ABSTRACT
For Presentation at the
Asia-North-American Waste Management Conference
Los Angeles, California December 9-11, 1998
Medical Waste Disposal (#22)
Michael Brown, P.E., PresidentThomas Vence, P .E., Corporate Vice President Brown, Vence & Associates, Inc. 120 Montgomery Street, Suite 1000
San Francisco, CA 94104 (415) 434-0900 [phone]
(415) 956-6220 [fax] bva-sf@brownvence.com
Since the washing-up of medical wastes on east coast beaches in the late '80s, jurisdictionS around the country have enacted various plans for medical waste management. The stricter regulatory environment over the last decade has lead to transformations in medical waste handling. This presentation summarizes both traditional and innovative techniques for treatment of medical waste, presents guidelines for selecting treatment options, and discusses recent trends towards medical waste reduction.
Generally, medical waste treatment techniques fall into three categories: thermal, chemical, or irradiation. Traditional treatment methods discussed include steam sterilization and incineration. Incineration, a common and widely used method for medical waste disposal, has been impacted by the release, last year, of the EPA's final rules governing national standards for hospital incinerators and other incinerators of hospital, medical, and/or infectious waste. Stringent emission standards have been defined for nine specific pollutants and a mandated training and qualifications program for incinerator operators has been established. Current and innovative treatment methods will be discussed with emphasis on practical, cost-effective solutions.
Considerations for selection of treatment techniques discussed include: waste stream characterization, technical evaluation of equipment, onsite vs. off site treatment, physical constraints, regulatory concerns, and life cycle costing.
The presentation will conclude with a discussion of future trends in medical waste management and the agreement between the American Hospital Association and the EPA to reduce medical waste, as well as eliminate mercury from waste streams by the year 2005.
G:\MKT\CONF\98ANACON\ASME2.wPD 912198 210
SECTION 1
MEDICAL WASTE DISPOSAL: INTRODUCTION
In the past decade, the cost of medical waste treatment has decreased, medical waste generators have learned how to better segregate and reduce their waste, the competition among companies in the medical waste industry has increased dramatically, treatment capacity has outpaced waste generation, and regulatory agencies have passed increasingly strict rules regarding the treatment and transportation of medical waste. In this dynamic environment, keeping abreast of recent changes and innovations in the medical waste disposal field becomes increasingly important. This paper gives an overview of pertinent regulatory issues regarding medical waste, generating entities, on-site and off-site treatment, treatment technologies, waste transportation, and recent developments in controlling medical waste streams.
SECTION 2
REGULATORY ISSUES
2.1 CALIFORNIA REGULATIONS
2.1.1 California Medical Waste Management Act
The California Medical Waste Management Act ("Act"), adopted by the legislature in late 1990 and put into effect on April 1, 1991, is enforced by the California Department of Health Services (DHS) in conjunction with selected local programs. The Act provides for regulation of medical waste generators (differentiated between small quantity generators and others), haulers, and treatment facilities and covers the following subjects:
• Powers and duties of local, state, and federal agencies
• Small quantity generator requirements • Large quantity generator requirements •
. Medical waste haulers
• Medical waste treatment facility permits • Medical waste treatment
• Containment and storage
• Trauma scene waste management • Enforcement
• Suspension or revocation of permits.
G:\MKlICONF\98ANACON\MEDWASTE.wPD 9/4/98
212
2.1.2
Definitions
Defining some pertinent tenns would be useful at this point. The following are based on definitions contained in the Act. A more comprehensive list of definitions can be found in Chapter Two of the Act.
• Bioharordous waste
(a) Laboratory waste, including, but not limited to, all of the following:
(1) Human or animal specimen cultures from medical and pathology laboratories.
(2) Cultures and stocks of infectious agents from research and industrial laboratories.
(3) Wastes from the production of bacteria, viruses, spores, discarded animal vaccines ... as identified by the department, and culture dishes and devices used to transfer, iIinoculate, and mix cultures.
(b) Human surgery specimens or tissues removed at surgery or autopsy, which are suspected by the attending physician and surgeon or dentist or being contaminated with infectious agents known to be contagious to humans.
(c) Animal· parts, tissues, fluids, or carcasses suspected by the attending veterinarian of being contaminated with infectious agents known to be contagious to humans.
(d) Waste, which at the point of transport from the generator's site, at the point of disposal, or thereafter, contains recognizable fluid blood, fluid blood products, containers, or equipment containing blood that is fluid, or blood from animals known to be infected with diseases which are highly communicable to humans.
(e) Waste containing discarded materials contaminated with excretion, exudate, or secretions from humans or animals that are required to be isolated by the infection control staff ...
(t)
(1 ) Waste which is hazardous only because it is comprised of human surgery specimens or tissues which have been fixed in fonnaldehyde or other fixatives, or only because the waste is contaminated through contact with, or having previously contained, chemotherapeutic agents, including, but not limited to, gloves, disposable gowns, towels, and intravenous solution bags and attached tubing which are empty ...
(g) Waste that is hazardous only because it is comprised of pharmaceuticals ...
• Infectious a"ent is a type of microorganism, bacteria, mold, parasite, or virus that nonnally causes, or significantly contributes to the cause of, increased morbidity or mortality of human beings.
• Medical waste
(a) Means waste which meets both of the following requirements:
(1) The waste is composed of waste which is generated or produced as a result of any of the following actions:
(A) Diagnosis, treatment, or immunization of human beings or animals. (B) Research pertaining to the activities specified [above].
(C) The production or testing of biologicals.
(D) The accumulation of properly contained home-generated sharps waste that is brought by a patient, a member of the patient's family, or by a person authorized by the enforcement agency, to a point of consolidation approved by the enforcement agency ... (2) The waste is either of the following:
(A) Biohazardous waste. (B) Sharps waste.
(b) ... "Biologicals" means medicinal preparations made from living organisms and their products, including, but not limited to, serums, vaccines, antigens, and antitoxins.
(c) Medical waste includes trauma scene waste. • Medical waste treatment facility
(a) Means all adjacent land and structures, and other appurtenances or improvements on the land, used for treating medical waste or for associated handling and storage of medical waste ...
(b) "Adjacent" ... means real property within 400 yards from the property boundary of the existing medical waste treatment facility.
• Mixed waste means mixtures of medical and nonmedical waste. Mixed waste is medical waste, except
for all of the following:
(a) Medical waste and hazardous waste is hazardous waste and is subject to regulation as specified in the statutes and regulations applicable to hazardous waste.
(b) Medical waste and radioactive waste is radioactive waste and is subject to regulation as specified in the statutes and regulations applicable to radioactive waste.
(c) Medical waste, hazardous waste, and radioactive waste is radioactive mixed waste and is subject to regulation as specified in the statutes and regulations applicable to hazardous waste and radioactive waSte.
G:\MKT\CONF\98ANACON\MEDWASTE.wPD 9/4/98
2.2
FEDERAL REGULATIONS
The most recent activity in medical waste disposal at the federal level has been the promulgation of the U.S. EPA's new rules regarding medical waste incinerators. These new rules, passed on August 15, 1997, could curtail from 50 percent to 80 percent of on-site burning of medical waste at hospitals (Malloy, 7/97). Section 3.2.1 addresses these new federal regulations in more detail.
SECTION 3
MEDICAL WASTE TREATMENT
3.1
ON-SITE VERSUS OFF-SITE TREATMENT
According to the "Medical Waste Issues Study" (California Integrated Waste Management Board, 6/94), the average large quantity medical waste generator in California produces about 5,900 lbs/mo of medical waste, whereas the average small quantity generator generates about 25 lbs/mo (the Act defmes small quantity generators as those that generate less than two hundred pounds per month of medical waste). Small quantity generators send the majority of their wastes C83%) off-site for treatment, while large quantity generators treat the majority of their wastes (> 63 %) on-site. A total of approximately 50,000 tons/yr of medical wastes were treated by off-site facilities in California in 1991192.
Off-site treatment facilities are not associated with the generating facility. According to Chapter 7 of the California Medical Waste Management Act, off-site medical waste treatment facilities must register with and obtain a permit from DHS.
The procedure to obtain and maintain a off-site treatment facility permit in California is summarized below: 1. Facility files an application with DHS.
2. Department reviews the compliance history of the applicant and denies, specifies additional permit conditions, or grants a permit to the applicant within 180 days of the time the application is deemed complete.
3. Applicant must pay an initial application fee equal to $100 for each hour the department spends processing the application, but not more than $50,000, or as provided in the regulations adopted by the department.
Permit holder must pay an annual permit fee equal to either $0.002 for each pound of medical waste treated or $10,000, whichever is greater.
4. Operating facilities must maintain individual records for a period of three years, that are accessible to the appropriate enforcement agency. Records must contain the following information: the type of treatment facility and its capacity,' all treatment facility operating records, and copies of the tracking documents for
all medical waste it receives for treatment from off-site generators or from medical waste haulers. 5. All permits shall be valid for five years, and renewal applications must be filed not less than 90 days prior
to the expiration date.
Prior to the passage of the Act, the acceptance of waste from smaller generators at the treatment facilities of larger generators was a common practice. However, only 17 % of the large quantity generators responding to the Medical Waste Issues Study ("Study") survey reported that they accept wastes from other generators for treatment at their facilities.
3.1.1 Small Quantity Generators
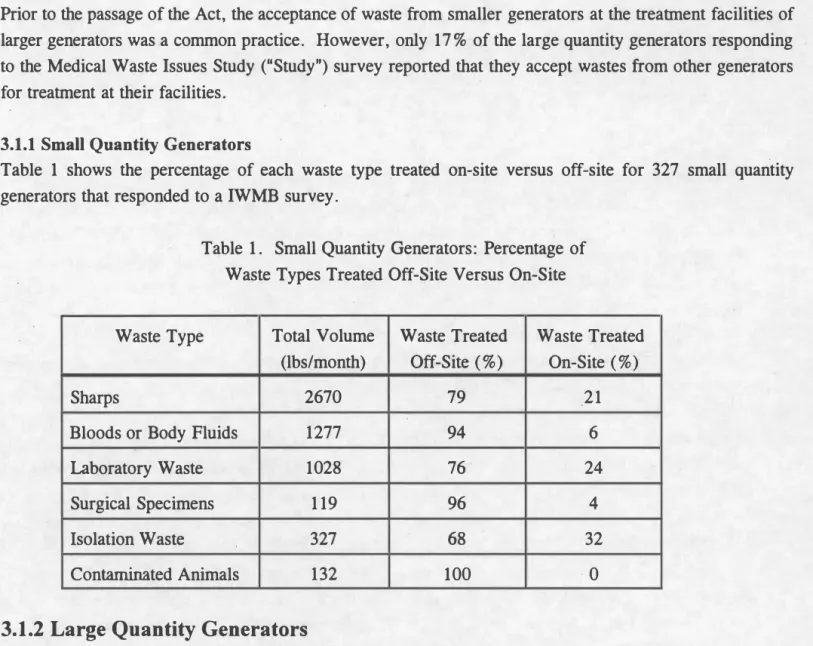
Table 1 shows the percentage of each waste type treated on-site versus off-site for 327 small quantity generators that responded to a IWMB survey.
Table 1. Small Quantity Generators: Percentage of Waste Types Treated Off-Site Versus On-Site
Waste Type Total Volume Waste Treated Waste Treated (lbs/month) Off-Site (%) On-Site (%)
Sharps 2670 79 21
Bloods or Body Fluids 1277 94 6
Laboratory Waste 1028 76 24
Surgical Specimens 119 96 4
Isolation Waste 327 68 32
Contaminated Animals 132 100 ·0
3.1.2
Large Quantity Generators
The Study data indicated that 23 % of 447 large quantity generators treated.&nne of their medical waste on
site. This 23% had an average.1Qla} medical waste generation rate of 9,500 lbs/month. The other 77% of
large quantity. generators reported no on-site waste treatment. The average reported waste volume for these large quantity generators was 4,900 lbs/monthl, indicating that on the average, facilities with no on-site treatment generate smaller volumes of waste than those with on-site treatment.
3.2 TRADITIONAL TREATMENT METHODS
Three medical waste treatment methods are defined and approved in the California statute: incineration, discharge of certain fluids and semisolid wastes to the sewer, and steam sterilization. Alternative methods must be approved by DHS. Common on-site treatment methods include incineration, steam sterilization, discharge to sanitary sewer, and
lOnly 285 (63%) of the large quantity generators that had no on-site treatment also reported waste volumes generated.
G:\MKliCONF\98ANACON\MEDW ASTE.wPD 9/4/98
216
disinfectioniencapsulization. Typical off-site treatment methods include incineration and steam sterilization, microwave, and radio frequency irradiation.
3.2.1 Incineration
3.2.1.1 Technology
According the Study, incineration was widely used in the past as a medical waste treatment technology. However, after the California Air Resources Board adopted dioxin control requirements for medical waste
incinerators, many on-site incinerators shut down. Others that retained their DHS off-site treatment facility permits upgraded their equipment to meet the dioxin requirement, modified operations or waste streams to control their dioxin emission levels, or were located in areas of the State that had not yet adopted dioxin control measures.
3.2.1.2 EPA Incinerator Regulations
On August 15, 1997, the U.S. EPA issued strict rules governing the operation of hospital incinerators for waste disposal. The rules set varying emission limits for new and existing incinerators with different waste-burning capacities. The rules establish limits for the following nine pollutants: particulate matter, carbon monoxide, dioxins and furans, hydrogen chloride, sulfur dioxide, nitrogen oxides, lead, cadmium, and mercury. New hospital incinerators, defmed as those that began construction after June 20, 1996, and incinerators modified· six months or more after the publication of the new rules, are subject to more stringent provisions than existing incinerators. Small, rural facilities that deal with small quantities of waste generally face less-stringent emission standards.
The schedule for implementation of these rules is as follows: for new incinerators implementation began in 1997; and for existing incinerators built on or before June 20, 1996 and for small rural units, implementation will begin two to five years following rule adoption, depending on the state. These rules were expected to affect approximately 2,400 existing incinerators, reduce toxics emissions (such as dioxin, lead, cadmium, and mercury) by more than 25 tons per year, reduce dioxins by over 90 percent from 1997 levels; and reduce other air pollutants (such as particulate matter, carbon monoxide, and hydrogen chloride) by over 7,000 tons per year. The rules also regulate stack opacity, f�cility monitoring and inspections, record keeping, incinerator operator training, and new incinerator siting.
Although the Amercian Hospital Association (AHA) stated that hospitals have the technology to comply with the strict requirements, these rules may have some implications for the use of off-site treatment facilities . . In 1997 the EPA projected that the high pollution abatement costs associated with complying
with the rules could lead to the closure of up to 80 % of the existing medical waste incinerators (Malloy, 7/97). By September 15, 1998, all states are to have either adopted the federal rules or established rules that met or exceeded the national guidelines.
3.2.2 Steam Sterilization
The process of steam sterilization treats waste by exposing it to steam at temperatures greater than 121 degrees Celsius for a minimum of 30 minutes. The benefits o'r on-site steam sterilization include its ease of operation, lack of air emissions, approved use in California regulations, and relative inexpensiveness. Self-contained steam sterilization units can treat between 20 and 1,000 pounds of waste per cycle. According to the Study, on-site costs for this treatment method average $0.04 per pound. Very small steam sterilization units, called autoclaves, are typically used to sterilize equipment.
3.3.3 Discharge to Sanitary Sewer
A study conducted for the Baxter Healthcare Corporation concluded that the most common disposal method of liquid medical wastes is discharge to the sewer. California state regulations do not prohibit all untreated liquid medical wastes from being discharged into the sewer. However, liquid wastes such as those containing any microbiological specimens, human or animal specimen cultures, cultures and stocks of Infectious agents, wastes from the producation of bacteria and viruses, or live and attenuated vaccines, are not allowed to be disposed in this manner. Furthermore, some local governments may have regulations pertaining to liquid waste disposal that are more stringent than the state rules.
3.3.4 DisinfectionlEncapsulization
A common method used to treat sharps utilizes a liquid chemical disinfectant in a heavy plastic container. As .
the container fills with sharps, the chemical disinfects them. Then a catalyst is added to convert the Hquid into a semi-solid polymer that is resistant to compression and tampering, and the entire container and contents can be disposed of as solid waste.
3.3.5 Microwave
In microwave treatment, medical waste is shredded in an environment of superheated steam. The steamed, shredded waste is then heated and disinfected using microwaves. Existing models can process from 200 to 900 pounds of waste per hour .. The treated waste is unrecognizable as medical waste and can be disposed of in a landfill as solid waste.
Advantages of this method include its adaptability for large or small facilities, the significant reduction in waste volume of up to 80 percent that is achievable (pEPCO, 8/97), and ease of operation due to automation and self containment. Disadvantages include the potential to emit exhausts of untreated volatile organics, an increase in the waste weight due to additional moisture content, worker health risks2, and unapplicability to wastes including animal carcasses, body parts, large pathology samples, large metal objects, or radioactive or chemotherapeutic agents. Lastly, disposal options may be limited due to the sharps contained in the treated waste.
2Health risks are associated with exposure to pathogens on the surfaces of the shredder or aerosolized during maintenance; the potential to emit exhaust of untreated volatile organics during loading, cleaning, or maintenance; and exposure to bio-aerosols and microwave radiation.
G:\MK1\CONF\98ANACON\MEDWASTE.wPD 9/4/98
218
3.3.6 Radio Frequency Irradiation
Treatment by radio-frequency irradiation involves shredding medical waste, increasing the moisture content in the shredded waste to ten percent, then heating the waste by exposing it to high-strength shortwave radio frequency radiation in a dielectric oven. The heating and disinfection process occurs as the waste absorbs the electrical energy, and the resulting treated waste can be disposed in a landfill as solid waste. According to the Study, this process is suitable for large off-site facilities and is not suitable for treating animal carcasses, body parts, large pathology samples, or radioactive material. Furthermore, workers in the treatment chamber must wear protective gear against potential airborne microorganisms.
3.2
NEW TREATMENT TECHNOLOGIES
A number of alternative treatment technologies have been approved for use in California, with new technologies being developed nationally. These alternative treatments must be approved by DHS and result in the destruction of pathogens, in order to qualify as a medical waste treatment. To share the cost of evaluation, vendors are required to develop a testing protocol, follow specified test procedures, and pay a
$1,000 application fee. Once a treatment process is approved, the vendor is issued a five-year permit to use that process in California.
Some alternative treatment methods are listed below:
3.3.1 Thermal Treatment
3.3.1.1 Dry heat sterilization
3.3.1.2 Plasma arc 3.3.1.3 Pyrolysis
3.3.2 ChemicallMechanical Treatment
3.3.2.1 Chlorination/chlorine derivatives 3.3.3 Irradiation Treatment
3.3.3.1 Electron beam
3.3.3.2 Gamma irradiation
3.3.3.3 Ultraviolet
3.3.4 Method Name
3.3.5 Other
3.3.5.1 Electric needle destroyers 3.3.5.2 Gas/vapor sterilization
G:\MKnCONF\98ANACON\MEDWASTE.wPD 9/4/98
Table 3. Medical Waste Hauling Rates for Selected Area Transporters
Medical Waste Transporter Cost of Service
All Chemical Disposal, Inc., San $75/hr for driver time, 2 hr minimum. Waste type
Jose, CA. does not affect rate.
Barnett Surgical Supply Company, $26 for the fIrst unit, $12 each additional umt. Walnut Creek, CA. Waste type does not affect rate.
Morgan Environmental, PacifIca, $ 1001 hr. CA.
BFI, Rancho Cordova, CA. Charge per container or per pound. Rate varies
according to the concentration of clients in the area.
SECTION 5
MEDICAL WASTE DISPOSAL SECTION 6
FUTURE TRENDS
6.1 CONTROLLING MERCURY IN THE WASTE STREAM
Mercury-containing products can be found in both hospital solid waste and medical waste. Because medical w�te contains fIve times more mercury than common municipal waste, medical waste incinerators are a large source of mercury to the environment. If mercury-containing products are incinerated in medical waste incinerators, the mercury becomes gaseous and can settle on land and in water, where there is an increased risk that it will accumulate in anima!" tissue, and in tum, adversely affect humans due to its high toxicity. In a June 24, 1998 memorandum of understanding between the EPA and the American Hospital Association (AHA), the AHA agreed to eliminate mercury from their waste stream by 2005. The association's 5,000 hospitals plan to replace mercury-containing products such as blood-pressure monitors and thermometers with mercury-free alternatives. The voluntary agreement also includes AHA plans to monitor hospitals' success in meeting goals, sponsor seminars on mercury reduction, and participate in an environmental leadership council.
Minimizing the amount of mercury that reaches the environment from the medical waste stream can be accomplished through the following practices: using alternate products that do not contain mercury, separating mercury-containing products from the rest of the medical waste before the waste is incinerated, and recycling mercury-containing products. The U.S. EPA recommends encouraging purchasers of medical supplies to select alternatives to mercury-containing products. Such products include thermometers, blood pressure
G:\MKTlCONF\98ANACON\MEDWASTE.wPD 9/4/98
222
gauges, sphygmomanometers, rechargeable and non-rechargeable batteries, tubing, fIxatives, reagents, solvents, and dental amalgam ..
Other recommendations to reduce mercury in the waste stream include separating medical wastes so that mercury-containing products do not go into a "red bag" earmarked for incineration, labelling instruments
containing mercury, familiarizing personnel with proper mercury clean-up and disposal procedures, and handling mercury in areas that will facilitate clean-up if a spill were to occur. Recycling dental amalgam has been very successful, and some recycling companies also accept various types of waste that contain mercury.
6.2 MEDICAL WASTE REDUCTION
The same June 24, 1998 memorandum of understanding between the U.S. EPA and the AHA discussed above, also calls for the association's 5,000 hospitals to reduce their waste streams by one-third by 2005 and one-half by 2010. Part of the plan to achieve this goal involves collecting baseline data to measure waste reduction progress and hospitals developing waste minimization plans targetting specifIc chemicals.
Steps to reduce the amount of medical waste generated should be taken beginning with supplies procurement, by purchasing fewer items that have large amounts of plastic and packaging, substituting other items for those that require much packaging, buying in bulk, and favoring the purchase of reusable equipment (Rhodes).
REFERENCES
AHA Regulatory Advisory, "EPA's Final Rules on Hospital Incinerators" , 9/97, http://www .aha.org/ar/epahospital.html.
California Integrated Waste Management Board, "Medical Waste Issues Study", 6/94.
California Medical Waste Management Act, California Health and Safety. Code, Sections 117600-118360. Infectious Wastes News, "Medical Waste: Still Healthy After All These Years," Vol. 13, No. 12, 8/8/98.
Malloy, Michael G, "Medical Waste Comes of Age," in Waste A�e, 7/97.
Potomac Electric Power Company (PEPCO) , "Medical Waste Treatment", 8/97,
http://www.pepco.com!bsemwt.htm.
Rhodes, David, "The Health Industry A http://altair.et.deakin.edu.au/courses/seb 1211fmaI2a.htm.
Report of Medical Wastes",
u.S. Environmental Protection Agency, "Mercury Fact Sheets", 2/98, http://www.epa.gov/ARD R51 glakes/hgfact. htm.
Waste News, "Mercury Sinking", Vol. 4, Issue 7, 6/29/98.
G:\MKT\CONF\98ANACON\MEDWASTEWPD 9/4/98