1-Benzyl-3-methyl-3
000,5
000-diphenylspiro-[quinoxaline-2(1
H
),2
000(3
000H
)-1,3,4-thiadiazole]
Caleb Ahoya Anothane,aRachid Bouhfid,bEl Mokhtar Essassiaand Seik Weng Ngc,d*
aLaboratoire de Chimie Organique He´te´rocyclique, Poˆle de Compe´tences
Pharmacochimie, Universite´ Mohammed V-Agdal, BP 1014 Avenue Ibn Batout, Rabat, Morocco,bInstitute of Nanomaterials and Nanotechnology, MAScIR, Avenue
de l’Arme´e Royale, Rabat, Morocco,cDepartment of Chemistry, University of
Malaya, 50603 Kuala Lumpur, Malaysia, anddChemistry Department, King
Abdulaziz University, PO Box 80203 Jeddah, Saudi Arabia Correspondence e-mail: seikweng@um.edu.my
Received 7 December 2011; accepted 7 December 2011
Key indicators: single-crystal X-ray study;T= 293 K; mean(C–C) = 0.003 A˚; disorder in main residue;Rfactor = 0.045;wRfactor = 0.135; data-to-parameter ratio = 24.5.
In the title spiro compound, C29H24N4S, the quinoxaline and
thiadiazole ring systems share a common C atom; their mean planes are aligned at 87.0 (1)in one molecule and at 84.1 (1)
in the other independent molecule. The thiazole ring possesses two aromatic ring substituents and is roughly coplanar with these rings [the dihedral angles between the thiadiazole and phenyl rings are 10.7 (1) and 11.7 (1) in one molecule, and 16.8 (1) and 17.7 (1) in the other]. The aromatic ring of the benzyl unit of one molecule is disordered over two orienta-tions in a 1:1 ratio.
Related literature
For the structure of a related molecule, see: Anothaneet al.
(2010).
Experimental
Crystal data
C29H24N4S Mr= 460.58 Triclinic,P1
a= 13.5441 (2) A˚
b= 14.8971 (2) A˚
c= 15.0149 (2) A˚ = 66.431 (1)
= 63.921 (1)
= 65.275 (1)
V= 2383.45 (6) A˚3 Z= 4
MoKradiation = 0.16 mm1 T= 293 K
0.350.340.17 mm
Data collection
Bruker APEX DUO diffractometer Absorption correction: multi-scan
(SADABS; Sheldrick, 1996)
Tmin= 0.946,Tmax= 0.973
69257 measured reflections 14918 independent reflections 10508 reflections withI> 2(I)
Rint= 0.030
Refinement
R[F2> 2(F2)] = 0.045 wR(F2) = 0.135 S= 1.01 14918 reflections 609 parameters
37 restraints
H-atom parameters constrained
max= 0.27 e A˚
3
min=0.22 e A˚
3
Data collection:APEX2(Bruker, 2010); cell refinement:SAINT
(Bruker, 2010); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 2008); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008); molecular graphics: X-SEED (Barbour, 2001); software used to prepare material for publication:publCIF(Westrip, 2010).
We thank Universite´ Mohammed V-Agdal and the University of Malaya for supporting this study.
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5152).
References
Anothane, C. A., Bouhfid, R., Zouihri, H., Essassi, E. M. & Ng, S. W. (2010).
Acta Cryst.E66, o3227.
Barbour, L. J. (2001).J. Supramol. Chem.1, 189–191.
Bruker (2010).APEX2andSAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
Sheldrick, G. M. (1996).SADABS. University of Go¨ttingen, Germany. Sheldrick, G. M. (2008).Acta Cryst.A64, 112–122.
Westrip, S. P. (2010).J. Appl. Cryst.43, 920–925. Acta Crystallographica Section E
Structure Reports
Online
supporting information
Acta Cryst. (2012). E68, o103 [doi:10.1107/S1600536811052731]
1-Benzyl-3-methyl-3
′
,5
′
-diphenylspiro[quinoxaline-2(1
H
),2
′
(3
′
H
)-1,3,4-thia-diazole]
Caleb Ahoya Anothane, Rachid Bouhfid, El Mokhtar Essassi and Seik Weng Ng
S1. Comment
A previous study reported 1-allyl-3-methyl-3′,5′-diphenylspiro[quinoxaline-2(1H),2′(3′H)-[1,3,4]thiadiazole] (Anothane
et al., 2010). The allyl substitutent is replaced by a benzyl substituent in the present study. The asymmetric unit of C29H24N4S (Scheme I) consists of two independent molecules, one of which is disordered in the benzyl substituent. The
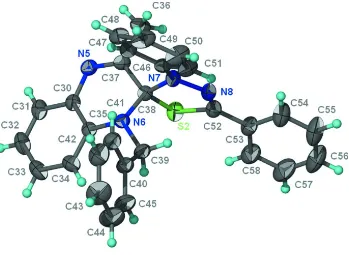
quinoxaline and thiadiazole ring systems share a common C atom; their mean planes are aligned at 87.0 (1)° in one molecule (Fig.1 ) and at 84.1 (1)° in the other independent molecule (Fig. 2). The thiazole ring possesses two aromatic ring substituents and is nearly coplanar with these rings. The aromatic ring of the benzyl unit of one molecule is disordered over two positions in a 1:1 ratio.
S2. Experimental
To a solution of1-benzyl-3-methylquinoxaline-2-thione (1 g, 3.75 mmole) and diphenylnitrilimine (1.28 g, 5.55 mmole) in THF (20 mL), was added triethylamine (0.78 ml, 5.55 mmol). The mixture was heated under reflux for 24 hours. The precipitate was recovered by filtration and was separated by chromatography on silica gel (hexane/ethylAcetate: 9/1). Colorless crystals were isolated when solvent was allowed to evaporate.
S3. Refinement
Carbon-bound H-atoms were placed in calculated positions (C–H 0.93–0.97 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2–1.5U(C).
One of the phenyl rings of the second independent molecule is disordered over two positions in 1:1 ratio. The ring was refined as a rigid hexagon of 1.39 Å sides. The temperature factors of the primed atoms were set to those of the unprimed ones, and all anisotropic temperature factors were restrained to be nearly isotropic. The pair of Cbenzyl–Cphenyl distances
Figure 1
Thermal ellipsoid plot (Barbour, 2001) of one C29H24N4S molecule at the 50% probability level; hydrogen atoms are
drawn as spheres of arbitrary radius.
Figure 2
Thermal ellipsoid plot (Barbour, 2001) of second C29H24N4S molecule at the 50% probability level; hydrogen atoms are
[image:3.610.133.482.380.635.2]1-benzyl-3-methyl-3′,5′-diphenyl-1H,3′H-spiro[quinoxaline-2,2′- [1,3,4]thiadiazole]
Crystal data
C29H24N4S Mr = 460.58
Triclinic, P1 Hall symbol: -P 1
a = 13.5441 (2) Å
b = 14.8971 (2) Å
c = 15.0149 (2) Å
α = 66.431 (1)°
β = 63.921 (1)°
γ = 65.275 (1)°
V = 2383.45 (6) Å3
Z = 4
F(000) = 968
Dx = 1.284 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 9950 reflections
θ = 2.5–30.7°
µ = 0.16 mm−1 T = 293 K Prism, colorless 0.35 × 0.34 × 0.17 mm
Data collection
Bruker APEX DUO diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin = 0.946, Tmax = 0.973
69257 measured reflections 14918 independent reflections 10508 reflections with I > 2σ(I)
Rint = 0.030
θmax = 30.8°, θmin = 1.6°
h = −19→19
k = −21→21
l = −21→21
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.045 wR(F2) = 0.135 S = 1.01
14918 reflections 609 parameters 37 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(Fo2) + (0.0622P)2 + 0.5234P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.27 e Å−3
Δρmin = −0.22 e Å−3
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1) S1 1.03210 (3) 0.16041 (3) 0.12571 (3) 0.04354 (9)
H28 1.3261 0.0069 −0.1630 0.071* C29 1.20409 (12) 0.10748 (12) −0.08214 (12) 0.0464 (3) H29 1.1980 0.0613 −0.0175 0.056* C30 0.28653 (11) 0.87214 (10) 0.18112 (10) 0.0397 (3) C31 0.37500 (13) 0.91778 (12) 0.12528 (13) 0.0528 (4) H31 0.3737 0.9673 0.0633 0.063* C32 0.46396 (14) 0.89049 (14) 0.16081 (15) 0.0618 (4) H32 0.5222 0.9219 0.1237 0.074* C33 0.46623 (14) 0.81610 (14) 0.25209 (15) 0.0592 (4) H33 0.5263 0.7977 0.2764 0.071* C34 0.38066 (13) 0.76849 (12) 0.30798 (13) 0.0493 (3) H34 0.3841 0.7179 0.3690 0.059* C35 0.28904 (11) 0.79598 (10) 0.27338 (10) 0.0373 (3) C36 0.01034 (15) 0.90929 (12) 0.15860 (13) 0.0549 (4) H36A 0.0288 0.9529 0.0899 0.082* H36B −0.0579 0.9466 0.2022 0.082* H36C −0.0025 0.8507 0.1584 0.082* C37 0.10810 (12) 0.87449 (9) 0.19810 (10) 0.0387 (3) C38 0.09188 (11) 0.81007 (9) 0.30892 (9) 0.0349 (2) C39 0.19346 (12) 0.68252 (11) 0.43048 (10) 0.0435 (3)
H39A 0.1181 0.6718 0.4638 0.052* 0.50 H39B 0.1996 0.7177 0.4691 0.052* 0.50 H39C 0.1176 0.6729 0.4641 0.052* 0.50 H39D 0.2009 0.7173 0.4687 0.052* 0.50 C40 0.2843 (4) 0.5780 (3) 0.4369 (5) 0.0450 (3) 0.50 C41 0.3129 (5) 0.5183 (5) 0.3729 (4) 0.0530 (11) 0.50 H41 0.2802 0.5438 0.3213 0.064* 0.50 C42 0.3902 (6) 0.4206 (5) 0.3860 (6) 0.0691 (12) 0.50 H42 0.4093 0.3807 0.3431 0.083* 0.50 C43 0.4389 (5) 0.3825 (3) 0.4631 (7) 0.0853 (7) 0.50 H43 0.4907 0.3171 0.4718 0.102* 0.50 C44 0.4104 (5) 0.4421 (5) 0.5271 (5) 0.0808 (16) 0.50 H44 0.4430 0.4167 0.5787 0.097* 0.50 C45 0.3330 (4) 0.5399 (4) 0.5140 (5) 0.0592 (11) 0.50 H45 0.3139 0.5798 0.5569 0.071* 0.50 C40′ 0.2816 (4) 0.5787 (3) 0.4372 (5) 0.0450 (3) 0.50 C41′ 0.3403 (5) 0.5333 (5) 0.3561 (4) 0.0530 (11) 0.50 H41′ 0.3291 0.5689 0.2927 0.064* 0.50 C42′ 0.4159 (5) 0.4347 (5) 0.3696 (6) 0.0691 (12) 0.50 H42′ 0.4552 0.4043 0.3152 0.083* 0.50 C43′ 0.4326 (5) 0.3815 (3) 0.4642 (7) 0.0853 (7) 0.50 H43′ 0.4831 0.3155 0.4732 0.102* 0.50 C44′ 0.3738 (5) 0.4269 (5) 0.5454 (5) 0.0808 (16) 0.50 H44′ 0.3851 0.3913 0.6087 0.097* 0.50 C45′ 0.2983 (4) 0.5255 (4) 0.5319 (4) 0.0592 (11) 0.50 H45′ 0.2590 0.5558 0.5862 0.071* 0.50 C46 0.05731 (12) 0.65744 (9) 0.30806 (10) 0.0373 (3)
H47 0.1696 0.7053 0.1694 0.053* C48 0.17631 (14) 0.56422 (12) 0.17923 (12) 0.0508 (3) H48 0.2300 0.5613 0.1143 0.061* C49 0.13428 (18) 0.48211 (12) 0.24180 (13) 0.0617 (4) H49 0.1604 0.4232 0.2203 0.074* C50 0.05311 (19) 0.48863 (13) 0.33660 (13) 0.0657 (5) H50 0.0243 0.4334 0.3791 0.079* C51 0.01315 (15) 0.57533 (11) 0.37033 (11) 0.0510 (4) H51 −0.0429 0.5787 0.4342 0.061* C52 −0.08744 (12) 0.82234 (10) 0.46872 (11) 0.0431 (3) C53 −0.17437 (13) 0.83765 (11) 0.56788 (11) 0.0500 (3) C54 −0.26402 (16) 0.79480 (15) 0.61287 (15) 0.0689 (5) H54 −0.2698 0.7567 0.5801 0.083* C55 −0.3453 (2) 0.80904 (18) 0.70713 (17) 0.0888 (7) H55 −0.4058 0.7807 0.7372 0.107* C56 −0.3365 (2) 0.86476 (19) 0.75598 (17) 0.0921 (8) H56 −0.3907 0.8735 0.8193 0.111* C57 −0.2486 (2) 0.90736 (18) 0.71186 (16) 0.0849 (7) H57 −0.2432 0.9451 0.7453 0.102* C58 −0.16709 (17) 0.89472 (14) 0.61754 (14) 0.0643 (4) H58 −0.1077 0.9244 0.5876 0.077*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C56 0.0928 (17) 0.0832 (15) 0.0558 (11) −0.0134 (13) 0.0087 (11) −0.0263 (11) C57 0.0926 (16) 0.0863 (15) 0.0602 (11) −0.0065 (12) −0.0130 (11) −0.0392 (11) C58 0.0647 (10) 0.0639 (10) 0.0544 (9) −0.0073 (8) −0.0138 (8) −0.0250 (8)
Geometric parameters (Å, º)
C12—C13 1.384 (2) C43′—C44′ 1.3900 C12—H12 0.9300 C43′—H43′ 0.9300 C13—C14 1.377 (3) C44′—C45′ 1.3900 C13—H13 0.9300 C44′—H44′ 0.9300 C14—C15 1.370 (3) C45′—H45′ 0.9300 C14—H14 0.9300 C46—C51 1.3906 (18) C15—C16 1.381 (2) C46—C47 1.3930 (19) C15—H15 0.9300 C47—C48 1.382 (2) C16—H16 0.9300 C47—H47 0.9300 C17—C22 1.392 (2) C48—C49 1.377 (2) C17—C18 1.398 (2) C48—H48 0.9300 C18—C19 1.385 (2) C49—C50 1.375 (3) C18—H18 0.9300 C49—H49 0.9300 C19—C20 1.374 (3) C50—C51 1.384 (2) C19—H19 0.9300 C50—H50 0.9300 C20—C21 1.377 (3) C51—H51 0.9300 C20—H20 0.9300 C52—C53 1.4699 (19) C21—C22 1.381 (2) C53—C54 1.386 (2) C21—H21 0.9300 C53—C58 1.389 (2) C22—H22 0.9300 C54—C55 1.391 (3) C23—C24 1.4675 (18) C54—H54 0.9300 C24—C29 1.387 (2) C55—C56 1.374 (4) C24—C25 1.3963 (19) C55—H55 0.9300 C25—C26 1.379 (2) C56—C57 1.365 (4) C25—H25 0.9300 C56—H56 0.9300 C26—C27 1.375 (3) C57—C58 1.387 (3) C26—H26 0.9300 C57—H57 0.9300 C27—C28 1.374 (3) C58—H58 0.9300
C31—C30—N5 117.99 (13) C57—C58—C53 119.9 (2) C35—C30—N5 121.97 (11) C57—C58—H58 120.1 C32—C31—C30 120.71 (16) C53—C58—H58 120.1