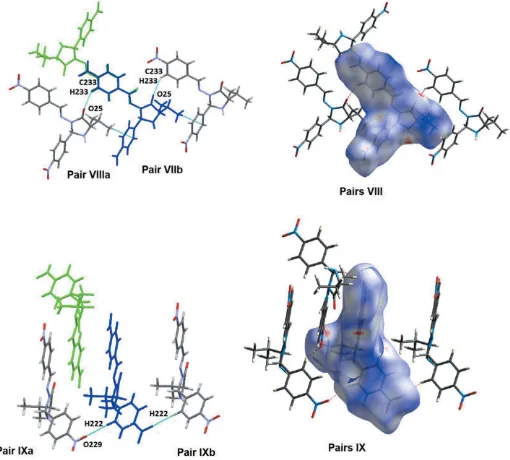
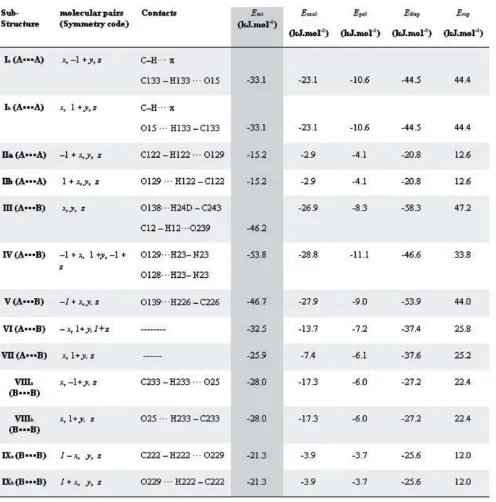
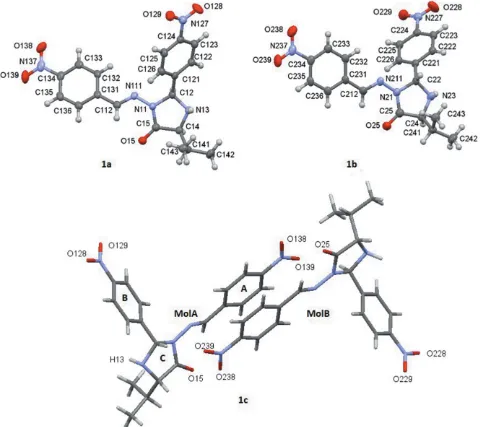
Crystal structure, Hirshfeld surface analysis and PIXEL calculations of a 1:1 epimeric mixture of 3 [(4 nitrobenzylidene)amino] 2(R,S) (4 nitrophenyl) 5(S) (propan 2 yl)imidazolidin 4 one
Full text
Figure

Related documents
The hydrogen atoms of the (prop-2-en-1-yl)sulfanyl group are involved in C—H N bonding with the tetrazole ring of an adjacent molecule; these bonds link independent molecules
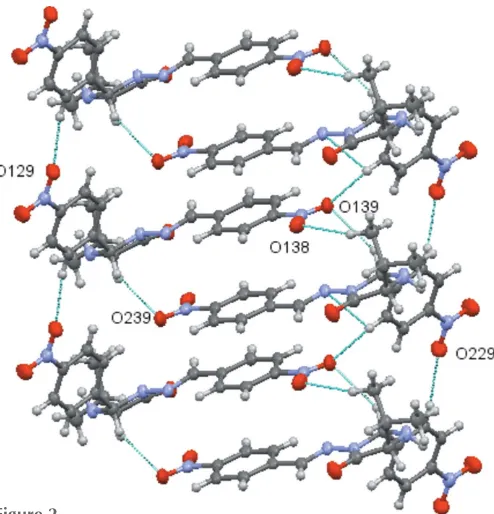
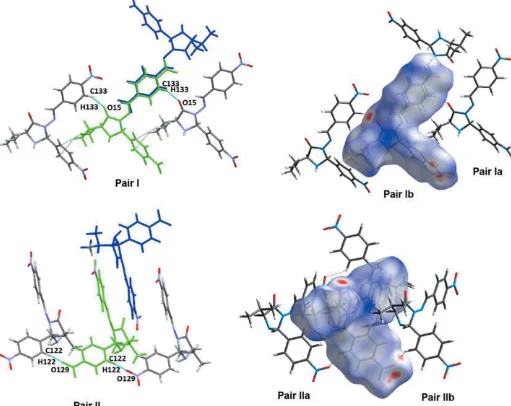
In the crystal, the molecules form sheets parallel to the b axis, which are supported by non-classical hydrogen-bonding interactions between C—H functionalities and the O atom
In the crystal, weak intermolecular C—H Cl hydrogen- bonding interactions between the C atoms of the benzene ring and the Cl atoms connect the complex molecules into a
The phenolic proton forms an intramolecular O—H O hydrogen bond with the adjacent carbonyl O atom.. In the crystal, molecules are linked by C—H O hydrogen bonds, forming
In the crystal structure, intra- and intermolecular C—H···S hydrogen bonds involving the disordered thienyl ring (Table 1) are
In each, the seven- membered diazepine ring adopts a boat conformation with the hydroxy-substituted C atom at the prow and fused-ring C atoms at the stern.. In the crystal,
In the crystal structure, pairs of molecules are connected into dimers via intermolecular N—H O hydrogen bonding between amine atom N3 and carbonyl atom O1.. These dimers are
Hindrance from the 5-phenyl ring to neighbouring mol- ecules and the conformation of the thiophenyl ring not only prevent any hydrogen-bonding associations to the S atom, but the