Original Article
The changes of serum levels of vaspin, adiponectin
and leptin in type 2 diabetic polyneuropathy
Aili Sun1, Cankun Xu2, Yihong Ni1, Jing Zhang3, Shihong Chen1
1Department of Endocrinology, The Second Hospital of Shandong University, Jinan, Shandong, P. R. China; 2The
Second Hospital of Shandong Traditional Chinese Medicine University, Jinan, Shandong, P. R. China; 3Shandong
Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, P. R. China
Received December 22, 2015; Accepted March 7, 2016; Epub May 1, 2016; Published May 15, 2016
Abstract: Diabetes mellitus is a major independent risk factor for polyneuropathy, but high polyneuropathy risk in diabetic patients is not completely explained by clustering traditional risk factors. We want to determine the levels of vaspin, adiponectin, leptin and their relationships with polyneuropathy. A total of 115 Chinese Han patients with type 2 diabetes mellitus according to with or without polyneuropathy were divided into 2 groups. Diagnosis of dia-betic polyneuropathy was primarily clinical and involves a thorough history and physical examination with a focus on cardiovascular and neurologic tests, and a detailed assessment of the feet. Vaspin and adiponectin levels were determined by enzyme linked immunosorbent assay, and leptin levels by radioimmunoassay. Multiple regression analysis revealed that vaspin (P=0.000), adiponectin (P=0.001) and BMI (P=0.042) were variables that influenced DPN significantly and independently. The preponderance of evidence presented here indicates that people with DPN have increased levels of serum vaspin, leptin and declined adiponectin. Serum vaspin and adiponectin are independent affecting variables of DPN, indicating that high vaspin level and low adiponectin level may be related to the process of DPN.
Keywords: Polyneuropathy in type 2 diabetes, vaspin, adiponectin, leptin
Introduction
With the increasing prevalence of diabetes, it has been a major cause of morbidity and mor-tality. Diabetic polyneuropathy (DPN) is one of the most common and complex chronic compli-cations of type 2 diabetes mellitus generating a huge economic burden. Adipose tissue is not only an inert energy-storing tissue, but also an active endocrine organ [1], which secrets many kinds of adipocytokines, such as adiponectin, leptin, TNF-a and so on. Leptin is an adipocyte-derived hormone discovered in 1994 which created dramatic interest in the field of white adipose tissue (WAT) research and related dis-eases such as hypertension, coronary athero-sclerosis, myocardial hypertrophy, diabetes, and dyslipidemia [2, 3].
Current studies show that basal plasma leptin and insulin concentrations parallel each other as leptin resistance can lead to insulin resis-tance and diabetes [4, 5].
Vaspin, a novel member of the adipokine family, is a 395 amino-acid product with structure simi-lar to serine protease inhibitors [6]. Vaspin mRNA expression is significantly increased in patients with obesity, insulin resistance. How- ever, vaspin was also found to improve gluco- se tolerance and insulin sensitivity in obese rodents and may also normalize altered expres-sion of genes relevant to insulin resistance [7]. Adiponectin, an adipokine, has several benefits related to its insulin-sensitizing, anti-inflamma-tory, and anti-atherosclerotic effects [8]. This peptide has primarily been studied in certain disease subsets, such as metabolic syndrome, obesity, and diabetes mellitus [9]. It has a major role in enhancing insulin sensitivity by stimulat-ing the phosphorylation of AMP-activated pro-tein kinase in insulin target organs, such as skeletal muscle and liver [10].
patients presenting with and without DPN, and then to define and assess the relative impor-tance of the three kinds of adipokines and DPN.
Patients and methods
Subject selection
A total of 115 Chinese Han patients (male 60 and female 55) with type 2 diabetes aged 30-70 y were recruited from the Second Hos- pital of Shandong University between June 2010 and January 2012 who were eligible for the study, and randomly assigned to 57 cases of simple diabetic group (DM group), 58 cases of diabetic polyneuropathy (DPN), and other selected age and gender matched 55 cases health examination persons as normal control group. Diagnosis of DPN was primarily clinical and involves a thorough history and physical examination with a focus on cardiovascular and neurologic tests, and a detailed assessment of
Fasting plasma glucose (FPG) was measured by enzymatic calorimetric method of glucose oxi-dase test. Fasting insulin (FINS) and serum leptin were determined by radioimmunoassay techniques. Hemoglobin A1c (HbA1c) was as- sessed via high performance liquid chromatog-raphy. Serum concentrations of adiponectin, vaspin, total cholesterol, high density lipopro-tein cholesterol (HDL-C), low density lipoprote- in cholesterol (LDL-C), and triglycerides were determined by enzyme-linked immunosorbent assay. Homeostasis model assessment (HO- MA-IR) was used to estimate insulin resistance: HOMA-IR=FPG*FINS/22.5.
Statistical analysis
[image:2.612.93.380.84.360.2]All experimental data were analyzed by SPSS software 16.0 (SPSS, Chicago, IL, USA). Me- asurement data were presented as mean ± standard deviation (mean ± SD). Single factor and multifactor ANOVA analysis were for the
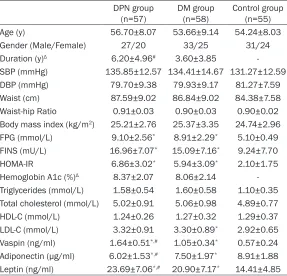
Table 1. Baseline characteristics of study and control groups
DPN group
(n=57) DM group (n=58) Control group (n=55) Age (y) 56.70±8.07 53.66±9.14 54.24±8.03 Gender (Male/Female) 27/20 33/25 31/24 Duration (y)Δ 6.20±4.96# 3.60±3.85
-SBP (mmHg) 135.85±12.57 134.41±14.67 131.27±12.59 DBP (mmHg) 79.70±9.38 79.93±9.17 81.27±7.59 Waist (cm) 87.59±9.02 86.84±9.02 84.38±7.58 Waist-hip Ratio 0.91±0.03 0.90±0.03 0.90±0.02 Body mass index (kg/m2) 25.21±2.76 25.37±3.35 24.74±2.96
FPG (mmol/L) 9.10±2.56* 8.91±2.29* 5.10±0.49
FINS (mU/L) 16.96±7.07* 15.09±7.16* 9.24±7.70
HOMA-IR 6.86±3.02* 5.94±3.09* 2.10±1.75
Hemoglobin A1c (%)Δ 8.37±2.07 8.06±2.14
-Triglycerides (mmol/L) 1.58±0.54 1.60±0.58 1.10±0.35 Total cholesterol (mmol/L) 5.02±0.91 5.06±0.98 4.89±0.77 HDL-C (mmol/L) 1.24±0.26 1.27±0.32 1.29±0.37 LDL-C (mmol/L) 3.32±0.91 3.30±0.89* 2.92±0.65
Vaspin (ng/ml) 1.64±0.51*,# 1.05±0.34* 0.57±0.24
Adiponectin (μg/ml) 6.02±1.53*,# 7.50±1.97* 8.91±1.88
Leptin (ng/ml) 23.69±7.06*,# 20.90±7.17* 14.41±4.85 Abbreviations: DM, diabetes mellitus; DPN, diabetic polyneuropathy; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FPG, Fasting plasma glucose; FINS, Fasting insulin; HOMA-IR, homeostasis model of as-sessment for insulin resistance index. Values are means ± SD. #P<0.05 compared to group DM. *P<0.05 compared to control group. Δwhen the variable duration was still not satisfied with the normal distribution, the nonparametric test of the Mann-Whitney U was used between the both groups.
the feet [11]. All patients were treated with hypoglyce-mic drugs to control blood glucose, and angiotensin-converting enzyme inhibitors (ACEI) and/or angiotensin-receptor blockers (ARB) to control blood pressure (or to reduce proteinuria). No addi-tional medicines were tak- en within 1 week of serum collection. Exclusion criteria were as follows: history of serious liver and kidney dis-ease, severe hypertension, cardiac dysfunction, cereb- rovascular accident, stress, acute or chronic infection, autoimmune disease, preg-nancy, etc.
Detection of indicators
mean difference among the three groups, and t test was used between both groups. Correlation was analyzed using Pearson correlation. The count data were analyzed by χ2 test, and logis-tic regression analysis was used for an inde-pendent risk factor of diabetic polyneuropathy. If parameters were still not satisfied with nor-mal distribution after variables converted, onparametric test was used between both groups. P<0.05 was considered statistically significant.
Results
Baseline demographics, biochemistry and clinical characteristics of all the subjects
A total of 160 subjects participated in this study with age ranging from 30 to 72 years. There was no significant difference in age, SBP, DBP, WC, WHR and BMI among the three groups (P>0.05). The duration of DPN group was longer than that of DM group (P<0.01). Patients in the two groups with diabetes had increased serum concentrations of FPG, FINS, HOMA-IR, vaspin, leptin, and decreased adiponectin compared to control group (P<0.01), but there were no sig-nificant differences in FPG, FINS and HOMA-IR between DM group and DPN group. In addition, significantly increased serum concentrations of vaspin, leptin and decreased adiponectin were
0.006), FPG (P<0.001), FINS (P=0.001), HO- MA-IR (P<0.001), TG (P=0.001), TC (P<0.001) and LDL-C (P=0.003) were obtained.
There were also significantly different between adiponectin, leptin and sex (P=0.009, P=0.000 respectively), WC (P=0.006, P=0.005 respec-tively), FPG (P=0.000, P=0.011 respectively), FINS (P=0.001, P=0.010 respectively), HOMA-IR P<0.001, P=0.002 respectively), TG (P= 0.002, P=0.000 respectively), TC (P=0.027, P=0.001 respectively) and LDL-C (P=0.017, P=0.000 respectively).
The stepwise multiple linear regression ana- lysis between vaspin, adiponectin, leptin and variables see Table 4. Multiple regression an- alysis revealed that sex (P=0.002), WC (P =0.001), FPG (P=0.000), WHR (P=0.022) and BMI (P=0.001) were variables that influenced vaspin values significantly, and sex (P=0.011), HOMA-IR (P=0.000) and TG (P=0.010) were variables that influenced adiponectin values significantly. The results also showed that sex (P=0.000), HOMA-IR (P=0.004), TG (P=0.028) and LDL-C (P=0.031) were significant determi-nants for serum leptin levels.
[image:3.612.90.367.97.151.2]Logistic analyses of influencing factors of DPN were shown in Table 5. The results revealed that vaspin (P=0.000), adiponectin (P=0.001)
Table 2. Comparison of serum vaspin, adiponectin and leptin in different sex
Female (n=79) Male (n=91) t P
Vaspin (ng/ml) 1.17±0.59 0.98±0.53 -2.096 0.038*
Adiponectin (μg/ml) 8.06±2.25 7.17±1.99 -2.646 0.0098*
Leptin (ng/ml) 22.15±7.77 17.46±6.58 -4.126 0.000* *compared between the two groups, P<0.05.
Table 3. Correlation between serum vaspin, adiponectin, leptin and variables in all subjects
Parameters R P
Vaspin Adiponectin Leptin Vaspin Adiponectin Leptin Sex 0.164 0.206 0.312 0.038 0.009 0.000 WC 0.216 -0.215 0.221 0.006 0.006 0.005 FPG 0.445 -0.384 0.200 0.000 0.000 0.011 FINS 0.260 -0.255 0.204 0.001 0.001 0.010 HOMA-IR 0.393 -0.386 0.247 0.000 0.000 0.002 TG 0.265 -0.245 0.287 0.001 0.002 0.000 TC 0.280 -0.175 0.261 0.000 0.027 0.001 LDL-C 0.231 -0.188 0.293 0.003 0.017 0.000
found in the patients of the DPN group than that of the DM group (Table 1).
Comparison of serum vaspin, adiponectin and leptin in differ-ent sex
Men comprised majority of ca- ses (56.88%, n=91). The value of serum vaspin, adiponectin, leptin of women increased sig-nificantly than that of men (P= 0.038; P=0.0098; P=000 res- pectively) (Table 2).
Correlations and multivariate analysis
[image:3.612.92.367.212.344.2]and BMI (P=0.042) were variables that influ-enced DPN significantly and independently.
Discussion
Our data showed that the three kinds of adipo-kines (vaspin, adiponectin and leptin) concen-trations of women were significantly increased than that of the men, maybe due to different kinds of fat content from different gender. HOMA-IR was also founded the mainly influenc-ing factor of the three kinds of adipokines (vaspin, adiponectin and leptin), which was accordance with adipokines improving insulin resistance. Longer duration of DPN patients than diabetic patients revealed that DPN was a long chronic damage process.
Increased visceral adiposity is associated with insulin resistance and type 2 diabetes [12-14]. Klöting et al [7] found that visceral vaspin mRNA expression was correlated with BMI, per-centage body fat, and 2-hour oral glucose toler-ance test plasma glucose. The authors con-cluded that human vaspin mRNA expression in adipose tissue could be associated with param-eters of obesity, insulin resistance, and glucose metabolism. Correlation between vaspin and diabetic microvascular [12-15] has been as- sessed, but the role of vaspin in DPN has not
clined significantly in DPN maybe be related with the discharge of anti-inflammatory media-tors [26].
Controversial results have been reported that serum leptin level tended to be associated with sensory conduction velocity [27] in diabetic patients, while another study did not find any relationship [28]. Kurajoh et al [29] found that hyperleptinemia was associated with cardiac autonomic dysfunction in patients with diabe-tes, with the relationship independent of the accumulation of visceral fat. Our study showed serum leptin levels were significantly increased in DM and DPN patients compared with control group, but there was no difference of leptin lev-els between DM and DPN patients, as reported before [29, 30].
Of all the clinical parameters, vaspin, adiponec-tin and BMI were the mainly influential factors of DPN. The specific mechanism is not clear, probably due to the inflammatory reaction or oxidative stress et al. The further confirmation is to be needed.
Limitations
First, the study had only a small sample size and needed to be repeated with a larger
popu-Table 4. The stepwise multiple linear regression analysis P values between vaspin, adiponectin, leptin and variables
Model T P
Vaspin Adiponectin Leptin Vaspin Adiponectin Leptin Constant -3.237 13.324 -2.637 0.001 0.000 0.009 Sex 3.147 2.575 5.406 0.002 0.011 0.000 WC 6.043 - - 6.043 -
-FPG 6.045 - - - -
-HOMA-IR - -4.829 2.928 0.000 0.000 0.004 TG - -2.622 2.222 - 0.010 0.028 LDL-C - - 2.179 - - 0.031 WHR 2.310 - - 0.022 - -BMI 3.342 - - 0.001 -
-Table 5. Logistic analysis of influencing factors of diabetic polyneuropathy
Model B SE Wald P OR (95% Confidence interval)
Vaspin 0.004 0.001 20.182 0.000 1.002 (1.002, 1.005) Adiponectin -0.552 0.171 10.395 0.001 0.576 (0.412, 0.805) Leptin -0.210 0.103 4.154 0.042 0.811 (0.663, 0.992)
been reported. The correlation between serum vaspin levels and markers of insulin sensitivity, glu-cose metabolism, and obesity is still controversial. In the current study, serum vaspin levels were correlated with markers of insulin sensitivity, glucose and lipid me- tabolism, including WC, fasting insulin, HOMA, TG, TC, LDL-C, as reported before. Vaspin serum lev-els markedly increased in DPN may be a defensive mechanism against insulin resistance.
[image:4.612.91.359.96.242.2] [image:4.612.91.358.290.357.2]lation. Although we identified patients prospec-tively, they were not randomly selected from a population with type 2 diabetes with or without DPN. In the paper, the relationships among decline adiponectin, elevated leptin, vaspin and the DPN are controversial, and further work is needed to prove a direct causal rela-tionship and the mechanism .
In conclusion, the preponderance of evidence presented here indicates that people with DPN have increased levels of serum vaspin, adipo-nectin and leptin. Serum vaspin and adiponec-tin were independent affecadiponec-ting variables of DPN, indicating that high vaspin level and low adiponectin level may be related to the process of DPN. Our results presented within this pilot study promote us to further determine if the use of serum vaspin and adiponectin are useful in the pathogenesis of DPN. While the relation-ship between leptin and DPN requires the fur-ther validation.
Acknowledgements
This study was supported by the seed funda-tion from the second hospital of Shandong University (S2013010004) and the Science and Technology Development Plan Project of Shandong Province (2014GSF 118041).
Disclosure of conflict of interest
None.
Address correspondence to: Dr. Shihong Chen, Department of Endocrinology, The Second Hospital of Shandong University, 247 Beiyuan Street, Jinan 250033, Shandong, P. R. China. Tel: +86 187531- 58197; Fax: +86 531 88962544; E-mail: chenshi-hong26@163.com
References
[1] Kershaw EE and Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548-2556.
[2] Mattu HS and Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol 2013; 216: T17-36.
[3] Liu J, Butler KR, Buxbaum SG, Sung JH, Campbell BW and Taylor HA. Leptinemia and its association with stroke and coronary hea- rt disease in the Jackson Heart Study. Clin Endocrinol (Oxf) 2010; 72: 32-37.
[4] Ahrén B, Larsson H, Wilhelmsson C, Näsman B and Olsson T. Regulation of circulating leptin in humans. Endocrine 1997; 7: 1-8.
[5] Yildiz BO and Haznedaroglu IC. Rethinking leptin and insulin action: Therapeutic Opp- ortunities for diabetes. Int J Biochem Cell Biol 2006; 38: 820-830.
[6] Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashi-moto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H and Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A 2005; 102: 10610-10615.
[7] Klöting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Stumvoll M, Blüher M. Vaspin gene expression in human adipose tissue: association with obesity and type 2 dia-betes. Biochem Biophys Res Commun 2006; 339: 430-436.
[8] Lara-Castro C, Fu Y, Chung BH and Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol 2007; 18: 263-270.
[9] Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, Ohnuma H, Igarashi M, Tominaga M, Kato T and Funagata S. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabe-tes in the Japanese Population: the Funagata study. Diabetes Care 2003; 26: 2015-2020. [10] Buff PR, Dodds AC, Morrison CD, Whitley NC,
McFadin EL, Daniel JA, Djiane J, Keisler DH. Leptin in horses: tissue localization and rela-tionship between peripheral concentrations of leptin and body condition. J Anim Sci 2002; 80: 2942-2948.
[11] Vinik A. The approach to the management of the patient with Neuropathic pain. J Clin Endocrinol Metab 2010; 95: 4802-4811. [12] Kaplan NM. The deadly quartet. Upper body
obesity, glucose intolerance, hypertriglyceride-mia and hypertension. Arch Intern Med 1989; 149: 1514-1520.
[13] Frayn KN. Visceral fatand insulin resistance-causative or correlative? Br J Nutr 2000; 83: S71-S77.
[14] Wajchenberg BL. Subcutaneous and visceral adipose tissue: their Relation to the metabolic syndrome. Endocr Rev 2000; 21: 697-738. [15] Gulcelik NE, Karakaya J, Gedik A, Usman A,
Gurlek A. Serum vaspin levels in type 2 diabet-ic women in relation to mdiabet-icrovascular compli-cations. Eur J Endocrinol 2009; 160: 65-70. [16] Cnop M, Havel PJ, Utzschneider KM, Carr DB,
inde-pendent roles of age and sex. Diabetologia 2003; 46: 459-469.
[17] Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Häring H and Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 2003; 52: 239-243.
[18] Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE and Tataranni PA. Hypoadiponectinemia in obesity and type 2 di-abetes: close association with insulin resis-tance and hyperinsulinemia. J Clin Endocrinol Metab 2001; 86: 1930-1935.
[19] Hanley AJ, Bowden D, Wagenknecht LE, Balasubramanyam A, Langfeld C, Saad MF, Rotter JI, Guo X, Chen YD, Bryer-Ash M, Norris JM and Haffner SM. Associations of adiponec-tin with body fat distribution and insulin sensi-tivity in nondiabetic Hispanics and African-Americans. J Clin Endocrinol Metab 2007; 92: 2665-2671.
[20] Bacha F, Saad R, Gungor N and Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell func-tion. Diabetes Care 2004; 27: 547-552. [21] Hivert MF, Sullivan LM, Fox CS, Nathan DM,
D’Agostino RB Sr, Wilson PW, Meigs JB and Hivert MF. Associations of adiponectin, resis-tin, and tumor necrosis factor alpha with insu-lin resistance. J Cinsu-lin Endocrinol Metab 2008; 93: 3165-3172.
[22] Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T and Matsuzawa Y. Plasma concen-trations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arte- rioscler Thromb Vasc Biol 2000; 20: 1595-1599.
[23] Gilardini L, McTernan PG, Girola A, da Silva NF, Alberti L, Kumar S and Invitti C. Adiponectin is a candidate marker of metabolic syndrome in obese children and adolescents. Atheroscle- rosis 2006; 189: 401-407.
[24] Mohan V, Deepa R, Pradeepa R, Vimaleswaran KS, Mohan A, Velmurugan K and Radha V. Association of low adiponectin levels with the metabolic syndrome-the Chennai Urban Rural Epidemiology Study (CURES-4). Metabolism 2005; 54: 476-481.
[25] Wang J, Li H, Franco OH, Yu Z, Liu Y and Lin X. Adiponectin and metabolic syndrome in mid-dle-aged and elderly Chinese. Obesity (Silver Spring) 2008; 16: 172-178.
[26] Ouchi N, Parker JL, Lugus JJ and Walsh K. Adipokines in inflammation and metabolic dis-ease. Nat Rev Immunol 2011; 11: 85-97. [27] Matsuda M, Kawasaki F, Inoue H, Kanda Y,
Yamada K, Harada Y, Saito M, Eto M, Matsuki M and Kaku K. Possible contribution of adipo-cytokines on diabetic neuropathy. Diabetes Res Clin Pract 2004; 66 Suppl 1: S121-123. [28] Sari R, Balci MK and Apaydin C. The
relation-ship between plasma leptin levels and chronic complication in patients with type 2 diabetes mellitus. Metab Syndr Relat Disord 2010; 8: 499-503.
[29] Kurajoh M, Koyama H, Kadoya M, Naka M, Miyoshi A, Kanzaki A, Kakutani-Hatayama M, Okazaki H, Shoji T, Moriwaki Y, Yamamoto T, Emoto M, Inaba M, Namba M. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc Diabetol 2015; 14: 117.