at high risk over a 20-year peri-od.24In another study, pulmonary and
neurologic disorders were reportedly the most common underlying condi-tions leading to hospitalization, ac-counting for 18% and 11%, respec-tively, of pediatric influenza-related admissions.25 More than 70% of
pa-tients in the present study had a his-tory of asthma (Table 3). Overall, these patients had a higher risk of an acute asthma event as a complication of in-fluenza. However, statistical analysis of acute asthma events or other non-pneumonia respiratory illnesses (eg, pharyngitis, allergic rhinitis, bronchi-tis, or bronchiolitis) was not conducted. Influenza occurs more frequently among and can have serious consequences for immunocompromised children, such as those who are undergoing or have undergone treatment for cancer.26,27
Similarly, influenza carries increased risks of morbidity, transplant rejection, and death for pediatric organ transplant recipients.28–30
An analysis of the US 2000 National Health Interview Survey data identified 5 to 10 million children 6 months to 17 years of age with high-risk conditions warranting influenza vaccination.31In
addition to achieving vaccination for as many of these patients as possible, effective timely treatment in the event of influenza is required. There has been a lack of data on the effective-ness of neuraminidase inhibitors for these patients, however. This study provides some evidence in addressing this need. The findings presented here are consistent with those of studies with otherwise healthy adults and chil-dren, which also demonstrated the ef-ficacy or effectiveness of oseltamivir in
reducing secondary complications of influenza.9,10,32
Some potential limitations of this study should be acknowledged. First, the database is limited primarily to pa-tients covered by employer-sponsored health insurance. Second, the time period of October 1 to March 31 was fixed to ensure consistency between seasons, and cases occurring later or earlier would not have been in-cluded. For example, influenza activity in the 2005–2006 season peaked in the middle of March and remained ele-vated for longer than in the preceding 3 seasons.33
Use of diagnostic coding also imposes limitations. Codes for influenza were assigned on the basis of physicians’ clinical diagnoses alone, and testing for influenza virus was not required. This is arguably an accurate reflection of real-world conditions because, when influenza is known to be circulat-ing in the community, most physicians still tend to use clinical diagnoses only. It could be argued that this approach to diagnosis is conservative, because clinicians seem to underrecognize or to underdiagnose influenza in chil-dren.34In addition, the weekly
distribu-tion of influenza cases in this study closely reflected the distribution of laboratory-confirmed influenza cases reported by the Centers for Disease Control and Prevention. Without labo-ratory confirmation, however, diag-nostic accuracy and possible differ-ences in the proportions of influenza A and B strain infections between the 2 patient groups might bias the out-comes. Adjustment for season and geographic region should have helped to decrease the likelihood of differ-ences in strains between the 2 groups, whereas the large sample size should have reduced the impact of minor vari-ations in diagnostic accuracy.
rec-ommended 48 hours, patients were in-cluded in the oseltamivir group only if the drug was prescribed within 1 day after the physician’s influenza diagno-sis; however, it was impossible to con-firm that patients then began treat-ment within the recommended time frame. If they did not always do so, as is probable, then there would have been bias against findings of a treatment ef-fect (ie, risk reduction) with oseltami-vir. It also could be speculated that some patients were not given oselta-mivir and thus were included in the no-antiviral group because their physi-cians decided that they were already outside the 48-hour limit. The impact of this factor on the results is unclear; any delay in these patients visiting their physicians might have resulted in more-severe influenza symptoms and thus a greater risk of complications. Conversely, patients with more-severe influenza symptoms might have visited a physician sooner, and patients who were outside the 48-hour period might not have sought advice earlier be-cause they had less-severe symptoms. Furthermore, the potential for
varia-tions in the timing of presentation would have been balanced because outcomes were assessed for both groups for the same time periods after diagnosis.
Identification of inpatient pneumonia and respiratory illnesses other than pneumonia through primary dis-charge diagnoses was required to en-sure accuracy of coding, although some cases might have been missed if they were recorded with secondary di-agnoses at discharge. Records of these diagnoses are thus likely to be conservative for both patient groups.
Finally, this was a retrospective study in which patients were neither as-signed randomly nor matched with re-spect to their propensity to be given oseltamivir. However, other than those already discussed, there were few po-tentially clinically significant differ-ences between the 2 patient cohorts, and multivariate analyses were used to adjust for those differences.
CONCLUSIONS
In this study, prescription of oseltami-vir was associated with reductions in
the relative risks of respiratory ill-nesses other than pneumonia, otitis media and its complications, and all-cause hospitalizations in children and adolescents considered to be at partic-ularly high risk of influenza complica-tions. These results provide the first evidence that, when it is prescribed at the time of presentation of clinically diagnosed influenza, oseltamivir may be of benefit to pediatric patients considered to be at high risk of mor-bidity and secondary infections after influenza because of chronic medical conditions.
ACKNOWLEDGMENTS
Funding for this study was provided by Roche (Nutley, NJ).
We thank Xue Song, PhD (Thomson Reuters), for work on the statistical analysis; Robert Sedgley, BS (Thomson Reuters), for programming and analy-sis; and Nicole Morandi, MS (Thomson Reuters), and Annie Rowe, PhD (Envi-sion Pharma, Horsham, United King-dom), for editorial assistance.
REFERENCES
1. Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep.2006;55(RR-10):1– 42
2. American Academy of Pediatrics, Committee of Infectious Diseases. Reduction of the influenza burden in children.Pediatrics.2002;110(6):1246 –1252
3. Louie JK, Schechter R, Honarmand S, et al. Severe pediatric influenza in California, 2003–2005: implications for immunization recommendations. Pediatrics.2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e610
4. Loughlin J, Poulios N, Napalkov P, Wegmuller Y, Monto AS. A study of influenza and influenza-related complications among children in a large US health insurance plan database. Pharmaco-economics.2003;21(4):273–283
5. Keren R, Zaoutis TE, Bridges CB, et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA.2005;294(17): 2188 –2194
6. Quach C, Piche-Walker L, Platt R, Moore D. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics. 2003;112(3). Available at: www.pediatrics.org/cgi/content/full/112/3/e197
7. Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuramini-dase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials.BMJ.2003;326(7401):1235–1238
9. Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J.2001;20(2):127–133
10. Barr CE, Schulman KS, Iacuzio D, Bradley JS. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza.Curr Med Res Opin.2007; 23(3):523–531
11. Machado CM, Boas LS, Mendes AV, et al. Use of oseltamivir to control influenza complications after bone marrow transplantation.Bone Marrow Transplant.2004;34(2):111–114
12. Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population.Curr Med Res Opin.2006;22(1):75– 82
13. Johnston SL, Ferrero F, Garcia ML, Dutkowski R. Oral oseltamivir improves pulmonary function and reduces exacerbation frequency for influenza-infected children.Pediatr Infect Dis J.2005; 24(3):225–232
14. Roche Clinical Trials Database. A double-blind, randomized, stratified, placebo-controlled study of oseltamivir in the treatment of influenza in children with asthma (protocol NV16871). Available at: www.roche-trials.com/patient/trialresults/stur40.html. Accessed June 30, 2008
15. Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial.JAMA.2000;284(13):1655–1663
16. Roche.Tamiflu: Summary of Product Characteristics. Nutley, NJ: Roche; 2006
17. Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders.Am J Epidemiol. 2003;158(3):280 –287
18. Centers for Disease Control and Prevention. Flu activity and surveillance. Available at: www. cdc.gov/flu/weekly/fluactivity.htm. Accessed January 23, 2008
19. Davis MM, Taubsert K, Benin AL, et al. Influenza vaccination as secondary prevention for cardio-vascular disease: a science advisory from the American Heart Association/American College of Cardiology.Circulation.2006;114(14):1549 –1553
20. Gaglani M, Riggs M, Kamenicky C, Glezen WP. A computerized reminder strategy is effective for annual influenza immunization of children with asthma or reactive airway disease.Pediatr Infect Dis J.2001;20(12):1155–1560
21. Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004.N Engl J Med.2005;353(24):2559 –2567
22. Centers for Disease Control and Prevention. Severe morbidity and mortality associated with influenza in children and young adults: Michigan, 2003.MMWR Morb Mortal Wkly Rep.2003;52(35): 837– 840
23. Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respi-ratory disease among infants and young children.N Engl J Med.2000;342(4):232–239
24. Neuzil KM, Wright PF, Mitchel EF Jr, Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions.J Pediatr.2000;137(6):856 – 864
25. Moore DL, Vaudry W, Scheifele DW, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediat-rics.2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e610
26. Kempe A, Hall CB, MacDonald NE, et al. Influenza in children with cancer.J Pediatr.1989;115(1): 33–39
27. Chisholm JC, Devine T, Charlett A, Pinkerton CR, Zambon M. Response to influenza immunisation during treatment for cancer.Arch Dis Child.2001;84(6):496 –500
28. Vilchez RA, Fung J, Kusne S. The pathogenesis and management of influenza virus infection in organ transplant recipients.Transpl Infect Dis.2002;4(4):177–182
29. Mauch TJ, Bratton S, Myers T, Krane E, Gentry SR, Kashtan CE. Influenza B virus infection in pediatric solid organ transplant recipients.Pediatrics.1994;94(2):225–259
30. Apalsch AM, Green M, Ledesma-Medina J, Nour B, Wald ER. Parainfluenza and influenza virus infections in pediatric organ transplant recipients.Clin Infect Dis.1995;20(2):394 –399
31. Erhart LM, Rangel MC, Lu PJ, Singleton JA. Prevalence and characteristics of children at increased risk for complications from influenza, United States, 2000.J Pediatr.2004;144(2):191–195
32. Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003; 163(14):1667–1672
33. Centers for Disease Control and Prevention. 2005– 06 US influenza season summary. Available at: www.cdc.gov/flu/weekly/weeklyarchives2005-2006/05-06summary.htm. Accessed June 22, 2007
DOI: 10.1542/peds.2008-0977
2009;124;170
Pediatrics
Pedro A. Piedra, Kathy L. Schulman and William A. Blumentals
Services
Updated Information &
http://pediatrics.aappublications.org/content/124/1/170 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/124/1/170#BIBL This article cites 27 articles, 5 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/therapeutics_sub Therapeutics
http://www.aappublications.org/cgi/collection/pharmacology_sub Pharmacology
http://www.aappublications.org/cgi/collection/influenza_sub Influenza
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su Infectious Disease
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2008-0977
2009;124;170
Pediatrics
Pedro A. Piedra, Kathy L. Schulman and William A. Blumentals
Chronic Medical Conditions
Effects of Oseltamivir on Influenza-Related Complications in Children With
http://pediatrics.aappublications.org/content/124/1/170
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.