organic papers
Acta Cryst.(2005). E61, o675–o677 doi:10.1107/S1600536805004435 Shinji Aramakiet al. C
44H38N42CHCl3
o675
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
(1
RS
,4
SR
,8
SR
,11
RS
,15
SR
,18
RS
,22
RS
,25
SR
)-1,4:8,11:15,18:22,25-Tetraethano-29
H
,31
H
-tetrabenzo[
b,g,l,q
]porphine chloroform disolvate
Shinji Aramaki,aYoshimasa Sakai,aHiroyuki Yanagisawa,b Takatoshi Senjuband
Jin Mizuguchib*
aMitsubishi Chemical Group Science and
Technology Research Center, Kamoshida-cho 1000, Aoba-ku, Yokohama 227-8502, Japan, andbDepartment of Applied Physics, Graduate
School of Engineering, Yokohama National University, Tokiwadai 79-5, Hodogaya-ku, Yokohama 240-8501, Japan
Correspondence e-mail: mizu-j@ynu.ac.jp
Key indicators
Single-crystal X-ray study
T= 93 K
Mean(C–C) = 0.008 A˚
Rfactor = 0.125
wRfactor = 0.311
Data-to-parameter ratio = 14.1
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
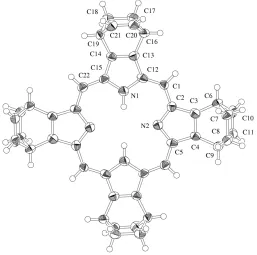
In the title compound, C44H38N42CHCl3, the porphine (CP) is
a soluble precursor of metal-free porphyrin which exhibits an excellent field-effect transistor characteristic. The CP skeleton is entirely flat and characterized by crystallographic Ci
symmetry. In the present geometrical isomer, the C—C single-bond linkages of the four peripheries are arranged in an above–above–below–below manner with respect to the CP skeleton.
Comment
Organic field-effect transistors (FET) are advantageous in lowering fabrication costs and being large-area devices compared with inorganic FETs. We have recently reported that the metal-free porphyrin, the so-called benzoporphyrin (BP), exhibits excellent FET characteristics (Aramaki et al., 2004). Our FET system is characterized by the use of a soluble BP-precursor (i.e.porphine in the title compound, called CP) and its thermal transformation into BP directly on the substrate at about 473 K. In order to improve the FET performance further, it is crucial to study the correlation between the structure and the solid-state properties. The structures of BP and toluene-solvated CP have previously been reported (Aramaki & Mizuguchi, 2003; Aramaki et al., 2005). The present paper deals with the structure of the title compound, (I), which is chloroform-solvated CP.
The skeleton of the centrosymmetric CP molecule is entirely planar (Fig. 1). As shown in the scheme, there is one C—C single bond and one double bond in the four groups at the periphery of the molecule, and the C—C single-bond linkages are arranged in an above–above–below–below manner with respect to the CP skeleton. This is clearly seen from the difference in bond lengths (Table 1): 1.519 (9)– 1.525 (9) A˚ for C7—C8 and C17—C18, and 1.339 (9)– 1.356 (9) A˚ for C10 C11 and C20 C21. By contrast, in toluene-solvated CP (Aramaki et al., 2005), the single and double bonds are averaged, giving an intermediate bond length. This provides a striking difference between the
chloroform and toluene-solvated crystals of CP. It is also to be noted that we have isolated two isomers by column chroma-tography (see Experimental) and their1H NMR spectra are different at themesosite. However, these isomers in toluene-solvated crystals showed similar disordered structures with similar cell constants. By contrast, in chloroform-solvated CPs, we still have a chance to study the structure of both isomers. Fig. 2 shows the packing arrangement of CP with the chloro-form molecules.
Experimental
CP was synthesized according to the method previously reported by Itoet al.(1998). The product was purified by column chromatography, using toluene as eluent. Two geometrical isomers of CP were clearly isolated, as indicated by 1H NMR data (fast-eluted component: 10.388 and 10.394 p.p.m.; slow-eluted component: 10.382 and 10.388 p.p.m.). Single crystals of (I) were grown from a chloroform solution of the fast-eluted component. After a week, a number of dark-red block-shaped crystals were isolated. The crystal used for analysis was found to include solvent molecules. Therefore, X-ray intensity data were collected at 93 K.
Crystal data
C44H38N42CHCl3
Mr= 861.52
Monoclinic, P21=c
a= 10.282 (2) A˚ b= 17.433 (3) A˚ c= 11.664 (2) A˚ = 106.51 (1)
V= 2004.5 (6) A˚3
Z= 2
Dx= 1.427 Mg m 3
Cu Kradiation Cell parameters from 9212
reflections = 4.5–68.0
= 4.22 mm1
T= 93.1 K Block, dark red 0.200.200.20 mm
Data collection
Rigaku R-AXIS RAPID diffractometer !scans
Absorption correction: multi-scan (ABSCOR; Higashi, 1995) Tmin= 0.400,Tmax= 0.430
18 024 measured reflections
3592 independent reflections 1286 reflections withF2> 2(F2)
Rint= 0.183
max= 68.2
h=11!11 k=20!20 l=13!14
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.125
wR(F2) = 0.311 S= 1.32 3592 reflections 254 parameters
H-atom parameters constrained w= 1/[2(F
o2) + (0.1P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.92 e A˚ 3
[image:2.610.311.564.70.284.2]min=0.67 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
N1—C12 1.381 (7) N1—C15 1.377 (8) N2—C2 1.375 (7) N2—C5 1.385 (9) C1—C2 1.401 (9) C1—C12 1.367 (9) C2—C3 1.446 (9) C3—C4 1.370 (9) C4—C5 1.446 (8) C5—C22i 1.404 (8)
C7—C8 1.525 (9) C10—C11 1.339 (9) C12—C13 1.443 (9) C13—C14 1.350 (8) C14—C15 1.434 (8) C15—C22 1.385 (8) C17—C18 1.519 (9) C20—C21 1.356 (9) C22—C5i
1.404 (8)
N1—C12—C1 126.5 (6) N1—C12—C13 104.7 (5) C15—N1—C12 111.8 (5) N1—C15—C14 105.6 (5) N1—C15—C22 125.2 (6) N2—C2—C1 124.4 (5) N2—C2—C3 110.4 (5) C5—N2—C2 105.3 (5) N2—C5—C4 111.1 (5) N2—C5—C22i
124.5 (5)
C12—C1—C2 127.4 (5) C1—C2—C3 125.2 (5) C1—C12—C13 128.8 (5) C2—C3—C4 107.3 (5) C3—C4—C5 105.8 (6) C4—C5—C22i 124.4 (6) C12—C13—C14 109.3 (5) C13—C14—C15 108.6 (6) C14—C15—C22 129.2 (6) C15—C22—C5i
128.9 (6)
Symmetry code: (i)x;y;z.
All the H atoms were positioned geometrically and included in the riding-model approximation, with N—H and C—H distances of 0.95 A˚ , and withUiso(H) = 1.2Ueq(parent atom). The position of the
organic papers
o676
Shinji Aramakiet al. C [image:2.610.43.300.72.327.2]44H38N42CHCl3 Acta Cryst.(2005). E61, o675–o677
Figure 2
The crystal structure of (I). H atoms of CP have been omitted.
Figure 1
[image:2.610.312.565.377.682.2]H atom bonded to N1 was calculated by assumingsp2hybridization, since a positive peak was found only near atom N1 (but not N2) in the difference-density map.
Data collection: PROCESS-AUTO (Rigaku, 1998); cell refine-ment:PROCESS-AUTO; data reduction:CrystalStructure (Rigaku/ MSC and Rigaku, 2004); program(s) used to solve structure:SIR2002
(Burlaet al., 2003); program(s) used to refine structure:SHELXL97
(Sheldrick, 1997); molecular graphics: ORTEPIII (Burnett & Johnson, 1996); software used to prepare material for publication:
CrystalStructure.
The authors express their sincere thanks to Mr I. Suzuki for experimental assistance.
References
Aramaki, S. & Mizuguchi, J. (2003).Acta Cryst.E59, o1556–o1558. Aramaki, S., Sakai, Y. & Ono, N. (2004).Appl. Phys. Lett.84, 2085–2087. Aramaki, S., Sakai, Y., Yanagisawa, H. & Mizuguchi, J. (2005).Acta Cryst.E61,
o659–o661.
Burla, M. C., Camalli, M., Carrozzini, B., Casarano, G. L., Giacovazzo, C., Polidori, G. & Spagna, R. (2003).J. Appl. Cryst.36, 1103.
Burnett, M. N. & Johnson, C. K. (1996).ORTEPIII. Report ORNL-6895. Oak Ridge National Laboratory. Tennessee, USA.
Higashi, T. (1995).ABSCOR.Rigaku Corporation, Tokyo, Japan.
Ito, S., Ochi, N., Murashima, T., Uno, H. & Ono, N. (1998).Chem. Commun. pp. 1661–1662.
Rigaku (1998).PROCESS-AUTO. Rigaku Corporation, Tokyo, Japan. Rigaku/MSC (2004).CrystalStructure.Version 3.6.0. MSC, 9009 New Trails
Drive, The Woodlands, TX 77381-5209, USA.
Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany.
organic papers
Acta Cryst.(2005). E61, o675–o677 Shinji Aramakiet al. C
supporting information
sup-1
Acta Cryst. (2005). E61, o675–o677
supporting information
Acta Cryst. (2005). E61, o675–o677 [https://doi.org/10.1107/S1600536805004435]
(1
RS
,4
SR
,8
SR
,11
RS
,15
SR
,18
RS
,22
RS
,25
SR
)-1,4:8,11:15,18:22,25-Tetra-ethano-29
H
,31
H
-tetrabenzo[
b,g,l,q
]porphine chloroform disolvate
Shinji Aramaki, Yoshimasa Sakai, Hiroyuki Yanagisawa, Takatoshi Senju and Jin Mizuguchi
(I)
Crystal data C44H38N4·2CHCl3 Mr = 861.52 Monoclinic, P21/c Hall symbol: -P 2ybc a = 10.282 (2) Å b = 17.433 (3) Å c = 11.664 (2) Å β = 106.51 (1)° V = 2004.5 (6) Å3
Z = 2
F(000) = 892.00 Dx = 1.427 Mg m−3
Cu Kα radiation, λ = 1.5418 Å Cell parameters from 9212 reflections θ = 4.5–68.0°
µ = 4.22 mm−1 T = 93 K Block, dark red 0.20 × 0.20 × 0.20 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Detector resolution: 10.00 pixels mm-1 48 frames, δω = 15° scans
Absorption correction: multi-scan (ABSCOR; Higashi, 1995) Tmin = 0.400, Tmax = 0.430 18024 measured reflections
3592 independent reflections 1286 reflections with F2 > 2σ(F2) Rint = 0.183
θmax = 68.2° h = −11→11 k = −20→20 l = −13→14
Refinement Refinement on F2 R[F2 > 2σ(F2)] = 0.125 wR(F2) = 0.311 S = 1.32 3592 reflections 254 parameters
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.1P)2] where P = (Fo2 + 2Fc2)/3 (Δ/σ)max < 0.001
Δρmax = 0.92 e Å−3 Δρmin = −0.67 e Å−3
Special details
Geometry. ENTER SPECIAL DETAILS OF THE MOLECULAR GEOMETRY
Refinement. Refinement using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R -factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt).
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-2
Acta Cryst. (2005). E61, o675–o677
Cl2 0.2157 (2) −0.1016 (1) 0.1748 (2) 0.0933 (9) Cl3 0.3934 (2) −0.0097 (1) 0.3548 (2) 0.0707 (7) N1 −0.0576 (5) 0.0457 (3) 0.1475 (4) 0.039 (1) N2 0.1182 (5) 0.0941 (3) 0.0037 (4) 0.035 (1) C1 0.0740 (6) 0.1650 (3) 0.1707 (5) 0.032 (1) C2 0.1376 (6) 0.1558 (3) 0.0801 (5) 0.034 (1) C3 0.2349 (6) 0.2085 (3) 0.0554 (5) 0.038 (2) C4 0.2768 (6) 0.1780 (3) −0.0364 (5) 0.033 (1) C5 0.2019 (7) 0.1073 (3) −0.0686 (5) 0.036 (1) C6 0.3068 (6) 0.2806 (3) 0.1063 (5) 0.040 (2) C7 0.4618 (6) 0.2576 (4) 0.1540 (5) 0.042 (2) C8 0.5097 (6) 0.2208 (4) 0.0546 (5) 0.041 (2) C9 0.3902 (6) 0.2208 (3) −0.0641 (5) 0.040 (2) C10 0.3048 (6) 0.3340 (3) 0.0036 (5) 0.041 (2) C11 0.3466 (6) 0.3028 (3) −0.0847 (5) 0.037 (2) C12 −0.0113 (6) 0.1145 (3) 0.2027 (5) 0.038 (2) C13 −0.0715 (6) 0.1197 (3) 0.3002 (5) 0.036 (1) C14 −0.1480 (6) 0.0568 (3) 0.3003 (5) 0.035 (1) C15 −0.1428 (6) 0.0088 (3) 0.2018 (5) 0.035 (1) C16 −0.0729 (6) 0.1782 (3) 0.3954 (5) 0.038 (2) C17 −0.2249 (7) 0.1988 (3) 0.3731 (6) 0.049 (2) C18 −0.3096 (6) 0.1278 (3) 0.3755 (5) 0.042 (2) C19 −0.2210 (6) 0.0548 (3) 0.3952 (5) 0.041 (2) C20 −0.0365 (7) 0.1324 (4) 0.5148 (5) 0.044 (2) C21 −0.1123 (6) 0.0687 (4) 0.5142 (5) 0.045 (2) C22 −0.2089 (6) −0.0599 (3) 0.1641 (5) 0.039 (2) C23 0.3160 (7) −0.0196 (4) 0.1996 (6) 0.056 (2)
H1 −0.0335 0.0261 0.0803 0.047*
H2 0.0921 0.2115 0.2148 0.038*
H3 0.2723 0.3034 0.1657 0.048*
H4 0.4154 0.1993 −0.1295 0.048*
H5 0.5143 0.3022 0.1819 0.050*
H6 0.4732 0.2221 0.2180 0.050*
H7 0.5376 0.1695 0.0759 0.049*
H8 0.5838 0.2492 0.0430 0.049*
H9 0.2760 0.3859 0.0018 0.049*
H10 0.3484 0.3303 −0.1545 0.045*
H11 −0.2712 0.0089 0.3945 0.050*
H12 −0.0161 0.2213 0.3962 0.046*
H13 −0.3782 0.1236 0.3015 0.050*
H14 −0.3505 0.1324 0.4387 0.050*
H15 −0.2337 0.2333 0.4336 0.059*
H16 −0.2571 0.2228 0.2971 0.059*
H17 −0.0993 0.0357 0.5813 0.054*
H18 0.0338 0.1479 0.5830 0.053*
H19 −0.2657 −0.0770 0.2081 0.046*
supporting information
sup-3
Acta Cryst. (2005). E61, o675–o677
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Cl1 0.086 (2) 0.156 (2) 0.056 (1) 0.036 (2) 0.035 (1) 0.022 (1) Cl2 0.084 (2) 0.073 (2) 0.099 (2) −0.009 (1) −0.013 (1) −0.018 (1) Cl3 0.082 (2) 0.082 (1) 0.051 (1) −0.015 (1) 0.023 (1) −0.0153 (9) N1 0.046 (3) 0.038 (3) 0.034 (3) −0.002 (3) 0.015 (2) −0.002 (2) N2 0.038 (3) 0.036 (3) 0.030 (3) −0.001 (2) 0.008 (2) 0.001 (2) C1 0.038 (4) 0.031 (3) 0.026 (3) −0.006 (3) 0.009 (3) −0.004 (2) C2 0.036 (4) 0.034 (3) 0.031 (3) −0.001 (3) 0.008 (3) 0.003 (3) C3 0.050 (4) 0.034 (3) 0.031 (3) 0.001 (3) 0.012 (3) 0.002 (3) C4 0.042 (4) 0.031 (3) 0.024 (3) 0.002 (3) 0.008 (3) 0.000 (2) C5 0.046 (4) 0.035 (3) 0.027 (3) 0.007 (3) 0.011 (3) 0.001 (3) C6 0.050 (5) 0.043 (4) 0.035 (3) −0.003 (3) 0.025 (3) −0.002 (3) C7 0.043 (5) 0.056 (4) 0.030 (3) −0.002 (3) 0.015 (3) 0.002 (3) C8 0.038 (4) 0.049 (4) 0.039 (4) −0.002 (3) 0.015 (3) 0.001 (3) C9 0.041 (4) 0.049 (4) 0.033 (3) −0.008 (3) 0.016 (3) −0.005 (3) C10 0.051 (4) 0.031 (3) 0.042 (4) −0.005 (3) 0.014 (3) 0.010 (3) C11 0.040 (4) 0.035 (3) 0.038 (3) −0.005 (3) 0.010 (3) −0.000 (3) C12 0.051 (4) 0.034 (3) 0.027 (3) 0.004 (3) 0.009 (3) −0.003 (3) C13 0.032 (4) 0.044 (4) 0.035 (3) 0.002 (3) 0.011 (3) −0.003 (3) C14 0.044 (4) 0.033 (3) 0.027 (3) 0.001 (3) 0.010 (3) −0.002 (3) C15 0.043 (4) 0.035 (3) 0.031 (3) 0.003 (3) 0.019 (3) 0.005 (3) C16 0.048 (4) 0.038 (4) 0.035 (3) 0.001 (3) 0.020 (3) −0.003 (3) C17 0.066 (5) 0.042 (4) 0.044 (4) 0.004 (3) 0.023 (3) −0.008 (3) C18 0.045 (4) 0.045 (4) 0.034 (3) 0.003 (3) 0.011 (3) 0.000 (3) C19 0.057 (5) 0.040 (4) 0.033 (3) −0.017 (3) 0.023 (3) −0.003 (3) C20 0.045 (4) 0.058 (4) 0.030 (3) −0.007 (3) 0.010 (3) −0.009 (3) C21 0.050 (4) 0.053 (4) 0.029 (3) 0.001 (4) 0.008 (3) 0.005 (3) C22 0.040 (4) 0.043 (4) 0.035 (3) −0.002 (3) 0.014 (3) 0.001 (3) C23 0.069 (5) 0.059 (5) 0.040 (4) 0.018 (4) 0.017 (4) 0.015 (3)
Geometric parameters (Å, º)
Cl1—C23 1.758 (8) C13—C16 1.511 (8)
Cl2—C23 1.738 (7) C14—C13 1.350 (8)
Cl3—C23 1.767 (6) C14—C15 1.434 (8)
N1—C12 1.381 (7) C14—C19 1.505 (9)
N1—C15 1.377 (8) C15—C22 1.385 (8)
N1—H1 0.9500 C16—C13 1.511 (8)
N2—C2 1.375 (7) C16—C17 1.551 (9)
N2—C5 1.385 (9) C16—C20 1.555 (8)
C1—C2 1.401 (9) C16—H12 0.9500
C1—C12 1.367 (9) C17—C18 1.519 (9)
C1—H2 0.9500 C17—H15 0.9500
C2—C3 1.446 (9) C17—H16 0.9500
C3—C4 1.370 (9) C18—C19 1.543 (8)
supporting information
sup-4
Acta Cryst. (2005). E61, o675–o677
C4—C5 1.446 (8) C18—H14 0.9500
C4—C9 1.495 (9) C19—C21 1.534 (7)
C5—C22i 1.404 (8) C19—H11 0.9500
C6—C7 1.583 (8) C20—C21 1.356 (9)
C6—C10 1.513 (8) C20—H18 0.9500
C6—H3 0.9500 C21—H17 0.9500
C7—C8 1.525 (9) C22—C5i 1.404 (8)
C7—H5 0.9500 C22—H19 0.9300
C7—H6 0.9500 C23—H20 0.9500
C8—C9 1.568 (7) H4—C9 0.9500
C8—H7 0.9500 H6—C7 0.9500
C8—H8 0.9500 H7—C8 0.9500
C9—C11 1.496 (8) H9—C10 0.9500
C9—H4 0.9500 H10—C11 0.9500
C10—C6 1.513 (8) H11—C19 0.9500
C10—C11 1.339 (9) H12—C16 0.9500
C10—H9 0.9500 H13—C18 0.9500
C11—C10 1.339 (9) H14—C18 0.9500
C11—H10 0.9500 H15—C17 0.9500
C12—C13 1.443 (9) H16—C17 0.9500
C13—C12 1.443 (9) H18—C20 0.9500
C13—C14 1.350 (8) H20—C23 0.9500
Cl1—C23—H20 109.1680 H7—C8—C9 109.5271
Cl2—C23—H20 109.1675 H8—C8—C9 109.5272
Cl3—C23—H20 109.1675 H8—C8—H7 109.4607
N1—C12—C1 126.5 (6) C9—C11—C10 114.3 (6)
N1—C12—C13 104.7 (5) H4—C9—C11 112.6158
C15—N1—C12 111.8 (5) C9—C11—H10 122.8553
H1—N1—C12 124.1125 H9—C10—C11 122.4574
N1—C15—C14 105.6 (5) C10—C11—H10 122.8555
N1—C15—C22 125.2 (6) C12—C13—C14 109.3 (5)
H1—N1—C15 124.1134 C12—C13—C16 135.8 (6)
N2—C2—C1 124.4 (5) C13—C14—C15 108.6 (6)
N2—C2—C3 110.4 (5) C16—C13—C14 114.9 (6)
C5—N2—C2 105.3 (5) C13—C14—C19 115.6 (5)
N2—C5—C4 111.1 (5) C13—C16—C17 104.6 (4)
N2—C5—C22i 124.5 (5) C13—C16—C20 105.1 (5)
C12—C1—C2 127.4 (5) C13—C16—H12 113.9563
C1—C2—C3 125.2 (5) C14—C15—C22 129.2 (6)
H2—C1—C2 116.3225 C19—C14—C15 135.7 (5)
C1—C12—C13 128.8 (5) C14—C19—C21 105.9 (5)
H2—C1—C12 116.3218 C14—C19—C18 105.5 (5)
C2—C3—C4 107.3 (5) C14—C19—H11 113.3321
C2—C3—C6 138.4 (6) C15—C22—H19 115.5218
C3—C4—C5 105.8 (6) C15—C22—C5i 128.9 (6)
C6—C3—C4 114.1 (6) C20—C16—C17 104.1 (5)
supporting information
sup-5
Acta Cryst. (2005). E61, o675–o677
C3—C6—C7 105.1 (5) C16—C17—H16 109.0174
C3—C6—C10 108.0 (4) C16—C17—C18 111.3 (5)
C3—C6—H3 113.3666 C16—C17—H15 109.0170
C4—C5—C22i 124.4 (6) C16—C20—C21 115.1 (5)
C9—C4—C5 139.5 (6) H12—C16—C20 113.9565
C4—C9—C11 107.2 (5) C16—C20—H18 122.4532
C4—C9—C8 105.8 (5) C17—C18—C19 111.0 (5)
C4—C9—H4 112.6163 H16—C17—C18 109.0171
C5—C22i—H19i 115.5284 C17—C18—H13 109.0910 C5—C22i—C15i 128.9 (6) H15—C17—C18 109.0168
C10—C6—C7 102.8 (5) C17—C18—H14 109.0908
H3—C6—C7 113.3665 H16—C17—H15 109.4604
C6—C7—H6 109.2220 C18—C19—C21 104.6 (5)
C6—C7—C8 110.5 (4) C18—C19—H11 113.3326
C6—C7—H5 109.2222 H13—C18—C19 109.0905
C6—C10—C11 115.1 (5) H14—C18—C19 109.0911
H3—C6—C10 113.3670 H14—C18—H13 109.4601
C6—C10—H9 122.4567 C19—C21—C20 113.9 (5)
C7—C8—C9 109.3 (5) H11—C19—C21 113.3321
H6—C7—C8 109.2223 C19—C21—H17 123.0720
C7—C8—H7 109.5267 H18—C20—C21 122.4533
H5—C7—C8 109.2222 C20—C21—H17 123.0726
C7—C8—H8 109.5265 H19—C22—C5i 115.5284
H6—C7—H5 109.4599 C22—C5i—N2i 124.5 (5) C8—C9—C11 105.4 (4) C22—C5i—C4i 124.4 (6)
C8—C9—H4 112.6162