META-ANALYSIS: BACTERIAL MENINGITIS IN CHILDREN WITH
A FIRST FEBRILE SEIZURE
Dr. Ziyad Faleh Alnofei1*, Homam Talal Abdullah Alsharif2, Sarah Obaid Dhafar3, Saad Mohamed Saad Alharthi4, Sultan Mohammed Saleh Alzahrani5, Emad Marzouq
Rashed Alsufyani6, Amal Saleh hamdi Alsofyany7, Abdulaziz Saeed Asiri8 and Rayan Khalid Almalki9
1*
General Pead. Consultant Ta’if Children Hospital, Ta’if, Saudi Arabia.
2,3,5,6,7,8,9,
Medical Intern, Al-Hada Armed Forces Hospital, Taif, Saudi Arabia.
4
Medical Student, Al-Hada Armed Forces Hospital, Taif, Saudi Arabia.
ABSTRACT
Background & Purpose: Seizures may be the sole presentation of
bacterial meningitis in febrile babies. Seizures are the primary
manifestation of meningitis in 16.7% of kids and in one-third of those
sufferers, whereas meningeal signs and signs might not be obvious.
Consequently, it is obligatory to exclude underlying meningitis in kids
providing with fever and seizure previous to making the analysis of FS.
The Aim of this work is to provide cumulative data about the
prevalence of Bacterial Meningitis (BM) among children with a first
seizure in the context of fever. Methods: A systematic search was
performed of PubMed, Cochrane library Ovid, Scopus & Google scholar to identify
Pediatrics clinical trials, and prevalence studies, which studied the outcome of the prevalence
of BM in young children presenting to emergency care with a first ‘‘seizure and fever’’. A
meta-analysis was done using fixed and random-effect methods. The primary outcome was
prevalence of BM. Secondary outcomes were prevalence of encephalitis and overall
prevalence of CNS infections. Results: A total of 7 studies were identified involving 1414
patients. Regarding primary outcome measures, I2 (inconsistency) was 84% with highly
significant Q test for heterogeneity (p < 0.01), so random-effects model was carried out; with
overall pooled prevalence of Bacterial Meningitis = 2% (95% CI 0.5 to 4.4). Regarding
secondary outcome measures, I2 (inconsistency) was 86% with highly significant Q test for
heterogeneity (p < 0.01), so random-effects model was carried out; with overall pooled Article Received on
21 Oct. 2019,
Revised on 11 Nov. 2019, Accepted on 01 Dec. 2019,
DOI: 10.20959/wjpr201913-16435
*Corresponding Author
Dr. Ziyad Faleh Alnofei
General Pead. Consultant Ta’if Children Hospital, Ta’if, Saudi Arabia.
prevalence of encephalitis = 0.8% (95% CI to 3.6). I2 (inconsistency) was 95% with highly
significant Q test for heterogeneity (p < 0.01), so random- effects model was carried out; with
overall pooled prevalence of overall CNS infections = 4.2% (95% CI 0.12 to 13.5).
Conclusion: To conclude, Meningitis is more common in patients less than 18 months
presenting with febrile seizures.
KEYWORDS: Bacterial Meningitis, Children, First Febrile Seizure.
INTRODUCTION
Febrile seizures affect 2% to 5% of children in Europe and North the united states. it is defined
as a seizure taking place in children aged 6 months to 5 years, in a context of fever, without a
history of an unprovoked seizure or concurrent central nervous system infection.[1]
Most febrile seizures are precipitated through fevers from viral upper respiratory infections,
ear infections, or roseola. but, meningitis, if bacterial in etiology, also can present with fever
and a single, self-limited seizure. therefore it is a priority to diagnose or exclude bacterial
meningitis.[2]
Febrile seizures are classified into simple and complex febrile seizures. Simple febrile seizures
are defined as generalized seizures occurring only once in a 24-hour duration and lasting less
than 15 mins. Whereas, complex/extraordinary febrile seizures are defined as focal seizures,
lasting more than 15 mins and occurring more than one time in 24 hours. Simple febrile
seizures are benign and self-restricting. They have higher diagnosis and carry very low threat
for further epilepsy. Probability of acute bacterial meningitis presenting as fever with seizures
varies from 0.6 % to 6.7 %.[3]
Seizures may be the sole presentation of bacterial meningitis in febrile babies. Seizures are
the primary manifestation of meningitis in 16.7% of kids and in one-third of those sufferers,
whereas meningeal signs and signs might not be obvious. Consequently, it is obligatory to
exclude underlying meningitis in kids providing with fever and seizure previous to making
the analysis of FS.[4]
The American Academy of Pediatrics in its consensus statement strongly recommends
performing lumbar puncture in infants elderly 6-365 days, and considering it in youngsters
aged 12-18 months, who manifest first simple febrile seizures, for the sake of diagnosing
observed. Recent studies demonstrate a variable prevalence of meningitis in patients with
first febrile seizures.[5]
Aim of the study: The Aim of this work is to provide cumulative data about the prevalence of Bacterial Meningitis (BM) among children with a first seizure in the context of fever.
METHODS
This review was carried out using the standard methods mentioned within the Cochrane
handbook and in accordance with the (PRISMA) statement guidelines.[6]
Identification of studies
An initial search carried out throughout the PubMed, Cochrane library Ovid, Scopus &
Google scholar using the following keywords: Bacterial Meningitis, Children, First
Febrile Seizure.
We will consider published, full text studies in English only. Moreover, no attempts were
made to locate any unpublished studies nor non-English studies.
Criteria of accepted studies
Types of studies
The review will be restricted to clinical trials, and prevalence studies, either prospective or
retrospective, which studied the outcome of A the prevalence of BM in young children
presenting to emergency care with a first ‘‘seizure and fever’’.
Types of participants
Participants will be children from 6 to 72 months.
Types of outcome measures
1. Prevalence of BM (1ry outcome)
2. Prevalence of encephalitis (2ry outcome)
3. Prevalence of overall CNS infections (2ry outcome)
Inclusion criteria
English literature.
Journal articles.
Between 2009 until 2019.
seizure in the context of fever.
Human studies.
Exclusion criteria
Articles less than 20 patients, because the likelihood that small studies would have
overestimated event outcome rates.
Irrelevance to our study.
Methods of the review
Locating studies
Abstracts of articles identified using the above search strategy will be viewed, and articles
that appear of fulfill our inclusion criteria will be retrieved in full, when there is a doubt, a
second reviewer will assess the article and consensus will be reached.
Data extraction
Using the following keywords: Bacterial Meningitis, Children, First Febrile Seizure, data will
be independently extracted by two reviewers and cross-checked.
Statistical analysis
Statistical analysis done using MedCalc ver. 18.11.3 (MedCalc, Ostend, Belgium). Data were
pooled and odds ratios (ORs) as well as standard mean differences (SMD), were calculated
with their 95 per cent confidence intervals (CI). A meta-analysis was performed to calculate
direct estimates of each treatment, technique or outcome. According to heterogeneity across
trials using the I2-statistics; a fixed-effect model (P ≥ 0.1) or random-effects model (P < 0.1)
was used.
Study selection
We found 68 records; 31 were excluded based on title and abstract review; 37 articles are
searched for eligibility by full text review; 11 articles cannot be accessed or obtain full text;
10 studies were reviews and case reports; 9 were not describing outcome; leaving 7 studies
Figure 1: Flow chart for study selection.
RESULTS
[image:5.595.128.523.441.572.2]Descriptive analysis of all studies included (Tables 1, 2) Table 1: Patients and study characteristics.
N Author Study setting Number of patients Age
(months) Total
1 Shaked et al., 2009 ED 56 9
2 Seltz et al., 2009 ED, inpatient 192 19
3 Kimia et al., 2010 ED 526 17
4 Guedj et al., 2015 ED 168 8
5 Siddiqui et al., 2017 ED 157 18.5
6 Sohail et al., 2018 Inpatient 165 8
7 Sivalingam et al., 2019 Inpatient 150 30
#Studies were arranged according to publication year. ED: emergency department.
Table 2: Summary of outcome measures in all studies.
N Author
Primary outcome Secondary outcomes
Prevalence of BM Prevalence of encephalitis
Prevalence of overall CNS infections
1 Shaked et al., 2009 0 0 0
2 Seltz et al., 2009 1 0 1
3 Kimia et al., 2010 3 0 15
4 Guedj et al., 2015 0 --- ---
5 Siddiqui et al., 2017 12 --- ---
6 Sohail et al., 2018 5 --- ---
[image:5.595.17.579.625.773.2]The included studies published between 2009 and 2019.
Regarding patients’ characteristics, the average age of all patients was (15 months). The total
number of patients in all the included studies was 1414 patients.
Meta-analysis of outcome measures
Meta-analysis study was done on 7 studies which described an overall number of patients
(N=1414).
Patients who achieved outcome measures were pooled:
Each outcome was measured by
Pooled Prevalence (Proportion)
Prevalence of BM (1ry outcome)
Prevalence of encephalitis (2ry outcome)
Prevalence of overall CNS infections (2ry outcome) Regarding primary outcome measure,
We found 7 studies reported Bacterial Meningitis with total number of patients (N=1414).
I2 (inconsistency) was 84% with highly significant Q test for heterogeneity (p < 0.01), so
random-effects model was carried out; with overall pooled prevalence of Bacterial Meningitis
[image:6.595.96.492.487.734.2]= 2% (95% CI 0.5 to 4.4).
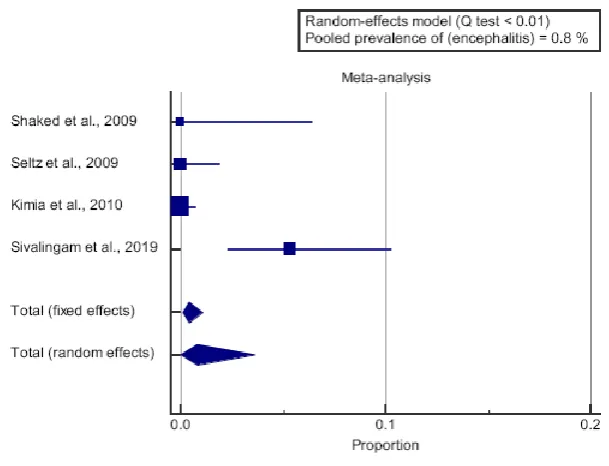
Regarding secondary outcome measure,
We found 4 studies reported encephalitis with total number of patients (N=924).
I2 (inconsistency) was 86% with highly significant Q test for heterogeneity (p < 0.01), so
[image:7.595.144.449.198.431.2]random-effects model was carried out; with overall pooled prevalence of encephalitis = 0.8%.
Figure 3: Forest plot of (encephalitis) - Proportion.
We found 4 studies reported overall CNS infections with total number of patients (N=924).
I2 (inconsistency) was 95% with highly significant Q test for heterogeneity (p < 0.01), so
random-effects model was carried out; with overall pooled prevalence of overall CNS
infections = 4.2% (95% CI 0.12 to 13.5).
[image:7.595.151.448.582.745.2]DISCUSSION
The Aim of this work is to provide cumulative data about the prevalence of Bacterial
Meningitis (BM) among children with a first seizure in the context of fever.
The included studies published between 2009 and 2019.
Regarding patients’ characteristics, the average age of all patients was (15 months). The total
number of patients in all the included studies was 1414 patients.
Regarding Meta-analysis of outcome measures; Meta-analysis study was done on 7 studies
which described an overall number of patients (N=1414).
Regarding primary outcome measure; We found 7 studies reported Bacterial Meningitis with
total number of patients (N=1414).
I2 (inconsistency) was 84% with highly significant Q test for heterogeneity (p < 0.01), so
random-effects model was carried out; with overall pooled prevalence of Bacterial Meningitis
= 2% (95% CI 0.5 to 4.4) which came in agreement with Khosroshahi et al. 2016[2] and with
Batra et al. 2011[5] and with Najaf-Zadeh et al. 2013[7] and disagreement with Reddy, Khan,
and Hegde 2016.[8]
Khosroshahi et al. 2016[2] reported that Bacterial meningitis was evident in 1.1% of patients
with first febrile seizures. 80 % of children with bacterial meningitis were presented with
complex febrile seizures with focal features. Another risk factor predicting bacterial
meningitis became duration of postictal drowsiness.
Batra et al. 2011[5] reported that the prevalence of meningitis was 2.4% in children with first
febrile seizures, 0.86% in simple febrile seizures, and 4.81% in complex febrile seizures.
Najaf-Zadeh et al. 2013[7] reported that the pooled prevalence of BM using a random effects
model was 2.6% (95%).
Reddy, Khan, and Hegde 2016[8] reported that The CSF analysis turned into normal in all the
kids who presented as simple febrile seizures. There has been 25.87% prevalence of
meningitis in children with extraordinary febrile seizures who underwent lumbar puncture.
The CSF yield suggestive of bacterial meningitis turned into as excessive as 50% in children
Regarding secondary outcome measure; We found 4 studies reported encephalitis with total
number of patients (N=924).
I2 (inconsistency) was 86% with highly significant Q test for heterogeneity (p < 0.01), so
random-effects model was carried out; with overall pooled prevalence of encephalitis = 0.8%
(95% CI 0.01 to 3.6) which came in disagreement with (Casasoprana et al. 2013)[9] and with
Guedj et al. 2017.[10]
(Casasoprana et al. 2013)[9] Reported that the diagnosis of meningitis/encephalitis become
selected in 8 cases: 3 instances of viral meningitis, three bacterial meningitis (Streptococcus
pneumoniae), and non-herpetic viral encephalitis. The prevalence of bacterial meningitis in
our study changed into 1.9%. The risk of significant infection, bacterial meningitis or
encephalitis, become expanded while there has been a complex FS (14% as opposed to 0%
with a simple FS, P=0.06). The presence of other suggestive clinical symptoms become
strongly related to a risk of bacterial meningitis/encephalitis (36% in case of scientific
orientation versus 0% within the absence of such signs, P<0.001).
Guedj et al. 2017[10] Reported that The proportions of children who underwent a lumbar
puncture amongst those with and without a medical examination end result suggestive of
meningitis or encephalitis were, respectively, 54% (113/209) and 23% (147/630).
We found 4 studies reported overall CNS infections with total number of patients (N=924).
I2 (inconsistency) was 95% with highly significant Q test for heterogeneity (p < 0.01), so
random-effects model was carried out; with overall pooled prevalence of overall CNS
infections = 4.2 % (95% CI 0.12 to 13.5) which came in agreement with Najaf-Zadeh et al.
2013[7] and with Mwipopo et al. 2016[11] and disagreement with Mahmood, Fareed, and
Tabbasum 2011.[12]
Najaf-Zadeh et al. 2013[7] Reported that the overall average prevalence of CNS infections was
3.9% (range 2.3 to 7.4%).
Mwipopo et al. 2016[11] reported that There were 109 (54.5%) males and 91 (45.5%) females.
among these patients, 193 (96.5%) were aged 1 month to 5 years and 182 (91.0%) presented
with seizures and fever. Generalized tonic-clonic seizure become the maximum common
175 (87.5%) children followed by epilepsy in 11 (5.5%) children. There were simplest three
(2%) kids with central nervous system infections.
Mahmood, Fareed, and Tabbasum 2011[12] reported that The most common cause of fever
leading to febrile convulsions was respiratory tract infections 40%, 2nd most common being
UTI that accounts for 24%, CNS infections being 8% and other causes 24%.
CONCLUSION
To conclude, Meningitis is more common in patients less than 18 months presenting with
febrile seizures.
ACKNOWLEDGMENTS Conflict of interest
None.
Authorship
All the listed authors contributed significantly to conception and design of study, acquisition,
analysis and interpretation of data and drafting of manuscript, to justify authorship.
Funding Self-funding.
REFERENCES
1. Guedj, R.; Chappuy, H.; Titomanlio, L.; Trieu, T.-V.; Biscardi, S.; Nissack-Obiketeki, G.;
Pellegrino, B.; Charara, O.; Angoulvant, F.; Villemeur, T. B. D.; et al. Risk of Bacterial
Meningitis in Children 6 to 11 Months of Age With a First Simple Febrile Seizure: A
Retrospective, Cross-Sectional, Observational Study. Acad Emerg Med, 2015; 22(11):
1290–1297. https://doi.org/10.1111/acem.12798.
2. Khosroshahi, N.; Kamrani, K.; Zoham, M. H.; Noursadeghi, H. Factors Predicting
Bacterial Meningitis in Children Aged 6-18 Months Presenting with First Febrile Seizure;
2016. https://doi.org/10.18203/2349-3291.ijcp20161033.
3. Reddy, D. S. S.; Khan, S. H.; Hegde, P. Predictors of Meningitis in Children Presenting
with First Episode of Febrile Seizure; 2016.
https://doi.org/10.18203/2349-3291.ijcp20164593.
4. Tavasoli, A.; Afsharkhas, L.; Edraki, A. Frequency of Meningitis in Children Presenting
neurology, 2014; 8(4): 51.
5. Batra, P.; Gupta, S.; Gomber, S.; Saha, A. Predictors of Meningitis in Children Presenting
With First Febrile Seizures. Pediatric Neurology, 2011; 44(1): 35–39.
https://doi.org/10.1016/j.pediatrneurol.2010.07.005.
6. Liberati, A.; Altman, D.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.; Ioannidis, J.; Clarke, M.;
Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic
Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions. Bmj
2009; 339.
7. Najaf-Zadeh, A.; Dubos, F.; Hue, V.; Pruvost, I.; Bennour, A.; Martinot, A. Risk of
Bacterial Meningitis in Young Children with a First Seizure in the Context of Fever: A
Systematic Review and Meta-Analysis. PLoS ONE, 2013; 8(1): e55270.
https://doi.org/10.1371/journal.pone.0055270.
8. Reddy, D. S. S.; Khan, S. H.; Hegde, P. Predictors of Meningitis in Children Presenting
with First Episode of Febrile Seizure; 2016.
https://doi.org/10.18203/2349-3291.ijcp20164593.
9. Casasoprana, A.; Hachon, C. L. C.; Claudet, I.; Grouteau, E.; Chaix, Y.; Cances, C.;
Karsenty, C.; Cheuret, E. [Value of lumbar puncture after a first febrile seizure in children
aged less than 18 months. A retrospective study of 157 cases]. Arch Pediatr, 2013; 20(6):
594–600. https://doi.org/10.1016/j.arcped.2013.03.022.
10.Guedj, R.; Chappuy, H.; Titomanlio, L.; De Pontual, L.; Biscardi, S.; Nissack-Obiketeki,
G.; Pellegrino, B.; Charara, O.; Angoulvant, F.; Denis, J.; et al. Do All Children Who
Present With a Complex Febrile Seizure Need a Lumbar Puncture? Annals of Emergency
Medicine, 2017; 70(1): 52-62.e6. https://doi.org/10.1016/j.annemergmed.2016.11.024.
11.Mwipopo, E. E.; Akhatar, S.; Fan, P.; Zhao, D. Profile and Clinical Characterization of
Seizures in Hospitalized Children. Pan Afr Med J, 2016; 24.
https://doi.org/10.11604/pamj.2016.24.313.9275.
12.Mahmood, K. T.; Fareed, T.; Tabbasum, R. Management of Febrile Seizures in Children;
2011.
Papers included in our meta-analysis
Shaked, O., Peña, B.M.G., Linares, M.Y. and Baker, R.L., Simple febrile seizures: are the
AAP guidelines regarding lumbar puncture being followed?. Pediatric emergency care,
2009; 25(1): 8-11.
meningitis/encephalitis in children with complex febrile seizures. Pediatric emergency
care, 2009; 25(8): 494-497.
Kimia, A., Ben-Joseph, E.P., Rudloe, T., Capraro, A., Sarco, D., Hummel, D., Johnston,
P. and Harper, M.B., Yield of lumbar puncture among children who present with their
first complex febrile seizure. Pediatrics, 2010; 126(1): 62-69.
Guedj, R., Chappuy, H., Titomanlio, L., Trieu, T.V., Biscardi, S., Nissack-Obiketeki, G.,
Pellegrino, B., Charara, O., Angoulvant, F., Villemeur, T.B.D. and Levy, C., Risk of
Bacterial Meningitis in Children 6 to 11 Months of Age with a First Simple Febrile
Seizure: A Retrospective, Cross-sectional, Observational Study. Academic Emergency
Medicine, 2015; 22(11): 1290-1297.
Siddiqui, H.B., Haider, N. and Khan, Z., Frequency of acute bacterial meningitis in
children with first episode of febrile seizures. JPMA. The Journal of the Pakistan Medical
Association, 2017; 67(7): 1054-1058.
Sohail, A., Das, C., Parkash, J. and Hotwani, P., Association of Meningitis among
Children with First Attack of Fever and Seizure without Clinical Manifestations of
Meningitis. Journal of Liaquat University of Medical & Health Sciences, 2018; 17(04):
241-244.
Sivalingam, B., Srinivasan, R. and Thilak, T., Clinicoetiological Profile Of First Episode
Seizure In Children 1 Month To 12 Years. Journal of Evolution of Medical and Dental