Acta Cryst.(2002). E58, m519±m520 DOI: 10.1107/S1600536802015787 Cornelis Lensinket al. [Ti(C21H26NO2S)Cl2]
m519
metal-organic papers
Acta Crystallographica Section E
Structure Reports
Online ISSN 1600-5368
Bis(
l
-
N
-(3-[3-indenyl]propyl-
p
-toluenesulfamido-N,O,O
000)-dimethylamidodichorotitanium)
Cornelis Lensink,* Graeme J. Gainsford and Menno J. R. Brandsma
Industrial Research Limited, PO Box 31-310, Lower Hutt, New Zealand
Correspondence e-mail: g.gainsford@irl.cri.nz
Key indicators Single-crystal X-ray study T= 170 K
Mean(C±C) = 0.007 AÊ Disorder in main residue Rfactor = 0.052 wRfactor = 0.140
Data-to-parameter ratio = 17.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
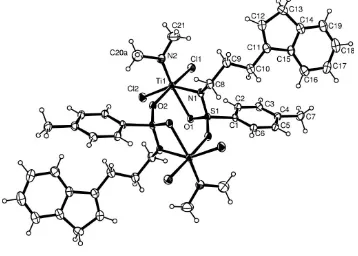
The title compound, [Ti(C21H26N2O2S)Cl2], crystallizes as a centrosymmetric dimer, with an eight-membered ring derived from the monomer sub-units by the formation of two TiÐ (N,O)ÐSÐO head-to-tail sequences around a crystallo-graphic inversion centre. The titanium atoms each have a distorted octahedral geometry through the nitrogen and one oxygen of the sulfonamido group [Ti1ÐO1, TiÐN1 2.280 (3), 2.091 (3) AÊ], one oxygen from the adjacent sulfonamide [TiÐ O2 2.170 (3) AÊ], a dimethylamido nitrogen and two chlorides.
Comment
The title compound, which is related to previously reported structures (Lensink, 1998, Lensinket al., 2001), crystallizes as a centrosymmetric dimer with an eight-membered ring derived from the monomer sub-units by the formation of two TiÐ (N,O)ÐSÐO head-to-tail sequences around a crystal-lographic inversion centre (Fig. 1, Table 1). There are no signi®cant intermolecular contacts in the crystal structure. The titanium atoms have a distorted octahedral geometry through the nitrogen and one oxygen of the sulfonamido group [Ti1Ð O1, TiÐN1 2.280 (3), 2.091 (3) AÊ], one oxygen from the adjacent sulfonamide [TiÐO2 2.170 (3) AÊ], a dimethylamido nitrogen and two chlorides. A similar ring structure has been reported for the yttrium compound, bis((2-trans -1,2-
bis(2,4,6-tri-isopropylbenzene-sulfonamidato)cyclohexane-N,N0,O,O0,O00)-bis(methylsilyl)-amido-yttrium(III))
(Goerlit-zeret al., 1998).
The TiÐN2 bond distance of 1.860 (4) AÊ is consistent with
N(p)±M(d) interaction expected for a dimethylamide, and the TiÐCl bond distances and relevant geometric parameters are similar to those found in dichloro-(4-methyl-2-(tri methylsilylamino)pyridine-N,N0
)-dimethylamino-dimethyl-amido-titanium (Fuhrmann et al., 1996). The SÐO bond
distances [1.471 (3), 1.477 (3) AÊ] re¯ect the equivalent dative binding of the O atoms to the titanium centres (Lensinket al., 2001). By comparison with the free 3-[3-indenyl]propyl group inN-(3-(3-indenyl)propyl)benzylammonium bromide (Groux
et al., 1999), it appears that the total geometry of the group is unaffected by its link to the complex, with the only signi®cant difference being close to the nitrogen N1, with an N1ÐC8Ð C9ÐC10 torsion angle 74.8 (5) in the complex, compared
withÿ54.4 (3)in the free group.
Experimental
A solution of C9H7(CH2)3N(H)SO2C6H4CH3(0.13 g, 0.40 mmol) in benzene-d6(2 ml) was added dropwise to a solution of Ti(NMe2)4 (89 mg, 0.40 mmol) dissolved in benzene-d6 (3 ml), turning the mixture from yellow to orange. The mixture was re¯uxed over a period of 4 days. Subsequently Me3SiCl (108 mg, 0.99 mmol) was slowly added. The mixture was stirred for 20 h, resulting in a dark-brown solution. Recrystallization from a CH2Cl2/pentane mixture resulted in crystals suitable for X-ray analysis. Yield: 20 mg (10%).
Crystal data [Ti(C21H26NO2S)Cl2]
Mr= 489.30
Triclinic,P1 a= 7.688 (3) AÊ b= 10.122 (5) AÊ c= 16.212 (7) AÊ = 94.808 (6) = 100.973 (5) = 109.200 (5) V= 1154.9 (9) AÊ3
Z= 2
Dx= 1.407 Mg mÿ3
MoKradiation Cell parameters from 6100
re¯ections = 2.6±25.8 = 0.71 mmÿ1
T= 170 (2) K Block, orange±brown 0.360.200.16 mm
Data collection
Siemens CCD area-detector diffractometer
'and!scans
Absorption correction: multi-scan (Blessing, 1995)
Tmin= 0.393,Tmax= 0.892
14834 measured re¯ections
4641 independent re¯ections 2627 re¯ections withI> 2(I) Rint= 0.078
max= 26.4
h=ÿ9!4 k=ÿ12!12 l=ÿ20!20 Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.052
wR(F2) = 0.140
S= 0.94 4641 re¯ections 264 parameters
H-atom parameters constrained w= 1/[2(F
o2) + (0.0747P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.73 e AÊÿ3
min=ÿ0.54 e AÊÿ3
Table 1
Selected geometric parameters (AÊ,).
Ti1ÐN2 1.860 (4)
Ti1ÐN1 2.091 (3)
Ti1ÐO2 2.170 (3)
Ti1ÐO1 2.280 (3)
Ti1ÐCl1 2.3010 (16)
Ti1ÐCl2 2.3196 (14)
S1ÐO1 1.471 (3)
S1ÐO2i 1.477 (3)
S1ÐN1 1.566 (3)
S1ÐC1 1.776 (4)
N2ÐTi1ÐN1 100.84 (15)
Cl1ÐTi1ÐCl2 95.64 (5)
C8ÐN1ÐS1 122.1 (3)
C8ÐN1ÐTi1 134.6 (3)
S1ÐN1ÐTi1 99.79 (16)
Symmetry code: (i) 2ÿx;1ÿy;1ÿz.
All H atoms except those on methyl C atoms were constrained with a riding model, with an isotropic thermal parameter 1.2 times that of the equivalentUof their parent atom. Atom C20 was disor-dered over two sites (a/b), with ®nal occupancies 0.78 (1)/0.22(1) and a commonUof 0.069 AÊ2.
Data collection: SMART (Siemens, 1996); cell re®nement:
SMART; data reduction: SAINT (Siemens, 1996) and SADABS
(Sheldrick, 1996); program(s) used to solve structure: SHELXS97 (Sheldrick, 1997); program(s) used to re®ne structure:SHELXL97 (Sheldrick, 1997); molecular graphics: ORTEP-3 (Farrugia, 1997); software used to prepare material for publication:SHELXL97.
We thank Dr J. Wikaira and Professor W. T. Robinson, University of Canterbury, for their assistance.
References
Blessing, R. H. (1995).Acta Cryst.A51, 33±38. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Fuhrmann, H., Brenner, S., Arndt, P. & Kempe R. (1996).Inorg. Chem.35, 6742±6745.
Goerlitzer, H. W., Spiegler, M. & Anwander, R. (1998).Eur. J. Inorg. Chem.7, 1009±1014.
Groux, L. F., Belanger-Gariepy, F. & Zargarian, D. (1999).Acta Cryst.C55, IUC9900130.
Lensink, C. (1998).J. Organomet. Chem.553, 387±392.
Lensink, C., Gainsford, G. J. & Baxter, N. I. (2001).Acta Cryst.C57, 366±367. Sheldrick, G. M. (1996).SADABS. University of GoÈttingen, Germany. Sheldrick, G. M. (1997). SHELXL97 and SHELXS97. University of
GoÈttingen, Germany.
Siemens (1996).SMARTandSAINT. Versions 4.0. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Figure 1
Molecular structure of [Ti(C21H26N2O2S)Cl2] (Farrugia, 1997).
supporting information
sup-1
Acta Cryst. (2002). E58, m519–m520
supporting information
Acta Cryst. (2002). E58, m519–m520 [doi:10.1107/S1600536802015787]
Bis(µ-
N
-(3-[3-indenyl]propyl-
p
-toluenesulfamido-
N,O,O
′
)-dimethylamido-dichorotitanium)
Cornelis Lensink, Graeme J. Gainsford and Menno J. R. Brandsma
S1. Comment
The title compound, which is related to previously reported structures (Lensink, 1998, Lensink et al., 2001), crystallizes
as a centrosymmetric dimer with an eight-membered ring derived from the monomer sub-units by the formation of two Ti
—(N,O)—S—O head-to-tail sequences around a crystallographic inversion centre (Fig. 1; Table 1). There are no
significant intermolecular contacts in the crystal structure. The titanium atoms have a distorted octahedral geometry
through the nitrogen and one oxygen of the sulfonamido group [Ti1—O1, Ti—N1 2.280 (3), 2.091 (3) Å], one oxygen
from the adjacent sulfonamide [Ti—O2 2.170 (3) Å], a dimethylamido nitrogen and two chlorides. A similar ring
structure has been reported for the yttrium compound, Bis((µ2-trans
-1,2-bis(2,4,6-tri-isopropylbenzene-sulfonamidato)cyclo hexane-N,N′,O,O′,O′′)-bis(methylsilyl)-amido-yttrium(III)) (Goerlitzer et al., 1998).
The Ti—N2 bond distance of 1.860 (4) Å is consistent with N(pπ\)–M(dπ\) interaction expected for a dimethylamide,
and the Ti—Cl bond distances and relative geometries are similar to those found in
dichloro-(4-methyl-2-(trimethylsilyl-amino)pyridine-N,N′)-dimethyl amino-dimethylamido-titanium (Fuhrmann et al., 1996). The S—O bond distances
[1.471 (3), 1.477 (3) Å] reflect the equivalent dative binding of the O atoms to the titanium centres (Lensink et al., 2001).
By comparison with the free 3-[3-indenyl]propyl group in N-(3-(3-indenyl)propyl)benzylammonium bromide (Groux et
al., 1995), it appears that the total geometry of the group is unaffected by its link to the complex, with the only significant difference being close to the nitrogen N1, with an N1—C8—C9—C10 torsion angle here of 74.8 (5)° compared with
−54.4 (3)°.
S2. Experimental
A solution of C9H7(CH2)3N(H)SO2C6H4CH3 (0.13 g, 0.40 mmol) in benzene-d6 (2 ml) was added dropwise to a solution
of Ti(NMe2)4 (89 mg, 0.40 mmol) dissolved in benzene-d6 (3 ml), turning the mixture from yellow to orange. The
mixture was refluxed over a period of 4 days. Subsequently Me3SiCl (108 mg, 0.99 mmol) was slowly added. The
mixture was stirred for 20 h, resulting in a dark-brown solution. Recrystallization from a CH2Cl2/pentane mixture resulted
in crystals suitable for X-ray analysis. Yield: 20 mg (10%).
S3. Refinement
All H atoms except those on methyl carbons were constrained to an isotropic thermal parameter 1.2 times that of the
equivalent U of their parent atom. Atom C20 was disordered over two sites (a/b), with final occupancies 0.78 (1)/0.22 (1)
Figure 1
Molecular structure of [Ti(C21H26N2O2S)Cl2] (Farrugia, 1997). Displacement ellipsoids are drawn at the 30% probability
level. H atoms have arbitrary radii.
(I)
Crystal data [Ti(C21H26NO2S)Cl2]
Mr = 489.30
Triclinic, P1 Hall symbol: -P 1 a = 7.688 (3) Å b = 10.122 (5) Å c = 16.212 (7) Å α = 94.808 (6)° β = 100.973 (5)° γ = 109.200 (5)° V = 1154.9 (9) Å3
Z = 2 F(000) = 508 Dx = 1.407 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 6100 reflections θ = 2.6–25.8°
µ = 0.71 mm−1
T = 170 K
Block, orange-brown 0.36 × 0.20 × 0.16 mm
Data collection
Make? CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 8.192 pixels mm-1
φ and ω scans
Absorption correction: multi-scan (Blessing, 1995)
Tmin = 0.393, Tmax = 0.892
14834 measured reflections 4641 independent reflections 2627 reflections with I > 2σ(I) Rint = 0.078
θmax = 26.4°, θmin = 2.3°
supporting information
sup-3
Acta Cryst. (2002). E58, m519–m520 Refinement
Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.052
wR(F2) = 0.140
S = 0.94 4641 reflections 264 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0747P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.73 e Å−3
Δρmin = −0.54 e Å−3
Special details
Experimental. Crystal decay was monitored by repeating the initial 10 frames at the end of the data collection and analyzing duplicate reflections. The standard 1.0 mm diameter collimator was used.
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
Ti1 0.94332 (10) 0.28639 (7) 0.38452 (4) 0.0292 (2)
Cl1 0.95819 (16) 0.25997 (11) 0.24397 (6) 0.0419 (3)
Cl2 1.17818 (16) 0.19587 (12) 0.43266 (7) 0.0456 (3)
S1 1.02898 (14) 0.58169 (10) 0.40401 (6) 0.0286 (3)
O1 1.1662 (4) 0.5083 (3) 0.40947 (16) 0.0305 (6)
O2 0.9501 (4) 0.3195 (3) 0.51925 (16) 0.0347 (7)
N1 0.8382 (4) 0.4523 (3) 0.37891 (19) 0.0299 (8)
N2 0.7201 (5) 0.1317 (4) 0.3684 (2) 0.0420 (9)
C1 1.0635 (5) 0.6921 (4) 0.3245 (2) 0.0273 (9)
C2 1.1104 (6) 0.6447 (4) 0.2518 (2) 0.0336 (10)
H2 1.1305 0.5569 0.2462 0.040*
C3 1.1275 (6) 0.7269 (4) 0.1875 (3) 0.0359 (10)
H3 1.1596 0.6945 0.1378 0.043*
C4 1.0986 (5) 0.8563 (4) 0.1941 (3) 0.0347 (10)
C5 1.0513 (6) 0.9014 (4) 0.2677 (3) 0.0388 (11)
H5 1.0303 0.9888 0.2733 0.047*
C6 1.0344 (6) 0.8202 (4) 0.3331 (2) 0.0344 (10)
H6 1.0031 0.8524 0.3831 0.041*
C7 1.1197 (7) 0.9453 (5) 0.1235 (3) 0.0502 (13)
H7A 1.1529 0.8967 0.0773 0.060*
H7B 1.2199 1.0376 0.1457 0.060*
H7C 1.0000 0.9591 0.1017 0.060*
H8A 0.6771 0.5638 0.4120 0.048*
H8B 0.5920 0.3994 0.4194 0.048*
C9 0.5186 (6) 0.4390 (5) 0.2963 (3) 0.0453 (12)
H9A 0.5168 0.3511 0.2638 0.054*
H9B 0.3891 0.4224 0.3045 0.054*
C10 0.5674 (7) 0.5560 (5) 0.2441 (3) 0.0502 (13)
H10A 0.6985 0.5750 0.2376 0.060*
H10B 0.5648 0.6431 0.2757 0.060*
C11 0.4381 (6) 0.5267 (5) 0.1564 (3) 0.0411 (11)
C12 0.2972 (6) 0.4079 (5) 0.1174 (3) 0.0423 (11)
H12 0.2561 0.3252 0.1427 0.051*
C13 0.2098 (6) 0.4207 (5) 0.0272 (3) 0.0454 (12)
H13A 0.2244 0.3515 −0.0154 0.054*
H13B 0.0739 0.4069 0.0199 0.054*
C14 0.3240 (6) 0.5704 (5) 0.0205 (3) 0.0416 (11)
C15 0.4566 (6) 0.6325 (5) 0.0976 (3) 0.0397 (11)
C16 0.5788 (7) 0.7745 (5) 0.1090 (3) 0.0539 (13)
H16 0.6688 0.8182 0.1612 0.065*
C17 0.5625 (8) 0.8490 (6) 0.0404 (4) 0.0618 (15)
H17 0.6428 0.9450 0.0461 0.074*
C18 0.4307 (8) 0.7844 (7) −0.0357 (4) 0.0680 (16)
H18 0.4244 0.8363 −0.0816 0.082*
C19 0.3092 (7) 0.6469 (6) −0.0457 (3) 0.0555 (14)
H19 0.2164 0.6050 −0.0972 0.067*
C20A 0.6843 (11) 0.0315 (9) 0.4275 (5) 0.069 (2)* 0.785 (11)
H20A 0.5903 0.0454 0.4571 0.083* 0.785 (11)
H20B 0.8023 0.0465 0.4691 0.083* 0.785 (11)
H20C 0.6361 −0.0653 0.3962 0.083* 0.785 (11)
C20B 0.612 (4) 0.092 (3) 0.4359 (17) 0.069 (2)* 0.215 (11)
H20D 0.5607 0.1657 0.4502 0.083* 0.215 (11)
H20E 0.6962 0.0830 0.4868 0.083* 0.215 (11)
H20F 0.5072 0.0015 0.4147 0.083* 0.215 (11)
C21 0.5616 (8) 0.0853 (6) 0.2922 (3) 0.0774 (18)
H21A 0.5378 −0.0132 0.2688 0.093*
H21B 0.5943 0.1460 0.2493 0.093*
H21C 0.4475 0.0922 0.3078 0.093*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Ti1 0.0380 (4) 0.0287 (4) 0.0230 (4) 0.0135 (3) 0.0078 (3) 0.0065 (3)
Cl1 0.0590 (7) 0.0422 (7) 0.0272 (6) 0.0186 (6) 0.0149 (5) 0.0062 (5)
Cl2 0.0586 (7) 0.0432 (7) 0.0424 (7) 0.0318 (6) 0.0050 (5) 0.0057 (5)
S1 0.0387 (6) 0.0300 (5) 0.0220 (5) 0.0166 (5) 0.0088 (4) 0.0085 (4)
O1 0.0380 (16) 0.0301 (15) 0.0287 (15) 0.0188 (13) 0.0071 (12) 0.0075 (12)
O2 0.0565 (18) 0.0312 (16) 0.0216 (14) 0.0199 (14) 0.0125 (13) 0.0060 (12)
N1 0.0385 (19) 0.0332 (19) 0.0256 (18) 0.0181 (16) 0.0134 (15) 0.0097 (15)
supporting information
sup-5
Acta Cryst. (2002). E58, m519–m520
C1 0.034 (2) 0.032 (2) 0.022 (2) 0.0150 (19) 0.0093 (17) 0.0110 (17)
C2 0.043 (2) 0.032 (2) 0.033 (2) 0.019 (2) 0.0133 (19) 0.0092 (19)
C3 0.038 (2) 0.047 (3) 0.026 (2) 0.016 (2) 0.0125 (18) 0.009 (2)
C4 0.034 (2) 0.037 (2) 0.034 (2) 0.011 (2) 0.0099 (19) 0.0121 (19)
C5 0.054 (3) 0.028 (2) 0.035 (3) 0.016 (2) 0.008 (2) 0.0090 (19)
C6 0.049 (3) 0.032 (2) 0.026 (2) 0.019 (2) 0.0091 (19) 0.0040 (18)
C7 0.070 (3) 0.041 (3) 0.038 (3) 0.013 (3) 0.017 (2) 0.015 (2)
C8 0.039 (2) 0.051 (3) 0.041 (3) 0.024 (2) 0.012 (2) 0.015 (2)
C9 0.045 (3) 0.050 (3) 0.045 (3) 0.021 (2) 0.007 (2) 0.015 (2)
C10 0.052 (3) 0.058 (3) 0.044 (3) 0.025 (3) 0.006 (2) 0.016 (2)
C11 0.044 (3) 0.050 (3) 0.037 (3) 0.025 (2) 0.012 (2) 0.010 (2)
C12 0.044 (3) 0.051 (3) 0.044 (3) 0.025 (2) 0.020 (2) 0.017 (2)
C13 0.042 (3) 0.059 (3) 0.037 (3) 0.023 (2) 0.009 (2) −0.001 (2)
C14 0.046 (3) 0.064 (3) 0.033 (2) 0.038 (3) 0.020 (2) 0.013 (2)
C15 0.038 (2) 0.048 (3) 0.044 (3) 0.025 (2) 0.015 (2) 0.016 (2)
C16 0.052 (3) 0.063 (3) 0.053 (3) 0.029 (3) 0.009 (2) 0.011 (3)
C17 0.072 (4) 0.052 (3) 0.079 (4) 0.032 (3) 0.030 (3) 0.032 (3)
C18 0.087 (4) 0.092 (5) 0.058 (4) 0.062 (4) 0.029 (3) 0.034 (3)
C19 0.065 (3) 0.075 (4) 0.043 (3) 0.040 (3) 0.019 (3) 0.021 (3)
C21 0.064 (4) 0.070 (4) 0.069 (4) −0.003 (3) 0.001 (3) −0.001 (3)
Geometric parameters (Å, º)
Ti1—N2 1.860 (4) C9—C10 1.502 (6)
Ti1—N1 2.091 (3) C9—H9A 0.9900
Ti1—O2 2.170 (3) C9—H9B 0.9900
Ti1—O1 2.280 (3) C10—C11 1.515 (6)
Ti1—Cl1 2.3010 (16) C10—H10A 0.9900
Ti1—Cl2 2.3196 (14) C10—H10B 0.9900
S1—O1 1.471 (3) C11—C12 1.331 (6)
S1—O2i 1.477 (3) C11—C15 1.482 (6)
S1—N1 1.566 (3) C12—C13 1.523 (6)
S1—C1 1.776 (4) C12—H12 0.9500
O2—S1i 1.477 (3) C13—C14 1.506 (6)
N1—C8 1.491 (5) C13—H13A 0.9900
N2—C20A 1.450 (7) C13—H13B 0.9900
N2—C21 1.479 (6) C14—C19 1.383 (6)
N2—C20B 1.50 (3) C14—C15 1.400 (6)
C1—C6 1.387 (5) C15—C16 1.408 (6)
C1—C2 1.389 (5) C16—C17 1.404 (6)
C2—C3 1.387 (5) C16—H16 0.9500
C2—H2 0.9500 C17—C18 1.389 (7)
C3—C4 1.397 (6) C17—H17 0.9500
C3—H3 0.9500 C18—C19 1.373 (7)
C4—C5 1.395 (6) C18—H18 0.9500
C4—C7 1.516 (5) C19—H19 0.9500
C5—C6 1.395 (5) C20A—H20A 0.9800
C6—H6 0.9500 C20A—H20C 0.9800
C7—H7A 0.9800 C20B—H20D 0.9800
C7—H7B 0.9800 C20B—H20E 0.9800
C7—H7C 0.9800 C20B—H20F 0.9800
C8—C9 1.533 (6) C21—H21A 0.9800
C8—H8A 0.9900 C21—H21B 0.9800
C8—H8B 0.9900 C21—H21C 0.9800
N2—Ti1—N1 100.84 (15) C9—C8—H8B 109.0
N2—Ti1—O2 89.40 (13) H8A—C8—H8B 107.8
N1—Ti1—O2 84.76 (11) C10—C9—C8 115.0 (4)
N2—Ti1—O1 164.90 (14) C10—C9—H9A 108.5
N1—Ti1—O1 64.48 (12) C8—C9—H9A 108.5
O2—Ti1—O1 85.83 (10) C10—C9—H9B 108.5
N2—Ti1—Cl1 94.28 (12) C8—C9—H9B 108.5
N1—Ti1—Cl1 95.94 (9) H9A—C9—H9B 107.5
O2—Ti1—Cl1 176.05 (8) C9—C10—C11 115.1 (4)
O1—Ti1—Cl1 90.96 (7) C9—C10—H10A 108.5
N2—Ti1—Cl2 103.80 (12) C11—C10—H10A 108.5
N1—Ti1—Cl2 151.82 (10) C9—C10—H10B 108.5
O2—Ti1—Cl2 82.06 (8) C11—C10—H10B 108.5
O1—Ti1—Cl2 89.73 (8) H10A—C10—H10B 107.5
Cl1—Ti1—Cl2 95.64 (5) C12—C11—C15 108.3 (4)
O1—S1—O2i 116.01 (16) C12—C11—C10 128.7 (4)
O1—S1—N1 100.58 (17) C15—C11—C10 122.9 (4)
O2i—S1—N1 114.78 (17) C11—C12—C13 111.9 (4)
O1—S1—C1 108.32 (17) C11—C12—H12 124.1
O2i—S1—C1 103.70 (18) C13—C12—H12 124.1
N1—S1—C1 113.70 (17) C14—C13—C12 102.1 (3)
S1—O1—Ti1 94.92 (13) C14—C13—H13A 111.4
S1i—O2—Ti1 148.35 (16) C12—C13—H13A 111.4
C8—N1—S1 122.1 (3) C14—C13—H13B 111.4
C8—N1—Ti1 134.6 (3) C12—C13—H13B 111.4
S1—N1—Ti1 99.79 (16) H13A—C13—H13B 109.2
C20A—N2—C21 109.9 (4) C19—C14—C15 120.9 (5)
C21—N2—C20B 99.8 (11) C19—C14—C13 130.2 (4)
C20A—N2—Ti1 123.8 (4) C15—C14—C13 108.8 (4)
C21—N2—Ti1 126.1 (3) C14—C15—C16 120.4 (4)
C20B—N2—Ti1 124.2 (11) C14—C15—C11 108.9 (4)
C6—C1—C2 120.6 (4) C16—C15—C11 130.7 (4)
C6—C1—S1 120.4 (3) C17—C16—C15 117.5 (5)
C2—C1—S1 118.9 (3) C17—C16—H16 121.3
C3—C2—C1 119.2 (4) C15—C16—H16 121.3
C3—C2—H2 120.4 C18—C17—C16 120.9 (5)
C1—C2—H2 120.4 C18—C17—H17 119.5
C2—C3—C4 121.5 (4) C16—C17—H17 119.5
C2—C3—H3 119.2 C19—C18—C17 121.3 (5)
supporting information
sup-7
Acta Cryst. (2002). E58, m519–m520
C5—C4—C3 118.1 (4) C17—C18—H18 119.4
C5—C4—C7 121.1 (4) C18—C19—C14 119.0 (5)
C3—C4—C7 120.7 (4) C18—C19—H19 120.5
C6—C5—C4 121.0 (4) C14—C19—H19 120.5
C6—C5—H5 119.5 N2—C20A—H20A 109.5
C4—C5—H5 119.5 N2—C20A—H20B 109.5
C1—C6—C5 119.5 (4) N2—C20A—H20C 109.5
C1—C6—H6 120.3 N2—C20B—H20D 109.5
C5—C6—H6 120.3 N2—C20B—H20E 109.5
C4—C7—H7A 109.5 H20D—C20B—H20E 109.5
C4—C7—H7B 109.5 N2—C20B—H20F 109.5
H7A—C7—H7B 109.5 H20D—C20B—H20F 109.5
C4—C7—H7C 109.5 H20E—C20B—H20F 109.5
H7A—C7—H7C 109.5 N2—C21—H21A 109.5
H7B—C7—H7C 109.5 N2—C21—H21B 109.5
N1—C8—C9 112.9 (4) H21A—C21—H21B 109.5
N1—C8—H8A 109.0 N2—C21—H21C 109.5
C9—C8—H8A 109.0 H21A—C21—H21C 109.5
N1—C8—H8B 109.0 H21B—C21—H21C 109.5
O2i—S1—O1—Ti1 120.47 (14) O1—S1—C1—C6 −146.5 (3)
N1—S1—O1—Ti1 −3.98 (15) O2i—S1—C1—C6 −22.7 (4)
C1—S1—O1—Ti1 −123.49 (15) N1—S1—C1—C6 102.6 (3)
N2—Ti1—O1—S1 −11.0 (5) Ti1—S1—C1—C6 155.6 (3)
N1—Ti1—O1—S1 3.24 (12) O1—S1—C1—C2 36.9 (3)
O2—Ti1—O1—S1 −82.93 (13) O2i—S1—C1—C2 160.7 (3)
Cl1—Ti1—O1—S1 99.37 (11) N1—S1—C1—C2 −74.0 (3)
Cl2—Ti1—O1—S1 −164.99 (11) Ti1—S1—C1—C2 −21.0 (4)
N2—Ti1—O2—S1i −130.9 (3) C6—C1—C2—C3 −0.1 (6)
N1—Ti1—O2—S1i −29.9 (3) S1—C1—C2—C3 176.5 (3)
O1—Ti1—O2—S1i 34.8 (3) C1—C2—C3—C4 0.0 (6)
Cl2—Ti1—O2—S1i 125.1 (3) C2—C3—C4—C5 −0.1 (6)
O1—S1—N1—C8 166.1 (3) C2—C3—C4—C7 179.5 (4)
O2i—S1—N1—C8 40.9 (3) C3—C4—C5—C6 0.4 (6)
C1—S1—N1—C8 −78.3 (3) C7—C4—C5—C6 −179.2 (4)
Ti1—S1—N1—C8 161.8 (4) C2—C1—C6—C5 0.4 (6)
O1—S1—N1—Ti1 4.39 (17) S1—C1—C6—C5 −176.2 (3)
O2i—S1—N1—Ti1 −120.90 (15) C4—C5—C6—C1 −0.5 (6)
C1—S1—N1—Ti1 119.93 (17) S1—N1—C8—C9 113.5 (4)
N2—Ti1—N1—C8 15.0 (4) Ti1—N1—C8—C9 −92.2 (4)
O2—Ti1—N1—C8 −73.4 (3) N1—C8—C9—C10 −74.8 (5)
O1—Ti1—N1—C8 −161.2 (4) C8—C9—C10—C11 178.0 (4)
Cl1—Ti1—N1—C8 110.5 (3) C9—C10—C11—C12 −5.7 (7)
Cl2—Ti1—N1—C8 −135.7 (3) C9—C10—C11—C15 176.2 (4)
N2—Ti1—N1—S1 173.17 (16) C15—C11—C12—C13 1.5 (5)
O2—Ti1—N1—S1 84.79 (15) C10—C11—C12—C13 −176.8 (4)
O1—Ti1—N1—S1 −3.08 (12) C11—C12—C13—C14 −0.8 (5)
Cl2—Ti1—N1—S1 22.5 (3) C12—C13—C14—C15 −0.4 (5)
N1—Ti1—N2—C20A −123.6 (5) C19—C14—C15—C16 −0.4 (7)
O2—Ti1—N2—C20A −39.1 (5) C13—C14—C15—C16 −178.4 (4)
O1—Ti1—N2—C20A −110.5 (7) C19—C14—C15—C11 179.2 (4)
Cl1—Ti1—N2—C20A 139.5 (5) C13—C14—C15—C11 1.3 (5)
Cl2—Ti1—N2—C20A 42.6 (5) C12—C11—C15—C14 −1.8 (5)
N1—Ti1—N2—C21 61.9 (4) C10—C11—C15—C14 176.6 (4)
O2—Ti1—N2—C21 146.4 (4) C12—C11—C15—C16 177.8 (5)
O1—Ti1—N2—C21 75.0 (7) C10—C11—C15—C16 −3.8 (8)
Cl1—Ti1—N2—C21 −35.0 (4) C14—C15—C16—C17 −0.5 (7)
Cl2—Ti1—N2—C21 −131.9 (4) C11—C15—C16—C17 180.0 (5)
N1—Ti1—N2—C20B −76.8 (16) C15—C16—C17—C18 −0.1 (8)
O2—Ti1—N2—C20B 7.7 (16) C16—C17—C18—C19 1.5 (8)
O1—Ti1—N2—C20B −63.7 (16) C17—C18—C19—C14 −2.4 (8)
Cl1—Ti1—N2—C20B −173.7 (16) C15—C14—C19—C18 1.9 (7)
Cl2—Ti1—N2—C20B 89.4 (16) C13—C14—C19—C18 179.3 (4)