R E S E A R C H
Open Access
Reduced expression of miR-22 in gastric cancer is
related to clinicopathologic characteristics or
patient prognosis
Weibin Wang
1, Fujun Li
1*, Yong Zhang
2, Yanyang Tu
3, Qi Yang
3and Xingchun Gao
3Abstract
Objective:Involvements of microRNA-22 (miR-22) in cancer development have attracted much attention, but its role in tumorigenesis of gastric cancer is still largely unknown. Therefore, the aim of this study was to investigate the expression patterns and clinical implications of miR-22 in gastric cancer.
Methods:Quantitative RT-PCR was performed to evaluate the expression levels of miR-22 in 98 pairs of gastric cancer and normal adjacent mucosa.
Results:Compared with normal adjacent mucosa, miR-22 expression was significantly downregulated in gastric cancer tissues (P < 0.001). Of 98 patients with gastric cancer, 58 (59.2%) were placed in the low miR-22 expression group and 40 (40.8%) were placed in the high miR-22 expression group. In addition, tumors with low miR-22 expression had greater extent of lymph node metastasis (P = 0.02) and distant metastasis (P = 0.01), and were at a worse stage (P = 0.01) than the tumors with high miR-22 expression. Moreover, the gastric cancer patients with low miR-22 expression had shorter overall survival than those with high miR-22 expression
(P = 0.03). MiR-22, determined by multivariate analysis, was an independent prognostic factor for patients with gastric cancer.
Conclusion: Our data offer the convincing evidence that the reduced expression of miR-22 was significantly associated with malignant development of gastric cancer and may be a novel prognostic marker of this disease. miR-22 might have potentials in the application of cancer therapy for patients with gastric cancer.
Keywords: MicroRNA-22, Gastric cancer, Prognosis, Quantitative RT-PCR
Introduction
Gastric cancer is the fourth most prevalent forms of human cancers and the second leading cause of cancer-related death in the world, especially in East Asian coun-tries. Its incidence rate is 20 per 100,000 annually [1]. According to its histological subtypes, gastric cancer can be divided into two groups: the intestinal type and the diffuse type. The former is characterized by expansive growth and liver metastasis; whereas the latter is charac-terized by infiltrative growth and peritoneal dissemin-ation [2-4]. They are both associated with Helicobacter
pylori infection that contributes to more than 80% of cases [5]. Nowadays, gastrectomy remains the mainstay treatment for gastric cancer, but the prognosis for ad-vanced stage patients is still very poor. The median survival time for patients with gastric cancer is only 6–9 months [6]. In China, the 5-year overall survival rate of patients with gastric cancer is lower than 40%, al-though recent advances in chemotherapy and surgical techniques [7]. This is primarily attributed to the follo-wing reasons: lack of diagnostic markers for early detec-tion, weak prognostic value of histological indicators, limited efficiency of current treatment for advanced dis-ease and lack of molecular markers utilized for targeted therapy [8-10]. Therefore, it is of great significance to make a better understanding of gastric carcinogenesis * Correspondence:18992891737@QQ.com
1
Department of General Surgery, The 323th Hospital of PLA, Xi’an 710054, China
Full list of author information is available at the end of the article
and to identify novel molecular markers for the im-provement of clinical management of patients with gas-tric cancer.
MicroRNAs (miRNAs) are a recently discovered ca-tegory of small (19 ~ 24 nucleotides), non-protein-coding and single-stranded endogenous RNA molecules [11]. miRNAs function as regulators of approximately 60%
protein-coding genes’ expression mainly at the
post-transcriptional level by binding to the sequences in the
3′ untranslated regions (3′-UTR) of their targeted
mRNAs resulting in translational repression or gene si-lencing [12]. As they are involved in regulation of wide array of biological processes including cell proliferation, differentiation, apoptosis, metastasis, angiogenesis and immune response, miRNAs have been considered to be new approaches of tumor biomarkers for early cancer diagnosis and prognosis. They may play roles in the de-velopment and progression of cancers similar to those played by oncogenes or tumor suppressor genes. Recent studies have identified a number of miRNAs with aber-rant expression in gastric cancer. For example, the com-parison of miRNAs deregulated in gastric cancer revealed a significant increase of several tumor-associated miRNAs such as miR-21, -25 and -106a and miRNAs from the miR-17-92 cluster [13]; based on the cluster analyses,
eight miRNAs (including miR-100, -143 and−145) were
upregulated specifically in diffuse-type, while four miRNA (miR-202, -373, -494 and −498) in intestinal-type gastric cancer [14]. In our previous study, we found that the downregulation of miR-206 was significantly correlated with tumor progression and may be a potent prognostic marker of gastric cancer [15]. According to our literature retrieval, miR-22 has been demonstrated to play important roles in different types of cancer, such as hepatocellular carcinoma, breast cancer, colon cancer, lung cancer, and prostate cancer [16-22]. However, its roles in tumorige-nesis of gastric cancer are still unknown. Because of its involvement in several tumors in digestive system (hepato-cellular carcinoma and colon cancer), we hypothesized that miR-22 might play a role in gastric cancer. Thus, the aim of the present study was to investigate the expression patterns and clinical implications of miR-22 in gastric cancer.
Materials and methods Patients and tissue samples
This study was approved by the Research Ethics Commit-tee of the 323th Hospital of PLA and Tangdu Hos-pital of the Forth Military Medical University, China. Written informed consent was obtained from all patients. All specimens were handled and made anonymous ac-cording to the ethical and legal standards.
Ninety-eight pairs of gastric cancer and matched nor-mal adjacent mucosa were resected from gastrectomy
with lymph node dissection between 1999 and 2007 at Department of General Surgery. These patients with gas-tric cancer included 62 males and 36 females, ranged in age from 21 to 86 years (mean 63 years). Clinicopatho-logic findings were based on the criteria of the tumor node metastasis (TNM) classification of the Internatio-nal Union against Cancer [23]. Histopathological types of gastric cancer were classified into two types, intestinal type and diffuse type. The intestinal type was further classified into three differentiated types: well-differenti-ated (tub1), moderately differentiwell-differenti-ated (tub2), and papil-lary differentiated (pap). The diffuse type was classified into two undifferentiated types: diffuse-adherent (por1) and diffuse-scattered (por2). None of these patients un-derwent endoscopic mucosal resection, palliative resec-tion, or preoperative chemotherapy, or had synchronous or metachronous multiple cancer in other organs. The clinicopathologic features of these patients with gastric cancer were summarized in Table 1.
All patients had follow-up after surgery, with X-ray examination and tumor marker assays
(carcinoembryo-nic antigen and carbohydrate antigen 19–9) performed
every 1–3 months, computed tomography performed
every 3–6 months, and ultrasonography performed every
6 months. Median follow up period was 38 (range 6 to 139) months for all patients. Overall survival was defined as the period between the time of surgery and death or was censored at the last follow-up. Patients, who died of diseases not directly related to their gastric cancers or due to unexpected events, were excluded from this study.
Quantitative RT-PCR
In order to detect the expression levels of miR-22 in gas-tric cancer and matched normal adjacent mucosa, quan-titative RT-PCR was performed. Briefly, total RNAs, including the miRNAs, were extracted from 98 primary gastric cancer tissues and matched normal adjacent mucosa using the miRNeasy Mini Kit (Qiagen Inc.,
Valencia, CA, USA) according to the user’s
instruc-tion. cDNA was synthesized from 10 ng of total RNA
using TaqMan™ MicroRNA hsa-miR-22 specific primer
(Applied Biosystems) and a TaqMan™ MicroRNA
Re-verse Transcription Kit (Applied Biosystems). RNU6B was used as an internal control. The reverse transcriptase reac-tions contained 10 ng of total RNAs, 50 nmol/stem-loop RT primer, 1X RT buffer, 0.25 mmol/L each of dNTP, 3.3 U/μL MultiScribe reverse transcriptase, and 0.2 U/μL
RNase inhibitor. The 15μL reaction samples were
10 min, followed by 44 cycles of denaturation at 95°C for 10 sec, annealing at 56°C for 10 sec, and extension at 60°C for 10 sec. Analysis was performed by the comparative threshold cycle (Ct) method according to User Bulletin no.2 (Applied Biosystems). Each sample was examined in triplicate and the amounts of the PCR products produced were normalized to RNU6B.
Statistical analysis
The software of SPSS version12.0 for Windows (SPSS Inc, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) was used for statistical analysis. Data were expressed as means ± standard deviation (SD). The differential expres-sion of miR-22 between gastric cancer and matched nor-mal adjacent mucosa was evaluated by paired sample
t test. The Χ2test was used to analyze the relationship between miR-22 expression and the clinicopathologic
characteristics. The Kaplan–Meier method was used for
survival analysis, and differences in survival were esti-mated using the log-rank test. Prognostic factors were examined by univariate and multivariate analyses (Cox proportional hazards regression model). Differences
were considered statistically significant when p was
less than 0.05.
Results
Reduced expression of microRNA-22 is associated with advanced clinicopathologic characteristics of patients with gastric cancer
Quantitative RT-PCR was performed to detect the differ-ential expression of miR-22 in 98 pairs of gastric cancer and matched normal adjacent mucosa tissues normalized to RNU6B. As a result, miR-22 expression in gastric can-cer was significantly lower than that in normal adjacent mucosa (mean ± SD: 2.1 ± 1.2 vs. 3.6 ± 1.3, P < 0.001, Figure 1).
The 98 patients with gastric cancer were classified into two groups according to the median expression level of miR-22 (2.2, normalized to RNU6B) as determined by quantitative RT-PCR. Of 98 patients with gastric cancer, 58 (59.2%) were placed in the low miR-22 expression group and 40 (40.8%) were placed in the high miR-22 expression group. The association between clinicopatho-logic features and miR-22 expression was summarized in Table 1. Tumors with low miR-22 expression had greater extent of lymph node metastasis (P = 0.02) and distant metastasis (P = 0.01), and were at a worse stage (P = 0.01) than the tumors with high miR-22 expression.
Reduced expression of microRNA-22 confers poor prognosis in patients with gastric cancer
All 98 patients with gastric cancer received follow-up after surgery. No patient died of postoperative complica-tions within 30 days at the beginning of the study period.
Table 1 Correlations of miR-22 expression with the clinicopathological features of primary gastric cancer
Features No. of cases
miR-22 expression P
High Low
Age (years) 98 62.8 ± 23.2 62.1 ± 23.9 NS
Gender
Male 62 (63.3) 25 (40.3) 37 (59.7) NS
Female 36 (36.7) 15 (41.7) 21 (58.3)
Histopathological type
Intestinal type
pap 5 (5.1) 2 (40.0) 3 (60.0) NS
tub1 20 (20.4) 9 (45.0) 11 (55.0)
tub2 25 (25.5) 8 (32.0) 17 (68.0)
Diffuse type
por1 15 (15.3) 6 (40.0) 9 (60.0) NS
por2 33 (33.7) 15 (45.5) 18 (54.5)
Tumor depth (pT)
pT1 40 (40.8) 20 (50.0) 20 (50.0) NS
pT2 30 (30.6) 10 (33.3) 20 (66.7)
pT3 20 (20.4) 8 (40.0) 12 (60.0)
pT4 8 (8.2) 2 (25.0) 6 (75.0)
Lymph node metastasis (pN)
pN0 50 (51.0) 27 (54.0) 23 (46.0) 0.02
pN1 20 (20.4) 8 (40.0) 12 (60.0)
pN2 15 (15.3) 4 (26.7) 11 (73.3)
pN3 13 (13.3) 1 (7.7) 12 (92.3)
Distant metastasis (pM)
pM0 86 (87.8) 39 (45.3) 47 (54.7) 0.01
pM1 12 (12.2) 1 (8.3) 11 (91.7)
pStage
I 50 (51.0) 29 (58.0) 21 (42.0) 0.02
II 15 (15.3) 5 (33.3) 10 (66.7)
III 20 (20.4) 5 (25.0) 15 (75.0)
IV 13 (13.3) 1 (7.7) 12 (92.3)
Lymphatic invasion
Negative 45 (45.9) 17 (37.8) 28 (62.2) NS
Positive 53 (54.1) 23 (43.4) 30 (56.6)
Venous invasion
Negative 68 (69.4) 23 (33.8) 45 (66.2) NS
Positive 30 (30.6) 17 (56.7) 13 (43.3)
Hematogenous recurrence
Negative 78 (79.6) 32 (41.0) 46 (59.0) NS
Positive 20 (20.4) 8 (40.0) 12 (60.0)
The 5-year survival rate of patients with tumors with high miR-22 expression was 82.5% (33/40), whereas the rate for patients with low miR-22 expression was 58.6% (34/58). Thus, the gastric cancer patients with low miR-22 expression had shorter overall survival than those with high miR-22 expression (P = 0.03, Figure 2).
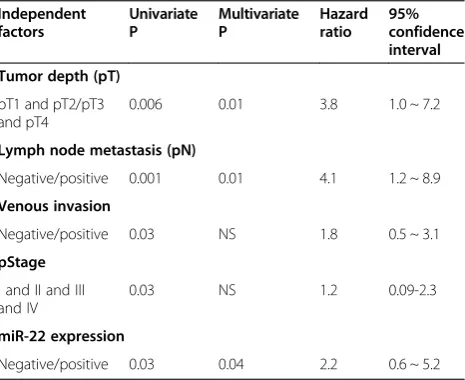
The univariate and multivariate analyses were also performed to identify factors related to patient progno-sis. As shown in Table 2, the univariate analysis showed that the depth of tumor invasion (P = 0.006), lymph node metastasis (P = 0.001), venous invasion (P = 0.03), tumor stage (P = 0.03) and miR-22 expression (P = 0.03) were significantly related to postoperative survival. Moreover, the multivariate regression analysis indicated that the depth of invasion (P = 0.01), lymph node metastasis (P = 0.01) and miR-22 expression (P = 0.04) were indepen-dent prognostic factors for patients with gastric cancer.
Discussion
Accumulating evidences have demonstrated that miRNAs play important roles in various physiological and patho-logical processes, and have a robust association with car-cinogenesis. miRNAs have been considered to be novel biomarkers for various cancers. Among human miRNAs, miR-22 is located at a fragile cancer-relevant genomic re-gion in chromosome 17 (17p13.3), and is mapped to an exon of the C17orf91 gene [24]. This miRNA plays unique roles in specific cell types. For example, it regulates PPAR-alpha and BMP7 signaling pathways in human chondro-cytes [25], and the differentiation of a monocyte cell line [26]. Recent studies have demonstrated that miR-22 is deregulated in many types of cancers and is involved in various cellular processes related to carcinogenesis, in-cluding cell growth, apoptosis, motility, and cell cycle. Zhang et al. [16] indicated that miR-22 was downregula-ted in hepatocellular carcinoma and had considerable
potential in identification of the prognosis; Xiong et al. [17] found that miR-22 was frequently downregulated in ERα-positive human breast cancer cell lines and clinical samples; Li et al. [18] identified miR-22 as a potential me-tastasis-inhibitor in ovarian cancer; Yamakuchi et al. [19] found that miR-22 expression in human colon cancer was lower than in normal colon tissue, and it might have an anti-angiogenic effect in this cancer; Ling et al. [20] ob-served the downregulation of miR-22 in lung cancer tis-sues and lung cancer cell lines, and also suggested that miR-22 might exhibit excellent anti-lung cancer activity in vitro and in vivo. All these studies suggest that miR-22 may act as a tumor suppressor. In contrast, Poliseno et al.
Figure 2Postoperative 5-year survival curves of patients
according to the expression of miR-22.The gastric cancer
patients with low miR-22 expression had shorter overall survival than those with high miR-22 expression (P = 0.03).
Figure 1Expression levels of miR-22 in 98 pairs of gastric
cancer tissues and normal adjacent gastric mucosa.miR-22
expression was significantly downregulated in gastric cancer tissues when compared with normal adjacent mucosa (P < 0.001).
Table 2 Univariate and multivariate analyses of prognostic factors in gastric cancer
Independent factors
Univariate P
Multivariate P
Hazard ratio
95% confidence interval
Tumor depth (pT)
pT1 and pT2/pT3 and pT4
0.006 0.01 3.8 1.0 ~ 7.2
Lymph node metastasis (pN)
Negative/positive 0.001 0.01 4.1 1.2 ~ 8.9
Venous invasion
Negative/positive 0.03 NS 1.8 0.5 ~ 3.1
pStage
I and II and III and IV
0.03 NS 1.2 0.09-2.3
miR-22 expression
Negative/positive 0.03 0.04 2.2 0.6 ~ 5.2
[21] showed that miR-22 was aberrantly overexpressed in human prostate cancer; Liu et al. [22] have reported that miR-22 might act as a micro-oncogene in transformed hu-man bronchial epithelial cells induced by anti-BPDE. These controversial findings of miR-22 in cancer develop-ment suggest the diverse roles of miR-22 in different types of cancer. In the present study, we confirmed that miR-22 expression was frequently reduced in gastric cancer tissues than in their normal adjacent mucosa. Moreover, the downregulation of miR-22 was found to be more fre-quently occurred in gastric cancer tissues with great ex-tent of lymph node and distant metastases, and with an advanced stage.
To our knowledge, the invasion and metastasis of tumor cells are major causes of mortality in cancer pa-tients. Therefore, the potential value of miR-22 as a prognostic marker is of interest. Yet, there has been only one study that has attempted to identify the prognostic value of miR-22 for hepatocellular carcinoma. Using 160 primary hepatocellular carcinoma cases, Zhang et al. [16] found that low miR-22 expression correlated with poor overall survival. In line with this finding, we ana-lyzed not only the Kaplan-Meier survival curve but also applied Cox multivariate analysis to clarify the prognos-tic value of miR-22 in gastric cancer. Notably, we dem-onstrated that low miR-22 expression in gastric cancer tissues significantly correlated with poorer overall sur-vival. Furthermore, in Cox multivariate analysis, miR-22 expression in gastric cancer tissues showed a significant association with overall survival.
In conclusion, our data offer the convincing evidence that the reduced expression of miR-22 was significantly associated with malignant development of gastric cancer and may be a novel prognostic marker of this disease. miR-22 might have potentials in the application of cancer therapy for patients with gastric cancer. How-ever, our study is limited by the number of study cases with relatively small subgroups. Further investi-gations with a larger number of cases would allow us to evaluate miR-22 in a variety of clinical settings and help us better understand its unique role in cancer progression.
Competing interests
The authors declare that they have no competing interests.
Authors’contributions
WW and FL designed the study, carried out the experiments and drafted the manuscript; YZ, YT, QY and XG participated in the experiments and data analysis. All authors read and approved the final manuscript.
Author details
1
Department of General Surgery, The 323th Hospital of PLA, Xi’an 710054, China.2Authorities outpatient Department of Lanzhou Military region, Lanzhou 730000, China.3Department of Experimental Surgery, Tangdu Hospital, Fourth Military Medical University, Xi’an City 710038, P.R. China.
Received: 25 April 2013 Accepted: 3 June 2013 Published: 21 June 2013
References
1. Siegel R, Naishadham D, Jemal A:Cancer statistics, 2013.CA Cancer J Clin
2013,63:11–30.
2. Ye YW, Dong RZ, Zhou Y, Du CY, Wang CM, Fu H, Shi YQ:Prognostic analysis of familial gastric cancer in Chinese population.J Surg Oncol
2011,104:76–82.
3. Shan L, Ying J, Lu N:HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population.Diagn Pathol2013,8:76.
4. Liu X, Xiong H, Li J, He Y, Yuan X:Correlation of hK6 expression with tumor recurrence and prognosis in advanced gastric cancer.
Diagn Pathol2013,8:62.
5. Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ:MicroRNA
dysregulation in gastric cancer: a new player enters the game.Oncogene
2010,29:5761–5771.
6. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D:Global cancer statistics.CA Cancer J Clin2011,61:69–90.
7. Zhang YZ, Zhang LH, Gao Y, Li CH, Jia SQ, Liu N, Cheng F, Niu DY, Cho WC, Ji JF, Zeng CQ:Discovery and validation of prognostic markers in gastric cancer by genome-wide expression profiling.World J Gastroenterol2011, 17:1710–1717.
8. Jia YF, Xiao DJ, Ma XL, Song YY, Hu R, Kong Y, Zheng Y, Han SY, Hong RL, Wang YS:Differentiated embryonic chondrocyte-expressed gene 1 is associated with hypoxia-inducible factor 1αand Ki67 in human gastric cancer.Diagn Pathol2013,8:37.
9. Jin J, Jin T, Quan M, Piao Y, Lin Z:Ezrin overexpression predicts the poor prognosis of gastric adenocarcinoma.Diagn Pathol2012,7:135. 10. Sotoudeh K, Hashemi F, Madjd Z, Sadeghipour A, Molanaei S, Kalantary E:
The clinicopathologic association of c-MET overexpression in Iranian gastric carcinomas; an immunohistochemical study of tissue microarrays.
Diagn Pathol2012,7:57.
11. Doench JG, Sharp PA:Specificity of microRNA target selection in translational repression.Genes Dev2004,18:504–511.
12. Esquela-Kerscher A, Slack FJ:Oncomirs-microRNAs with a role in cancer.
Nat Rev Cancer2006,6:259–269.
13. Link A, Kupcinskas J, Wex T, Malfertheiner P:Macro-role of microRNA in gastric cancer.Dig Dis2012,30:255–267.
14. Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY, Yuasa Y, Kang D, Kim YS, You WC:Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer.PLoS One2012,7:e33608.
15. Yang Q, Zhang C, Huang B, Li HY, Zhang R, Huang YX, Wang JJ: Downregulation of microRNA-206 is a Potent Prognostic Marker for Patients with Gastric Cancer.Eur J Gastroenterol Hepatol2013. In press. 16. Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu S, Wu M, Pan Z, Zhou W:
MicroRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity.Br J Cancer
2010,103:1215–1220.
17. Xiong J, Yu D, Wei N, Fu H, Cai T, Huang Y, Wu C, Zheng X, Du Q, Lin D, Liang Z:An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples.FEBS J2010,277:1684–1694.
18. Li J, Liang S, Yu H, Zhang J, Ma D, Lu X:An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer.Gynecol Oncol2010, 119:543–548.
19. Yamakuchi M, Yagi S, Ito T, Lowenstein CJ:MicroRNA-22 regulates hypoxia signaling in colon cancer cells.PLoS One2011,6:e20291.
20. Ling B, Wang GX, Long G, Qiu JH, Hu ZL:Tumor suppressor miR-22 suppresses lung cancer cell progression through post-transcriptional regulation of ErbB3.J Cancer Res Clin Oncol2012,138:1355–1361. 21. Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM,
Sportoletti P, Varmeh S, Egia A, Fedele G, Rameh L, Loda M, Pandolfi PP: Identification of the miR-106b 25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation.Sci Signal2010,3:ra29.
22. Liu L, Jiang Y, Zhang H, Greenlee AR, Yu R, Yang Q:MiR-22 functions as a micro-oncogene in transformed human bronchial epithelial cells induced by anti-benzo[a]pyrene-7,8-diol-9,10-epoxide.Toxicol In Vitro
23. Sobin LH, Wittekind C:TNM classification of malignant tumours.6th edition. New York: Wiley, International Union Against Cancer; 2002:121–126. 24. Gurha P, Abreu-Goodger C, Wang T, Ramirez MO, Drumond AL, van
Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, Kelm RJ Jr, Reddy AK, Taffet GE, Bradley A, Wehrens XH, Entman ML, Rodriguez A:Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction.Circulation2012,125:2751–2761.
25. Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A:Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks.PLoS One2008, 3:e3740.
26. Ting Y, Medina DJ, Strair RK, Schaar DG:Differentiation-associated miR-22 represses Max expression and inhibits cell cycle progression.Biochem Biophys Res Commun2010,394:606–611.
doi:10.1186/1746-1596-8-102
Cite this article as:Wanget al.:Reduced expression of miR-22 in gastric cancer is related to clinicopathologic characteristics or patient prognosis.Diagnostic Pathology20138:102.
Submit your next manuscript to BioMed Central and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution