organic papers
o1174
R. V. Krishnakumaret al. C30H22O6S DOI: 10.1107/S1600536802017634 Acta Cryst.(2002). E58, o1174±o1176 Acta Crystallographica Section EStructure Reports
Online ISSN 1600-5368
2,2
000-Sulfonylbis(2-benzoyl-3-phenyloxirane)
R. V. Krishnakumar,aM. Subha
Nandhini,bS. Renuga,b
S. Natarajan,b* S. Selvarajcand
S. Perumalc
aDepartment of Physics, Thiagarajar College,
Madurai 625 009, India,bDepartment of Physics, Madurai Kamaraj University, Madurai 625 021, India, andcDepartment of Chemistry, Madurai Kamaraj University, Madurai 625 021, India
Correspondence e-mail: s_natarajan50@yahoo.com
Key indicators
Single-crystal X-ray study
T= 293 K
Mean(C±C) = 0.011 AÊ
Rfactor = 0.084
wRfactor = 0.221
Data-to-parameter ratio = 14.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
The X-ray crystallographic study of the title compound, C30H22O6S, demonstrates the relative con®guration between
the aryl and aroyl groups (cis) on one hand, and that between the two oxirane rings (cis) on the other. The crystal packing is
characterized by a CÐH O interaction and C O short
contacts.
Comment
Organic synthesis of small ring heterocycles, such as oxiranes, assumes great importance in view of their reactivity towards a host of reagents and the synthetic potential associated with them. Details of chemical synthesis, NMR studies and preliminary crystallographic data for the title compound, (I), have already been reported (Renuga et al., 1999). Interest-ingly, (I) exhibits stereoisomerism and the dif®culty in ascer-taining the con®guration arises because one of the C atoms of each oxirane ring is a chiral center, as a result of which the molecule further exhibits diastereoisomerism with respect to the oxirane rings. Recently, the crystal structure of 2.20
-thio-bis[2-benzoyl-3-(4-chlorophenyl)oxirane] (Krishnakumar et
al., 2002) was elucidated in our laboratory.
Fig. 1 shows the atom-numbering scheme adopted for (I). This study demonstrates the relative con®guration between the aryl and aroyl groups (cis) on one hand and that between the two oxirane rings (cis) on the other. The con®guration of (I), which is symmetrical in solution in the absence of inter-molecular interactions, assumes an unsymmetrical form in the solid state. The absence of symmetry is probably necessitated by the optimum packing considerations, which bring different oxirane rings in proximity, unlike in solution.
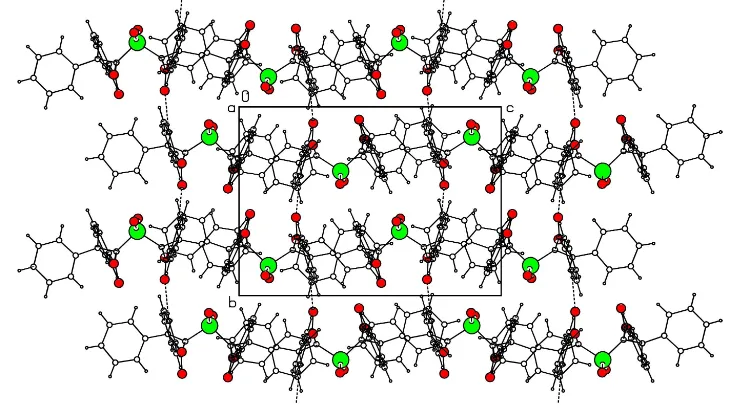
Fig. 2 shows the arrangement of molecules in layers parallel
to the (020) plane, viewed down the a axis. The packing
features of (I) are distinctly different from those of 2,20
-thio-bis[2-benzoyl-3-(4-chlorophenyl)oxirane], as its packing is determined, not only by the CÐH O, but also by the Cl Cl interactions. The molecules of (I) do not interact directly
among themselves, except for the presence of a CÐH O
hydrogen bond. In addition, the structure is stabilized by a
number of C O short contacts (see Table 1). Since the
molecules of (I) have aromatic rings, CÐH interactions
are expected to play a dominant role in stabilizing the crystal packing. An investigation (Maloneet al., 1997) on the nature
of CÐH interactions, using the Cambridge Structural
Database (Allen & Kennard, 1993) and theoretical calcula-tions, suggest six possible forms of interactions between an H atom and an aromatic ring. A recent database study (Umezawaet al., 1998) on the nature of CÐH interactions shows that these interactions also contribute signi®cantly to the optimum packing modes observed in the crystal structures
of organic compounds. The observed H distances in (I)
are 3.002 and 3.003 AÊ (below a cut off value of 3.05 AÊ), with
CÐH angles 158 and 133.
Experimental
Colourless single crystals of (I) were obtained as transparent needles from a saturated solution in methanol, by slow evaporation at room temperature.
Crystal data C30H22O6S
Mr= 510.54 Monoclinic,P21=c
a= 13.228 (3) AÊ
b= 11.754 (6) AÊ
c= 18.659 (5) AÊ
= 119.05 (2)
V= 2536.2 (16) AÊ3
Z= 4
Dx= 1.337 Mg mÿ3 CuKradiation Cell parameters from 25
re¯ections
= 14±27
= 1.50 mmÿ1
T= 293 (2) K Needle, colourless 0.400.340.24 mm Data collection
Enraf±Nonius CAD-4 diffractometer
!±2scans
Absorption correction: scan (Northet al., 1968)
Tmin= 0.576,Tmax= 0.698 5012 measured re¯ections 4795 independent re¯ections 3365 re¯ections withI> 2(I)
Rint= 0.019
max= 69.9
h= 0!16
k=ÿ14!0
l=ÿ22!19
2 standard re¯ections every 100 re¯ections intensity decay: <1%
Re®nement Re®nement onF2
R[F2> 2(F2)] = 0.084
wR(F2) = 0.221
S= 1.12 4795 re¯ections 335 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + 14.8815P] whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.43 e AÊÿ3
min=ÿ0.45 e AÊÿ3
Extinction correction:SHELXL97 Extinction coef®cient: 0.00103 (5)
Table 1
Contact distances (AÊ).
C4 O30i 3.25 (1) C5 O30i 3.24 (1) C7 O10ii 3.27 (1) C8 O2iii 3.46 (1) C12 C140iv 3.41 (1)
C14 O1iii 3.47 (1) C70 O1v 3.29 (1) C80 O1v 3.37 (1) C140 C12vi 3.41 (1)
Symmetry codes: (i) 1ÿx;1
2y;32ÿz; (ii)x;32ÿy;21z; (iii) 1ÿx;yÿ12;32ÿz; (iv)
1x;3
2ÿy;12z; (v)x;32ÿy;zÿ12; (vi)xÿ1;23ÿy;zÿ12.
Table 2
Hydrogen-bonding geometry (AÊ,).
DÐH A DÐH H A D A DÐH A
C15ÐH15 O2i 0.93 2.60 3.458 (8) 154 Symmetry code: (i) 1ÿx;yÿ1
2;32ÿz.
All H atoms were included in calculated positions with distances of 0.93 (forsp2CÐH) and 0.98 AÊ (forsp3CÐH). In the re®nement,
they were included as riding, withUisovalues equal to 1.2Ueqof the
carrier atom.
Data collection: CAD-4 Software (Enraf±Nonius, 1989); cell re®nement: CAD-4 Software; data reduction: CAD-4 Software; program(s) used to solve structure: SHELXS97 (Sheldrick, 1990); program(s) used to re®ne structure:SHELXL97 (Sheldrick, 1997); molecular graphics:PLATON(Spek, 1999); software used to prepare material for publication:SHELXL97.
SP thanks the Council of Scienti®c and Industrial Research (CSIR), India, for ®nancial assistance. The authors thank the UGC for the DRS programme.
References
Figure 2
Packing diagram, showing the arrangement of molecules in layers parallel to the (020) plane, viewed down theaaxis.
Figure 1
organic papers
o1176
R. V. Krishnakumaret al. C30H22O6S Acta Cryst.(2002). E58, o1174±o1176 Enraf±Nonius (1989).CAD-4Software. Version 5.0. Enraf±Nonius, Delft, TheNetherlands.
Krishnakumar, R. V., Subha Nandhini, M., Renuga, S., Natarajan, S., Selvaraj, S. & PErumal, S. (2002).Acta Cryst.E58, o504±o505.
Malone, J. F., Murray, C. M., Charlton, M. H., Docherty, R. & Lavery, A. J. (1997).J. Chem. Soc. Faraday Trans.93, 3429±3436.
North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968).Acta Cryst.A24, 351± 359.
Renuga, S., Selvaraj, S., Perumal, S. & Hewlins, M. J. E. (1999).Tetrahedron, pp. 9309±9316.
Sheldrick, G. M. (1990).Acta Cryst.A46, 467±473.
Sheldrick, G. M. (1997).SHELXL97. University of GoÈttingen, Germany. Spek, A. L. (1999). PLATON for Windows. Utrecht University, The
Netherlands.
Umezawa, Y., Tsuboyama, S., Honda, K., Uzawa, J. & Nishio, M. (1998).Bull.
supporting information
Acta Cryst. (2002). E58, o1174–o1176 [doi:10.1107/S1600536802017634]
2,2
′
-Sulfonylbis(2-benzoyl-3-phenyloxirane)
R. V. Krishnakumar, M. Subha Nandhini, S. Renuga, S. Natarajan, S. Selvaraj and S. Perumal
S1. Comment
Organic synthesis of small ring heterocycles, such as oxiranes, assume great importance in view of their reactivity
towards a host of reagents and the synthetic potential associated with them. Details of chemical synthesis, NMR studies
and preliminary crystallographic data for the title compound, (I), have already been reported (Renuga et al., 1999).
Interestingly, the (I) exhibits stereoisomerism and the difficulty in ascertaining the configuration arises because one of the
C atoms of each oxirane ring is a chiral center, as a result of which the molecule further exhibits diastereoisomerism with
respect to the oxirane rings. Recently, the crystal structure of 2.2′-thiobis[2-benzoyl-3-(4-chlorophenyl)oxirane]
(Krishnakumar et al., 2002) was elucidated in our laboratory.
Fig. 1 shows the atom-numbering scheme adopted for (I). This study demonstrates the relative configuration between
the aryl and aroyl groups (cis) on one hand and that between the two oxirane rings (cis) on the other. The configuration of
(I), which is symmetrical in solution in the absence of intermolecular interactions, assumes an unsymmetrical
configuration in the solid state. The absence of symmetry is probably necessitated by the optimum packing considerations
which bring different oxirane molecules in proximity, unlike in solution.
Fig. 2 shows the arrangement of molecules in layers parallel to the (020) plane, viewed down the a axis. The packing
features of (I) are distinctly different from those of the 2.2′-thiobis[2-benzoyl-3-(4-chlrophenyl)oxirane], as its packing is
determined, not only by the C—H···O, but also by the Cl···Cl interactions. The molecules of (I) do not directly interact
among themselves, except for the presence of a C—H···O hydrogen bond. In addition, the structure is stablized by a
number of C···O short contacts (see Table 1). Since the molecules of (I) have aromatic rings, C—H···π interactions are
expected to play a dominent role in stablizing the crystal packing. An investigation (Malone et al., 1997) on the nature of
C—H···π interactions using the Cambridge Structural Database (Allen & Kennard, 1993) and theoretical calculations
suggest six possible forms of interactions between an H atom and an aromatic ring. A recent database study (Umezawa et
al., 1998) on the nature of C—H···π interactions shows that these interactions also contribute sufficiently to the optimum
packing modes observed in the crystal structures of organic compounds. The observed H···π distances are 3.002 and
3.003 Å (below a cut off value of 3.05 Å), with C—H···π angles 158 and 133°.
S2. Experimental
Colourless single crystals of (I) were obtained as transparent needles from a saturated solution in methanol by slow
evaporation at room temperature.
S3. Refinement
All H atoms were included in calculated positions with distances of 0.93 (for sp2 C—H) and 0.98 Å (for sp3 C—H). In the
supporting information
[image:5.610.128.485.70.319.2]sup-2
Acta Cryst. (2002). E58, o1174–o1176Figure 1
View of (I), showing the atom-numbering scheme adopted, with the displacement ellipsoids drawn at the 50% probability
level.
Figure 2
Packing diagram, showing the arrangement of molecules in layers parallel to the (020) plane viewed down the a axis.
2,2′-Sulfonylbis(2-benzoyl-3-phenyloxirane)
Crystal data
C30H22O6S
Mr = 510.54
Monoclinic, P21/c Hall symbol: -P 2ybc
a = 13.228 (3) Å
b = 11.754 (6) Å
c = 18.659 (5) Å
[image:5.610.120.486.373.579.2]V = 2536.2 (16) Å3
Z = 4
F(000) = 1064
Dx = 1.337 Mg m−3
Cu Kα radiation, λ = 1.54180 Å Cell parameters from 25 reflections
θ = 14–27°
µ = 1.50 mm−1
T = 293 K Needle, colourless 0.40 × 0.34 × 0.24 mm
Data collection
Enraf-Nonius CAD-4 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω–2θ scans
Absorption correction: ψ scan (North et al., 1968)
Tmin = 0.576, Tmax = 0.698 5012 measured reflections
4795 independent reflections 3365 reflections with I > 2σ(I)
Rint = 0.019
θmax = 69.9°, θmin = 3.8°
h = 0→16
k = −14→0
l = −22→19
2 standard reflections every 100 reflections intensity decay: <1%
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.084
wR(F2) = 0.221
S = 1.12 4795 reflections 335 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + 14.8815P] where P = (Fo2 + 2Fc2)/3 (Δ/σ)max < 0.001
Δρmax = 0.43 e Å−3 Δρmin = −0.45 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 Extinction coefficient: 0.00103 (5)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
S1 0.34843 (13) 0.65913 (13) 0.61230 (9) 0.0489 (4)
O1 0.3329 (3) 0.7980 (4) 0.7185 (3) 0.0511 (10)
O2 0.5153 (4) 0.9143 (4) 0.7170 (3) 0.0610 (12)
O3 0.2596 (4) 0.5918 (4) 0.6147 (3) 0.0655 (13)
O1′ 0.3611 (4) 0.8300 (4) 0.5232 (3) 0.0614 (12)
O2′ 0.1911 (4) 0.9329 (4) 0.5424 (4) 0.0777 (15)
O3′ 0.4373 (4) 0.6069 (4) 0.6007 (3) 0.0643 (13)
supporting information
sup-4
Acta Cryst. (2002). E58, o1174–o1176C2 0.3932 (5) 0.7034 (5) 0.7715 (4) 0.0451 (13)
H2 0.3484 0.6330 0.7600 0.054*
C3 0.4710 (5) 0.7266 (5) 0.8579 (4) 0.0477 (14)
C4 0.5008 (6) 0.8379 (6) 0.8894 (4) 0.0564 (16)
H4 0.4728 0.9000 0.8541 0.068*
C5 0.5711 (6) 0.8550 (7) 0.9719 (4) 0.0673 (19)
H5 0.5904 0.9287 0.9921 0.081*
C6 0.6135 (6) 0.7645 (8) 1.0253 (4) 0.073 (2)
H6 0.6602 0.7766 1.0812 0.087*
C7 0.5853 (6) 0.6553 (7) 0.9943 (4) 0.070 (2)
H7 0.6144 0.5933 1.0295 0.083*
C8 0.5156 (6) 0.6380 (6) 0.9128 (4) 0.0581 (17)
H8 0.4972 0.5638 0.8934 0.070*
C9 0.5236 (5) 0.8109 (5) 0.7213 (3) 0.0420 (13)
C10 0.6313 (5) 0.7487 (5) 0.7431 (3) 0.0439 (13)
C11 0.7208 (5) 0.8060 (6) 0.7392 (4) 0.0564 (16)
H11 0.7119 0.8819 0.7233 0.068*
C12 0.8221 (6) 0.7499 (8) 0.7592 (5) 0.074 (2)
H12 0.8815 0.7880 0.7561 0.089*
C13 0.8369 (6) 0.6368 (8) 0.7839 (5) 0.075 (2)
H13 0.9061 0.5998 0.7978 0.090*
C14 0.7496 (6) 0.5800 (7) 0.7877 (4) 0.0668 (19)
H14 0.7594 0.5041 0.8038 0.080*
C15 0.6467 (5) 0.6346 (5) 0.7679 (4) 0.0496 (15)
H15 0.5878 0.5957 0.7710 0.060*
C1′ 0.2779 (5) 0.7659 (5) 0.5325 (3) 0.0437 (13)
C2′ 0.2976 (5) 0.7468 (6) 0.4613 (4) 0.0562 (16)
H2′ 0.3405 0.6775 0.4640 0.067*
C3′ 0.2155 (6) 0.7870 (7) 0.3774 (4) 0.0643 (19)
C4′ 0.1838 (7) 0.8986 (8) 0.3593 (5) 0.085 (3)
H4′ 0.2110 0.9532 0.4006 0.102*
C5′ 0.1088 (8) 0.9298 (11) 0.2767 (7) 0.109 (4)
H5′ 0.0866 1.0052 0.2630 0.131*
C6′ 0.0693 (9) 0.8468 (14) 0.2168 (7) 0.121 (5)
H6′ 0.0212 0.8671 0.1624 0.145*
C7′ 0.0994 (8) 0.7365 (13) 0.2360 (6) 0.110 (4)
H7′ 0.0693 0.6812 0.1953 0.132*
C8′ 0.1735 (7) 0.7067 (9) 0.3145 (5) 0.084 (3)
H8′ 0.1967 0.6313 0.3267 0.101*
C9′ 0.1763 (5) 0.8315 (5) 0.5273 (4) 0.0502 (15)
C10′ 0.0649 (5) 0.7747 (5) 0.5037 (4) 0.0497 (14)
C11′ −0.0185 (6) 0.8325 (7) 0.5134 (4) 0.0646 (19)
H11′ −0.0026 0.9050 0.5361 0.078*
C12′ −0.1249 (7) 0.7845 (8) 0.4900 (6) 0.086 (3)
H12′ −0.1803 0.8243 0.4970 0.103*
C13′ −0.1489 (6) 0.6763 (8) 0.4557 (5) 0.083 (3)
H13′ −0.2202 0.6426 0.4402 0.099*
H14′ −0.0833 0.5472 0.4221 0.083*
C15′ 0.0398 (5) 0.6670 (6) 0.4679 (4) 0.0567 (16)
H15′ 0.0942 0.6277 0.4594 0.068*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
S1 0.0419 (8) 0.0425 (8) 0.0499 (8) −0.0008 (6) 0.0126 (6) −0.0018 (7)
O1 0.046 (2) 0.055 (3) 0.056 (2) 0.0112 (19) 0.027 (2) 0.003 (2)
O2 0.066 (3) 0.038 (2) 0.083 (3) −0.002 (2) 0.040 (3) 0.002 (2)
O3 0.055 (3) 0.056 (3) 0.063 (3) −0.018 (2) 0.011 (2) 0.008 (2)
O1′ 0.048 (2) 0.068 (3) 0.067 (3) −0.008 (2) 0.027 (2) 0.003 (2)
O2′ 0.073 (3) 0.045 (3) 0.111 (4) 0.001 (2) 0.041 (3) −0.010 (3)
O3′ 0.059 (3) 0.059 (3) 0.063 (3) 0.020 (2) 0.020 (2) −0.008 (2)
C1 0.034 (3) 0.039 (3) 0.050 (3) −0.001 (2) 0.020 (2) −0.001 (3)
C2 0.040 (3) 0.043 (3) 0.055 (3) −0.001 (2) 0.025 (3) 0.002 (3)
C3 0.046 (3) 0.058 (4) 0.050 (3) −0.001 (3) 0.032 (3) 0.005 (3)
C4 0.067 (4) 0.053 (4) 0.057 (4) −0.002 (3) 0.036 (3) 0.006 (3)
C5 0.078 (5) 0.077 (5) 0.052 (4) −0.014 (4) 0.036 (4) −0.009 (4)
C6 0.068 (5) 0.101 (6) 0.047 (4) −0.004 (4) 0.026 (4) 0.010 (4)
C7 0.074 (5) 0.076 (5) 0.059 (4) 0.014 (4) 0.032 (4) 0.016 (4)
C8 0.065 (4) 0.055 (4) 0.061 (4) 0.008 (3) 0.036 (3) 0.004 (3)
C9 0.044 (3) 0.043 (3) 0.040 (3) −0.004 (3) 0.021 (3) 0.000 (2)
C10 0.035 (3) 0.053 (3) 0.042 (3) −0.006 (3) 0.017 (2) 0.003 (3)
C11 0.054 (4) 0.061 (4) 0.061 (4) −0.012 (3) 0.034 (3) −0.001 (3)
C12 0.051 (4) 0.092 (6) 0.091 (6) −0.012 (4) 0.043 (4) −0.005 (5)
C13 0.045 (4) 0.099 (6) 0.080 (5) 0.015 (4) 0.029 (4) 0.001 (5)
C14 0.055 (4) 0.069 (5) 0.073 (5) 0.014 (4) 0.029 (4) 0.013 (4)
C15 0.044 (3) 0.048 (3) 0.053 (4) 0.003 (3) 0.021 (3) 0.007 (3)
C1′ 0.037 (3) 0.045 (3) 0.046 (3) −0.008 (2) 0.017 (2) 0.000 (3)
C2′ 0.045 (3) 0.071 (4) 0.052 (4) −0.004 (3) 0.023 (3) −0.004 (3)
C3′ 0.050 (4) 0.093 (6) 0.059 (4) −0.008 (4) 0.035 (3) 0.011 (4)
C4′ 0.057 (4) 0.099 (7) 0.088 (6) −0.015 (4) 0.027 (4) 0.024 (5)
C5′ 0.073 (6) 0.131 (9) 0.115 (8) −0.007 (6) 0.039 (6) 0.060 (8)
C6′ 0.075 (7) 0.196 (15) 0.089 (8) −0.006 (8) 0.038 (6) 0.054 (9)
C7′ 0.082 (6) 0.187 (13) 0.065 (6) −0.008 (8) 0.038 (5) −0.003 (7)
C8′ 0.068 (5) 0.124 (8) 0.061 (5) 0.006 (5) 0.032 (4) −0.006 (5)
C9′ 0.053 (4) 0.040 (3) 0.056 (4) 0.002 (3) 0.026 (3) −0.005 (3)
C10′ 0.041 (3) 0.053 (4) 0.051 (3) 0.006 (3) 0.019 (3) 0.009 (3)
C11′ 0.058 (4) 0.066 (5) 0.072 (5) 0.021 (4) 0.033 (4) 0.021 (4)
C12′ 0.058 (5) 0.103 (7) 0.112 (7) 0.024 (5) 0.052 (5) 0.036 (6)
C13′ 0.042 (4) 0.095 (7) 0.101 (6) −0.005 (4) 0.026 (4) 0.034 (5)
C14′ 0.047 (4) 0.069 (5) 0.073 (5) −0.007 (3) 0.014 (3) 0.014 (4)
supporting information
sup-6
Acta Cryst. (2002). E58, o1174–o1176Geometric parameters (Å, º)
S1—O3′ 1.432 (4) C13—C14 1.366 (10)
S1—O3 1.435 (4) C13—H13 0.9300
S1—C1 1.801 (6) C14—C15 1.383 (8)
S1—C1′ 1.820 (6) C14—H14 0.9300
O1—C1 1.414 (6) C15—H15 0.9300
O1—C2 1.443 (7) C1′—C2′ 1.490 (8)
O2—C9 1.220 (7) C1′—C9′ 1.511 (8)
O1′—C1′ 1.413 (7) C2′—C3′ 1.484 (9)
O1′—C2′ 1.433 (8) C2′—H2′ 0.9800
O2′—C9′ 1.218 (7) C3′—C4′ 1.369 (11)
C1—C2 1.490 (8) C3′—C8′ 1.394 (11)
C1—C9 1.523 (7) C4′—C5′ 1.417 (12)
C2—C3 1.457 (8) C4′—H4′ 0.9300
C2—H2 0.9800 C5′—C6′ 1.381 (16)
C3—C8 1.376 (9) C5′—H5′ 0.9300
C3—C4 1.410 (9) C6′—C7′ 1.352 (16)
C4—C5 1.374 (9) C6′—H6′ 0.9300
C4—H4 0.9300 C7′—C8′ 1.355 (12)
C5—C6 1.376 (10) C7′—H7′ 0.9300
C5—H5 0.9300 C8′—H8′ 0.9300
C6—C7 1.383 (11) C9′—C10′ 1.476 (8)
C6—H6 0.9300 C10′—C11′ 1.381 (8)
C7—C8 1.357 (9) C10′—C15′ 1.394 (9)
C7—H7 0.9300 C11′—C12′ 1.374 (10)
C8—H8 0.9300 C11′—H11′ 0.9300
C9—C10 1.472 (8) C12′—C13′ 1.390 (12)
C10—C11 1.393 (8) C12′—H12′ 0.9300
C10—C15 1.401 (8) C13′—C14′ 1.368 (11)
C11—C12 1.373 (10) C13′—H13′ 0.9300
C11—H11 0.9300 C14′—C15′ 1.377 (9)
C12—C13 1.389 (11) C14′—H14′ 0.9300
C12—H12 0.9300 C15′—H15′ 0.9300
C4···O3′i 3.25 (1) C14···O1iii 3.47 (1)
C5···O3′i 3.24 (1) C7′···O1v 3.29 (1)
C7···O1′ii 3.27 (1) C8′···O1v 3.37 (1)
C8···O2iii 3.46 (1) C14′···C12vi 3.41 (1)
C12···C14′iv 3.41 (1)
O3′—S1—O3 120.8 (3) C15—C14—H14 119.8
O3′—S1—C1 108.1 (3) C14—C15—C10 119.9 (6)
O3—S1—C1 107.9 (3) C14—C15—H15 120.0
O3′—S1—C1′ 107.7 (3) C10—C15—H15 120.0
O3—S1—C1′ 107.7 (3) O1′—C1′—C2′ 59.1 (4)
C1—S1—C1′ 103.3 (3) O1′—C1′—C9′ 116.2 (5)
C1′—O1′—C2′ 63.1 (4) O1′—C1′—S1 110.3 (4)
O1—C1—C2 59.5 (3) C2′—C1′—S1 112.5 (4)
O1—C1—C9 116.8 (5) C9′—C1′—S1 119.5 (4)
C2—C1—C9 123.0 (5) O1′—C2′—C3′ 118.3 (6)
O1—C1—S1 110.5 (4) O1′—C2′—C1′ 57.8 (4)
C2—C1—S1 115.0 (4) C3′—C2′—C1′ 122.5 (6)
C9—C1—S1 117.7 (4) O1′—C2′—H2′ 115.3
O1—C2—C3 118.2 (5) C3′—C2′—H2′ 115.3
O1—C2—C1 57.6 (3) C1′—C2′—H2′ 115.3
C3—C2—C1 122.9 (5) C4′—C3′—C8′ 119.5 (8)
O1—C2—H2 115.3 C4′—C3′—C2′ 123.0 (8)
C3—C2—H2 115.3 C8′—C3′—C2′ 117.4 (8)
C1—C2—H2 115.3 C3′—C4′—C5′ 119.0 (10)
C8—C3—C4 117.4 (6) C3′—C4′—H4′ 120.5
C8—C3—C2 120.0 (6) C5′—C4′—H4′ 120.5
C4—C3—C2 122.6 (6) C6′—C5′—C4′ 119.2 (11)
C5—C4—C3 120.2 (6) C6′—C5′—H5′ 120.4
C5—C4—H4 119.9 C4′—C5′—H5′ 120.4
C3—C4—H4 119.9 C7′—C6′—C5′ 121.0 (11)
C4—C5—C6 121.0 (7) C7′—C6′—H6′ 119.5
C4—C5—H5 119.5 C5′—C6′—H6′ 119.5
C6—C5—H5 119.5 C6′—C7′—C8′ 120.0 (12)
C5—C6—C7 118.9 (7) C6′—C7′—H7′ 120.0
C5—C6—H6 120.6 C8′—C7′—H7′ 120.0
C7—C6—H6 120.6 C7′—C8′—C3′ 121.3 (10)
C8—C7—C6 120.4 (7) C7′—C8′—H8′ 119.4
C8—C7—H7 119.8 C3′—C8′—H8′ 119.4
C6—C7—H7 119.8 O2′—C9′—C10′ 122.5 (6)
C7—C8—C3 122.2 (7) O2′—C9′—C1′ 116.5 (6)
C7—C8—H8 118.9 C10′—C9′—C1′ 121.0 (5)
C3—C8—H8 118.9 C11′—C10′—C15′ 119.3 (6)
O2—C9—C10 123.9 (5) C11′—C10′—C9′ 118.9 (6)
O2—C9—C1 118.0 (5) C15′—C10′—C9′ 121.7 (6)
C10—C9—C1 118.1 (5) C12′—C11′—C10′ 121.1 (8)
C11—C10—C15 119.3 (6) C12′—C11′—H11′ 119.4
C11—C10—C9 118.6 (6) C10′—C11′—H11′ 119.4
C15—C10—C9 122.1 (5) C11′—C12′—C13′ 119.5 (8)
C12—C11—C10 119.7 (7) C11′—C12′—H12′ 120.3
C12—C11—H11 120.2 C13′—C12′—H12′ 120.3
C10—C11—H11 120.2 C14′—C13′—C12′ 119.4 (7)
C11—C12—C13 120.8 (7) C14′—C13′—H13′ 120.3
C11—C12—H12 119.6 C12′—C13′—H13′ 120.3
C13—C12—H12 119.6 C13′—C14′—C15′ 121.7 (8)
C14—C13—C12 119.9 (7) C13′—C14′—H14′ 119.2
C14—C13—H13 120.0 C15′—C14′—H14′ 119.2
C12—C13—H13 120.0 C14′—C15′—C10′ 119.0 (7)
C13—C14—C15 120.4 (7) C14′—C15′—H15′ 120.5
supporting information
sup-8
Acta Cryst. (2002). E58, o1174–o1176C2—O1—C1—C9 −114.3 (6) C2′—O1′—C1′—C9′ −115.2 (6)
C2—O1—C1—S1 107.6 (4) C2′—O1′—C1′—S1 104.7 (5)
O3′—S1—C1—O1 178.7 (4) O3′—S1—C1′—O1′ −44.1 (5)
O3—S1—C1—O1 −49.2 (4) O3—S1—C1′—O1′ −175.9 (4)
C1′—S1—C1—O1 64.7 (4) C1—S1—C1′—O1′ 70.1 (4)
O3′—S1—C1—C2 −116.4 (4) O3′—S1—C1′—C2′ 19.9 (5)
O3—S1—C1—C2 15.8 (5) O3—S1—C1′—C2′ −111.9 (4)
C1′—S1—C1—C2 129.6 (4) C1—S1—C1′—C2′ 134.1 (4)
O3′—S1—C1—C9 41.0 (5) O3′—S1—C1′—C9′ 177.3 (4)
O3—S1—C1—C9 173.1 (4) O3—S1—C1′—C9′ 45.5 (5)
C1′—S1—C1—C9 −73.0 (5) C1—S1—C1′—C9′ −68.4 (5)
C1—O1—C2—C3 113.0 (6) C1′—O1′—C2′—C3′ 112.4 (6)
C9—C1—C2—O1 104.0 (6) C9′—C1′—C2′—O1′ 102.6 (6)
S1—C1—C2—O1 −100.0 (4) S1—C1′—C2′—O1′ −101.0 (4)
O1—C1—C2—C3 −104.8 (6) O1′—C1′—C2′—C3′ −105.3 (7)
C9—C1—C2—C3 −0.8 (9) C9′—C1′—C2′—C3′ −2.7 (10)
S1—C1—C2—C3 155.2 (5) S1—C1′—C2′—C3′ 153.7 (6)
O1—C2—C3—C8 169.5 (5) O1′—C2′—C3′—C4′ −10.7 (10)
C1—C2—C3—C8 −122.6 (6) C1′—C2′—C3′—C4′ 57.2 (10)
O1—C2—C3—C4 −8.4 (8) O1′—C2′—C3′—C8′ 166.5 (6)
C1—C2—C3—C4 59.4 (8) C1′—C2′—C3′—C8′ −125.5 (7)
C8—C3—C4—C5 −0.4 (9) C8′—C3′—C4′—C5′ 0.0 (11)
C2—C3—C4—C5 177.6 (6) C2′—C3′—C4′—C5′ 177.2 (7)
C3—C4—C5—C6 −0.1 (11) C3′—C4′—C5′—C6′ 0.3 (13)
C4—C5—C6—C7 0.8 (11) C4′—C5′—C6′—C7′ 1.1 (16)
C5—C6—C7—C8 −1.1 (12) C5′—C6′—C7′—C8′ −2.9 (17)
C6—C7—C8—C3 0.6 (11) C6′—C7′—C8′—C3′ 3.2 (14)
C4—C3—C8—C7 0.2 (10) C4′—C3′—C8′—C7′ −1.8 (12)
C2—C3—C8—C7 −177.8 (6) C2′—C3′—C8′—C7′ −179.1 (7)
O1—C1—C9—O2 −24.7 (8) O1′—C1′—C9′—O2′ −23.3 (8)
C2—C1—C9—O2 −94.3 (7) C2′—C1′—C9′—O2′ −92.2 (8)
S1—C1—C9—O2 110.3 (6) S1—C1′—C9′—O2′ 113.0 (6)
O1—C1—C9—C10 154.0 (5) O1′—C1′—C9′—C10′ 155.8 (5)
C2—C1—C9—C10 84.5 (7) C2′—C1′—C9′—C10′ 86.9 (8)
S1—C1—C9—C10 −70.9 (6) S1—C1′—C9′—C10′ −67.9 (7)
O2—C9—C10—C11 −16.3 (9) O2′—C9′—C10′—C11′ −12.2 (10)
C1—C9—C10—C11 165.1 (5) C1′—C9′—C10′—C11′ 168.8 (6)
O2—C9—C10—C15 163.3 (6) O2′—C9′—C10′—C15′ 164.3 (7)
C1—C9—C10—C15 −15.4 (8) C1′—C9′—C10′—C15′ −14.7 (9)
C15—C10—C11—C12 0.5 (10) C15′—C10′—C11′—C12′ 1.4 (10)
C9—C10—C11—C12 −180.0 (6) C9′—C10′—C11′—C12′ 178.0 (7)
C10—C11—C12—C13 −0.6 (11) C10′—C11′—C12′—C13′ −0.2 (12)
C11—C12—C13—C14 0.6 (13) C11′—C12′—C13′—C14′ −0.6 (12)
C12—C13—C14—C15 −0.6 (12) C12′—C13′—C14′—C15′ 0.2 (12)
C11—C10—C15—C14 −0.4 (9) C11′—C10′—C15′—C14′ −1.8 (10)
C9—C10—C15—C14 −179.9 (6) C9′—C10′—C15′—C14′ −178.3 (6)
Symmetry codes: (i) −x+1, y+1/2, −z+3/2; (ii) x, −y+3/2, z+1/2; (iii) −x+1, y−1/2, −z+3/2; (iv) x+1, −y+3/2, z+1/2; (v) x, −y+3/2, z−1/2; (vi) x−1, −y+3/2,
z−1/2.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C15—H15···O2iii 0.93 2.60 3.458 (8) 154