Copyright 0 1990 by the Genetics Society of America
Genetic Evidence That the
ovo
Locus Is Involved in Drosophila
Germ Line Sex Determination
Brian Oliver,’ Daniel Pauli and Anthony
P.
Mahowald
Department of Genetics, School of Medicine, Case Western Reserve University, Cleveland, Ohio 44106 Manuscript received December 21, 1989
Accepted for publication March 26, 1990
ABSTRACT
Zygotically contributed ovo gene product is required for the survival of female germ cells in
Drosophila melanogaster. Trans-allelic combinations of weak and dominant ovo mutations (ovoD) result in viable germ cells that appear to be partially transformed from female to male sexual identity. The O V O ” ~ mutation is partially suppressed by many Sex-lethal alleles that affect the soma, while those that
affect only the germ line fail to interact with ovoD2. One of two loss-of-function ovo alleles is suppressed
by a loss-of-function Sex-lethal allele. Because ovo mutations are germ line dependent, it is likely that ovo is suppressed by way of communication between the somatic and germ lines. A loss-of-function
allele of ovo is epistatic to germ line dependent mutations in Sex-lethal. The germ line dependent sex
determination mutation, sans Jille, and ovoD mutations show a dominant synergistic interaction resulting in partial transformation of germ line sexual identity. The ovo locus appears to be involved
in germ line sex determination and is linked in some manner to sex determination in the soma.
F
ERTILITY depends on the proper sexual devel- opment of somatic tissues and the germ line. A great deal has been learned about the genetic control of somatic sex determination in Drosophila melano- gaster (see reviews by BAKER and BELOTE 1983; CLINE1985, 1988a; NOTHIGER and STEINMANN-ZWICKY
1985, 1987). Very little is known about the genetic control of germ line sex determination.
Sex determination in the soma can be briefly sum- marized as follows. T h e primary determinant of sex- ual identity is the relative abundance of X-linked genes or sites (numerator elements) as compared to auto- some sets (denominator elements). This relationship is commonly denoted as the X:A ratio. Flies with X:A ratios of one or greater develop as females while flies with X:A ratios of one-half or less develop as males (MORGAN and BRIDGES 19 19; BRIDGES 192 1, 1925a, b; HINTON 1955; HANNAH-ALAVA and STERN 1957; STERN 1966; SANTAMARIA 1983; CLINE 1976, 1979, 1983a, 1986, 1988b; FUYAMA 1987; TORRES and SANCHEZ 1989). Under wild-type diploid conditions the X:A ratio for female development is one (e.g., 2X:2A). In 2X:2A zygotes a number of gene products stored in the egg and the X:A ratio activate and/or maintain expression of Sex-lethal ( S x l ) (CLINE 1978,
1983a, 1984, 1986, 1988b; CHRONMILLER and CLINE 1987; OLIVER, PERRIMON and MAHOWALD 1988; STEINMANN-ZWICKY 1988). T h e Sxl gene product is required for the regulation of the downstream sex determination genes transformer ( t r a ) and doublesex
Stanford, California 94305.
Genetics 125: 535-550 (July, 1990)
’
Present address: Department of Biological Sciences, Stanford University,(dsx) and for the repression of X-linked dosage com- pensation (LUCCHESI and SKRIPSKY 198 1 ; SANCHEZ and NOTHIGER 1982; SKRIPSKY and LUCCHESI 1982; UENOYAMA et al. 1982; CLINE 1984; GERGEN 1987; LUCCHESI and MANNING 1987; NAGOSHI et al. 1988). T h e tra gene product, and the transformer-:! ( t r a - 2 ) gene product, control the function of the dsx gene (BAKER and RIDGE 1980; BELOTE and BAKER 1982, 1983; NAGOSHI et al. 1988). T h e female specific dsx gene product, and the intersex ( i x ) gene product, re- press the expression of male specific terminal differ- entiation genes, resulting in female development (BAKER and RIDGE 1980; BELOTE and BAKER 1983). In chromosomal males S x l , tra, tra-2 and ix are not required for somatic sexual identity. T h e absence of these functions results in the expression of male spe- cific dsx gene product, which represses the expression of female specific terminal differentiation genes. Mo- lecular data indicate that the gene activities of somatic sex determination genes are regulated, at least in part, by pre-mRNA splicing (reviewed by BAKER 1989; HODGKIN 1989).
536 B. Oliver, D. Pauli and A. P. Mahowald
(2X:2A) homozygous for mutations in tra, tra-2, dsx, i x or another maternal regulator of S x l , daughterless ( d a ) , can form fully functional eggs in wild-type 2X:2A female hosts, indicating that the corresponding wild- type gene products are not required in germ cells for the production of eggs (MARSH and WIESCHAUS 1978; SCHUPBACH 1982; CRONMILLER and CLINE 1987).
It appears that cells of the germ line and soma must not only know their own sex, but must also commu- nicate with each other to ensure the proper prolifer- ation and differentiation of the germ cells. Some of the evidence for this idea comes from the analysis of the germ line phenotypes of sexually transformed flies. Because tra or tra-2 2X:2A germ cells can form eggs in a wild-type female, one might expect to see germ line structures resembling eggs in 2X:2A flies homozygous for either tra or tra-1. This is not the case. These 2X:2A “males” contain few or no germ cells and the germ cells that are seen have alternatively been reported to be arrested primary spermatocytes, or a mix between spermatocytes and oocytes [BROWN and KING 1961; R. NOTHIGER, T. WEBER and M. JONGLEZ (cited in NOTHIGER and STEINMANN-ZWICKY
1985); LEUTHOLD 1986; NOTHIGER et al. 19891. T h e 2X:2A “males” resulting from homozygosity for some somatic line specific alleles of Sxl also exhibit male germ line morphology (CLINE 1984). In none of these 2X:2A “males” are advanced stages of spermatogene- sis seen [irrespective of the presence of a Y chromo- some which bears male fertility genes (see LINDSLEY and TOKUYASU 1980)], and S x l , tra or tra-2 2X:2A “males” fail to express at least some male germ line dependent transcripts (SCHAFER 1986; DIBENEDETTO et al. 1987; W. MATTOX, personal communication). These results suggest that S x l , tra, and tra-2 can have a somatic line dependent influence on the final num- ber of 2X:2A germ cells in the adult gonad and the sexual identity of those cells. T h e 2X:2A intersexes resulting from homozygosity for dsx or ix can form early egg chambers at low frequency (SCHUPBACH 1982), suggesting that female sexual identity is main- tained in at least some of the 2X:2A germ cells of dsx or i x intersexes. This may be a function of mixed somatic signals, since 2X:2A flies trans-allelic for dom- inant dsx mutations and loss of function dsx mutations develop as phenotypic males with germ line pheno- types similar to 2X:2A tra- or tra-2- flies (NOTHIGER et al. 1987; B. OLIVER and R. NAGOSHI, unpublished data). All of these results suggest that 2X:2A germ cells can partially switch sexual identity (female to male) in a male soma.
Further evidence that the somatic sexual environ- ment is important for germ line development comes from mosaic studies. Somatic sex determination is cell autonomous. When a 2X:2A embryo loses an X chro- mosome during early nuclear divisions large patches
of 1X:2A cells elaborate male structures, while the 2X:2A cells elaborate female structures (BRIDGES 1921). Because the somatic part of the gonad is formed in a different location than the germ cells, the germ cells and the somatic cells of a 1X:2A/2X:2A mosaic are often of different chromosomal sex (GEHR-
ING, WIESCHAUS and HOLLIGER 1976). T h e gonads of these 1X:2A/2X:2A mosaic intersexes are often rudi- mentary. Further, the gonads that do contain differ- entiating gametes are always associated with somatic gonads of matching sexual identity (DOBZHANSKY
193 1; BROWN and KING 1962; GEHRING, WIESCHAUS and HOLLIGER 1976; SZABAD and FAJSZI 1982; B. OLIVER, unpublished data). Some atrophic ovaries contain cells of apparent male sexual identity (B. OLIVER, unpublished data), but other atrophic gonads contain no germ cells (SZABAD and FAJSZI 1982; B. OLIVER, unpublished data). It was suggested that the atrophic nature of many of the gonads of mosaics is due to a germ line/somatic line interaction resulting in the death or retarded development of germ cells that become enclosed in a gonad of the opposite sex (GEHRING, WIESCHAUS and HOLLIGER 1976).
Transplantation data support the idea that the sex- ual identity of the soma influences the sexual identity of the germ line. 1X:2A germ cells transplanted into female hosts do not form functional eggs and can not even be found in the adult ovary (VAN DEUSEN 1976; SCHUPBACH 1985). It is a reasonable suggestion that
1X:2A germ cells die, or have such a growth disad- vantage in a 2X:2A soma that they are effectively eliminated. In other experiments, using hosts that have no germ cells of their own, it has been shown that both germ cell autonomous and somatic cues are important for germ cell sexual identity (STEINMANN- ZWICKY, SCHMID and NOTHIGER 1989). 1X:2A germ cells transplanted into 2X:2A hosts with no endoge- nous germ cells appear to be arrested as early sper- matocytes suggesting that the 1X:2A germ cell sexual identity is not switched in a female soma. In contrast, 2X:2A germ cells are switched to a malelike identity in a 1X:2A host. This latter observation is consistent with the somatic line dependent transformation of the germ line observed in 2X:2A flies transformed to male by S x l , tra, tra-2 or dsx mutations.
There should be genes responsible for reception of
The ovo Locus 537
NOTHIGER 1989). Another germ line sex determina- tion gene is probably snf, which also results in the production of cells resembling early spermatocytes in homozygous 2X:2A females or snf, Sxl trans-hetero- zygotes (OLIVER, PERRIMON and MAHOWALD 1988). This study focuses on a third gene that seems to be involved in germ line sex determination-the ovo locus. Mutations in a gene involved in germ line sex de- termination might be expected to: (1) result in germ line sexual transformation; (2) interact with mutations involved in providing somatic sexual cues; and (3) interact with mutations in other genes active in germ line sex determination. We show that: (1) ovo germ cells that survive in 2X:2A flies resemble spermato- cytes; (2) ovo is suppressed by somatic line dependent sex determination mutations; and (3) ovo acts syner- gistically with the germ line dependent snfmutation.
MATERIALS AND METHODS
Flies were grown on standard Drosophila media at 25", unless otherwise noted. See LINDSLEY and GRELL (1968) for visible markers. Mutant alleles of sex determination loci are described in the text, table footnotes and figure legends.
Females used for ovary dissections were transferred to fresh vials following eclosion and allowed to mate and feed for 6-7 days. The ovaries of aged females were dissected in phosphate saline and viewed immediately under a com- pound microscope. The egg chambers of ouoD2 females usually arrest at stage 6. Egg chambers reaching at least stage 10 were counted as vitellogenic eggs. See MAHOWALD and KAMBYSELLIS (1 980) for descriptions of Drosophila mel-
anogaster oogenesis stages. The nonparametric Smirnov test was used to analyze egg/ovary distributions (CONNOVER
1980). Embryos for scanning electron microscopy were col- lected and processed as previously described (TURNER and
MAHOWALD 1976).
RESULTS
Some combinations of ovo alleles appear to result in germ line sex transformation: Known ovo muta- tions result in three germ line phenotypes. T h e prop- erties of the alleles extensively used in this study are summarized in Table 1. T h e most common phenotype is the complete absence of germ cells in the 2X:2A adult (BUSSON et al. 1983; OLIVER, PERRIMON and MAHOWALD 1987; M~VEL-NINIO, MARIOL and GANS 1989; this study). For at least two loss-of-function alleles, O V O ~ ' ~ ~ ' and Eorenge-likeofc""s ( I d ' ) , adult steril-
ity is the result of germ cell death during early em- bryogenesis. T h e second phenotype, produced by the dominant ovoD', ovoD2, and ovoD3, and the partial loss- of-function alleles, ovorM', ovorM2, and O V O ~ Iis ar- ~ ~ ~ ~ ,
rested or defective oogenesis (MOHLER 1977; KOMI- TOPOULOU et al. 1983; BUSSON et al. 1983; OLIVER,
PERRIMON and MAHOWALD 1987; M~VEL-NINIO,
MARIOL and GANS 1989; this study). This phenotype results in the failure to differentiate an oocyte (result- ing in egg chambers with greater than 15 nurse cells),
arrested oogenesis (prior to vitellogenesis), and/or chorion defects. T h e third phenotype seen in trans- allelic combinations of dominant and hypomorphic alleles is the production of small "ovarian tumors" (OLIVER, PERRIMON and MAHOWALD 1987; J. D. MOHLER, personal communication; this study).
T h e term "ovarian tumor" may be a misnomer as females trans-heterozygous for ovoD2 or ovoD3 and either ovorM', ovorM2 or have germ cells closely resembling early spermatocytes (Figure 1, c and d).
These cells are indistinguishable from the germ line dependent ovarian tumor cells resulting from muta- tions in female sterile alleles of Sxl, SxZf*' or S x F (Figure 1, A and B). We believe that the ovo sex transformation phenotype is due to extreme hypo- morphy. T h e dominant alleles are antimorphic (Bus- SON et al. 1983) and have phenotypes (in ovoD/
+
females) similar to the partial-loss-of-function alleles (in ovor/ovor or O V O ~ / O V O - females, where ovor is ovorM',ovorM2 or O V O D " ' ~ ~ ) . In ovoD/ovor females the domi- nant gene product probably inactivates much of the already low level of ovo gene activity from mor. Like S x l , we suggest that ovo mutations can transform 2X:2A germ cells towards a male sexual identity. T h e difference between Sxl and ovo in terms of germ line sex transformation is that the ovo sex transformation is only detected when there is enough ovo activity to allow for germ cell survival but insufficient ovo activity for female sexual identity.
ovo is upstream or independent of Sxl: It has been suggested that ovo acts downstream of Sxl in the germ line (STEINMANN-ZWICKY 1988). If Sxl is an obligatory positive regulator of all ovo functions, Sxl mutations would be expected to result in an ovo-like germ line phenotype. T h e female ovo- germ line phenotype is death. All known alleles of Sxl are viable in germ line clones, and result in either egg production or ovarian tumors (SCHUPBACH 1985; SALZ, CLINE and SCHEDL
1987; STEINMANN-ZWICKY, SCHMID and NOTHIGER
1989). Because loss of Sxl function results in organis- mal lethality, it is not known if Sxl is required for early germ cell viability in non-mosaics. Crosses generating females homozygous for the loss-of-function alleles, Sxl@', or O V O ~ ' ~ ~ ' , or lzl' were made, and the germ
cells of the early embryos (between the end of the cellular blastoderm stage and prior to the invagination of the posterior midgut) were counted (Figure 2). T h e crosses generated 25% homozygous mutant female embryos. No individuals showing reductions in germ cell number were seen in populations containing homozygous Sxlf#' females. This was in contrast to crosses generating females homozygous for either of
538 B. Oliver, D. Pauli and A. P. Mahowald
TABLE 1
Summary of m o alleles u s e d in this study
l?e111;11e germ linr phcwotvpr
;\Il<*k
Arr1.rtc.d
oogrwsis“ hid/da~h S0lll;l Ilillllrc’ Kef.‘
Eggs ( k ~ ~ c t i c
ovolJ1 Arrested None Wild-type 1)otnin;lnt ; u ~ i n l o r p l ~ 1-3
0votJ2 Arre~tetl Few Wild-type 1)olnin;lnt ;lntinlorph 1-3
ollolJ 7 “Nornlal” Many Wild-type L)onlinant ;mtimorph 1-3
ovo..lIz “Normal” Many Wiltl-tyl)e Recessive h y ~ m l t o r p h 3 . 4
oVOrJll Arrested Fe\v Viable, few denticle Recessive I y m n o r p h (ouo and soh)”
3.
4belt s e t x
o u o l J l r ~ : ? ? “Norm;tl”
Xl;unv 1,eth;d. few tlenticle Recessive ovo l ~ y ~ ~ o ~ n o r p l ~ sub amorph? 3
1.et11a1 None M’iltl-type Recessive amorph 3
belt setae
ovolJlr.Vl
1 : P 1.etll;ll Nonr Rough or glazed Recessive ovo ;unorph. setnidonlinant IzI neon~orph? 2 ,
S.
5 eves” Arrested oogenesis inc1ic;ltes t l l x t nwst of the egg chambers fiil to proceed past early oogenesis. “Nornlal” oogenesis indicates t h a t most h A fcw eggs laid is less t l l a n 30% of the wild-type ntrnlher per day. M a n y eggs is greater t l w l 30% o f wild tvpe.
‘ Rcferertces: I , BUSSON P I 01. ( I 983); 2. Kow.ropomu e f al. (1983); 3 , OI.IVER, PERRIMON ;~nd MAHOWALD ( 1 987); 4, MOHLER ( 1 977); 5 .
“ suh is shavenhahv. sub nlutations generally show :I polyphasic let11;d phase. We believe t h a t this constitutes the amorphic condition. OVO“”~ ‘ This is I);~secl on the larval cuticular phenotype ant1 the polvp1l;lsic lethality exhilited by henlizygotes.
’ I ; / is lozenge-like. I d n l y be allelic to rugose. The phenotype of I d ” i n frans to various deficiencies gives inconsistent results, making the
of the egg cllamlm-s de\v4op into eggs t h a t are laid These eggs are usually pernleable to neutral red and show fused appendages.
M$VEI.-NINIO, M A R I O I . and G A N S ( 1 989).
larvae show ;I very weak svh phenotype but show no overt letllalitv.
Ilronlorlhic designation tenuous. The eye phenotype is cold sensitive.
FIGURE 1 .--<:onlbinations O ~ O L I O ;tllclc\ ; I ~ ~ X Y I Y to causr ~ ~ ~ t ~ s l O t ~ t l l ~ ~ ~ i o ~ l o f ’ femalc germ cells t o ;I male identity. (;ernl cclls l i - o m sclu;tsllcd
gonads o f ; ~ t l u l t : ( A ) Honlozygous y N .S.d’’”’ 1 1 f fen1;lles. (1%) Honwzygous SxIf”” fenlales. ( C ) ouol’? V ~ ’ / O U O ” ‘ ‘ ~ ~ ~ u” females. (1)) OVOI” d ‘ / y ov0 r.lll
N vffetnales. (E) Wild-type ~nales. Bar = 1 0 p i .
ing that Sxl- does not result in a germ line phenotype similar to the ovo- phenotype makes it unlikely that Sxl is a regulator of all ovo functions.
To look at epistatic interactions, ovonfrS’ Sxlf”’,
were constructed. In crosses generating the above double mutant females we found that the germ cell death associated with OVO”~~” during early gastrulation
ov0 n I rS I Sxlfi”’ and ouoDfrS’ SxlJ’”’ double homozygotes
is suppressed by homozygosity for Sxlf“’. In crosses giving rise to homozygous ovoDfrS’ Sxlf*’ females, few embryos have less than 18 germ cells (Figure 2e). In contrast, O V O ~ “ ~ ~ ~ Sxlfi”’ and ovonfrS’ S ~ l f ’ ” ~ adult fe-
x
0 F
E M
E
R V 0 S
N I
C
L A
S S
2 0 ~
1 0
3 0
1
ovo N = 205 C.n
. E
3 0
-I
ovo Sxl N = 74
2 0
1 0
The ovo Locus
1
I
F./.?/ SX/ N = 81
p - 3 4 7
539
FIGURE 2.--Sxl- can suppress the death o f ovo- germ cells. T h e vertical axis shows the frequency o f a given number o f germ cells in embryos during early gastrulation. T h e first bar is the zero class, the remaining bars are o f width 6. T h e number ofembryos scored ( N ) is indicated. T h e filled bars and the arrows show the region o f the popula- tions containing ovo- embryos that approx- imates 25% (C and D). T h e same interval is indicated in the remaining panels for illus- trative purposes. T h e crosses are: (A) ovo-
''"
v z J / F M 3 X + / Y . (B) cm Sxlf"' ct/FMS X cm Sxlf"' ct/Y. ( C ) O V O ' ~ ' ' ~ ' v 2 ' / F M 3 XoVODlr.sl v z 4 / Y . (D) l z l c / F M 3 X lrl"/Y. (E) O V O D l r S l cm Sxlf" c t l F M 3 X O V O ' ~ ~ ' ~ ' cm Sxlf" c t / Y . (F) I d " cm Sxlf"' c t l F M 3 X lzl" cm-
Sxlf"'/Y. Embryos were scored by scanning electron microscopy. Scored embryos were 3.5-3.75 hr old when harvested.
0 3 0 6 0 0 3 0 6 0
CLASS (NUMBER OF GERM CELLS PER EMBRYO)
Sxlf#' allele is epistatic to O Y O ~ ' ' ~ ~ . Another example
of allele specific results comes from the analysis of lrlG
Sxlf#' double mutants. T h e germ cells of lrlG females die regardless of the presence of the loss-of-function Sxlf*' mutation. About 25% of the embryos from the cross of heterozygous lrlC Sxlf*' females to hemizygous lzl' Sxlf*' males have few or no germ cells (Figure 20. T h e data from lzlc Sxlf"', O I J O ~ ~ ' ~ ' ~ Sxlfi"' and O U O ~ ' ' ~ '
S ~ l f i * ~ double homozygotes are consistent with the idea that ouo acts either upstream of Sxl or in a different pathway. T h e suppression of germ cell death in OuODlrSl Sxlf#' embryos could indicate that these
mutations are compensatory in some way-specifi- cally that this result is due to the absence of somatic Sxl expression. It has been noted that the O U O ~ ~ ' ~ ~ embryonic phenotype (but not the adult phenotype) depends on genetic background (OLIVER, PERRIMON,
and MAHOWALD 1987; N. PERRIMON and B. OLIVER,
unpublished data). I t is also possible that the germ cell death in OUO"'~'' Sxlf#' females is delayed until later in development. It is difficult to determine which of these possibilities is correct given that females homo- zygous for S x l - die during embryogenesis.
Dominant interactions between ovo and Sxl: To avoid the problem of Sxl embryonic death we have looked for dominant interactions between ouo and Sxl. This type of gene dosage assay can be very sensitive and has been used extensively in the analysis of so-
matic sex determination (CLINE 1978, 1980, 1983a, 1984, 1986, 1988b; SKRIPSKY and LUCCHESI 1982;
UENOYAMA et al. 1982; BELOTE et al. 1985; CRON-
MILLER and CLINE 1986; OLIVER, PERRIMON and MA-
HOWALD 1988; STEINMANN-ZWICKY 1988).
For these interactions we have used the dominant ouoD mutations (Table 1). Like all other tested ouo alleles, ouoD mutations have no known effect on the male germ line and are fully penetrant for female sterility. Any genetic interactions with OUO' alleles are
likely to be related to the interactions of wild-type gene products because of the nature of the OUO" mu-
tations: all three are antimorphic and do not appear to cause novel gene activity unrelated to the ouo locus
(KOMITOPOULOU et al. 1983; BUSSON et al. 1983;
OLIVER, PERRIMON and MAHOWALD 1987). Unlike
females homozygous for ouo- alleles, many ouo"/+
female germ cells survive to adulthood (Figure 3) allowing for the assessment of the number of germ cells reaching advanced stages of oogenesis and the final phenotype of those cells in adults. T h e egg chambers of females heterozygous for OUO"' always
540 B. Oliver, D. Pauli and A. P. Mahowald
FIGURE 3.-l’he ova"' phenot)pes. (A) Part of a wild-type ovary (Oregon R strain). (B) A n ovary of a O V O ” ~ v z 4 / + female (+ = Oregon R). One vitellogenic egg is seen. The anterior part of the chorion is missing. ( C ) An ovary of a OVO‘”’~” vZ4 homozygote. Note its size smaller than that of a normal oocyte. (D) A higher magnification of a OVO”’ v2‘/+ ovary. One egg chamber has failed to develop an oocyte (open arrow) while other egg chambers are filled by undifferentiated cells (filled arrows). (E) An egg from a ovon2 vZ4/FM6 female showing fused dorsal appendages. Compare this egg to the one shown in (A). Similar fused dorsal appendages have been reported in snf’”’/Sxf- females (STEINMANN-ZWICKY 1988). Btr = 100 gm (A, B. C . E). Bar = 20 pm (D).
dages are usually fused and the vitelline membrane is permeable). T h e ouoD2 allele is particularly useful for
looking at genetic interactions because the dominant phenotype is intermediate. In addition to failure to initiate vitellogenesis, the more advanced egg cham- bers of o m D 2 females can show other developmental irregularities such as permeability, flaccidity, absence of the anterior end of the chorion, fused dorsal ap-
pendages, failure in the oocyte/nurse-cells choice re- sulting in egg chambers containing 16 or more poly- ploid nuclei, a?d less frequently, small ovarian tumors of unknown sexual identity (Figure 3).
The ouo Locus 54 1
TABLE 2
The ovoD2 phenotype in different genetic backgrounds
Effect on ouoD2 Sensitivity to sis-a, sis-b duplications'
Male viability Female viability
Strain"
Ovaries
Eggs/ovary scored # DD/+ # +/+ (%) # DD/+ #
+/+
(%)Rel. via. Rei. via.
Crimea Oregon-R Samarkand Oregon-RC 1 Canton6 Florida-9 Swedish-B6 Amherst-3 Lausanne-S Wageningen Oregon-RC2 Swedish-C Seto Hikone-R Urbana-S 0.2 0.2 0.2 0.3 0.4 0.6 0.9 1.5 2.0 2.1 2.7 3.0 3.4 3.8 8.4 81 68 57 54 66 121 123 68 110 103 91 79 109 97 90 93 1 1 0 30 0 27
N D ~
1 3 1 1 2 4 33 179 80 149 185 183 87 143 ND 166 150 78 61 163 101 102 52 <1 <1 <1 16 <1 19 <1 2 1 2 1 4 32 ND 152 65 89 97 111 113 122 123 102 40 36 128 118 99 ND 16 113 147 190 169 140 157 ND 133 151 96 93 170 147 127 950 58 61 51 66 81 78 92 68 42 39 75 80 78 N D
" The wild-type background used for testing the ouon2 phenotype and the sensitivity to duplications of both s k u and sis-b (=DD). The wild-type strains were obtained from the Bowling Green Stock Center. Oregon-RC1 corresponds to stock a17. Oregon-RC2 corresponds to stock a 16.
'
Males of the genotype OUO''~ u2.'/Y were crossed to the wild-type females indicated in the first column. The mean number of vitellogeniceggs seen in each overy of the resulting progeny is listed. The number of ovaries scored is also indicated.
' The number of DD/+ and
+/+
flies recovered as viable adults is indicated. The relative viability (rel. via.) for males and females is tletermined by dividing the number of DD/+ flies by the number of+/+
flies. See CLINE (1988b) for details on the construction of DD/+ flies.Not done.
(Urbana-S) the females heterozygous for ovoD2 (and male derived autosomes) have, on average, less than 3.8 vitellogenic eggs per ovary (also see KOMITOPOU- LOU et al. 1983; BUSSON et al. 1983; OLIVER, PERRI-
MON and MAHOWALD 1987). A 50% change in genetic
background causes significant changes in ovoD2/+egg production, but the phenotypic range is narrow. T h e cause of sensitivity to genetic background is unknown, but it does not appear to be due to the same genetic factors that sensitize the soma to perturbations of the X:A ratio. There is not a significant correlation be- tween the ovoD2 ovarian phenotype and the male-lethal phenotype caused by duplications of the X-linked numerator elements, sisterless-a (sis-a) and sisterless-b (sis-b) (CLINE 1986, 198813) (Table
2).
For example, the wild-type strains at each end of the ovoD2 pheno- typic range, Crimea and Urbana-S, show the least sensitivity to sis-a, sis-b duplication mediated male lethality. T h e narrow phenotypic range of ovoD2/+ ovarian development seen in 14 of 15 genetic back- grounds [and in the vast majority of backgrounds used to test the effect of deficiencies, totaling about 60% of the genome, on the ovoD2 phenotype (D. PAULI, unpublished data)] is important since the number of eggs per ovary in experimental crosses can be refer- enced against internal controls and the wild-type phe- notypic range (0.2-3.8 eggs/ovary).T h e central zygotically contributed gene product
involved in sex determination in the soma is Sxl (CLINE 1988a). Many mutations in Sxl result in significant suppression of ovoD2. T h e ovarian size of many ovoD2/ Sxl females approaches wild-type (Figure 4) due to the production of more vitellogenic eggs (Figure 5, Table 3). For example, when females heterozygous for the loss-of-function allele, Sxlf#', or the deletion,
Df(
I ) S X ~ ' ~ ~ , are crossed to ovoD2/Y males, the female progeny are of two distinct classes with respect to the number of vitellogenic eggs that are found. Female progeny trans-heterozygous forDf(
1)Sxl and ovoD2 have means of 5-6 vitellogenic eggs per ovary. In contrast, the female siblings trans-heterozygous for Sxl+ and ovoD2 have fewer than one vitellogenic egg per ovary. These results suggest that reducing the dose of S x l + in both the soma and germ line partially suppresses the ovoD2 phenotype. T h e eggs of ovoD2/ S x l - females have never been observed to develop and often show the range of defects associated with ovoD2/+
eggs ( c j Figure 3) suggesting that Sxl has a quanti- tative, not qualitative effect on the ovoD2 phenotype.542 B. Oliver, D. Pauli and A. P. Mahowald
-I-
-
I:IC.URF. 4."7'he o\.;tri;tn development of ovo"' heterozygotes i s influerlcetl by Yygotic S x l dose. Ovaries are from the fbllo\\,ing: (A) Wild tvpe (Oregon R). (B) ovo"? v"/+ (+ = Oregon R). ( C ) b ' i l d type (Amherst-3). (1)) ovo''' v2"/+ (+ = Anlherst-3). (E) O V O " ~ v"/cm S'xl'"' cl. (F) ovoD2 2.1
v / F W a [siblings of females in panel (E)]. ( G ) ovo"' d 4 / y Sxlf"' oc v f . ( H ) ova/" vZf/FM7a [siblings of females in panel ( G ) ] . (I ) ovorJ2
sn cf ( J ) o ~ o ' ' ~ v"/Binsc [siblings of females in panel (I)]. (K) ovoD2 ~ ~ ~ / . c j x l f " ' ~ . (1,) ovorJ2 v2'/F,M3 [siblings of females in panel (K). Bar = 500 pm.
v 2 4 / ~ c j x ~ / m , 7 , .N.f
of Sxl that have different effects on the soma and the germ line. In general, alleles of Sxl that are defective in somatic functions (ie., result in female lethality or sexual transformation in homozygotes) but are wild type for germ line functions (ie., are able to promote the production of wild-type eggs in germ line clones) suppress the ovon2 phenotype (Table 4). For example, the females trans-heterozygous for the somatic line dependent Sxlfm7* Ma' mutation, and ovon2 have a mean number of vitellogenic eggs that exceeds 14, while the
control sibling females have only about three vitello- genic eggs per ovary (Figures 4 and 5 , Table 3). T h e suppression of w o D 2 is clearly a result of the Sxlfm7. '"*I
mutation as ovon2/SxlJm7~ " I ; Dp( l:?)SxI+ sni3/+ fe-
The o m Locus 543
FIGURE 5.-The effect of various S x l al- leles on the O V O ” ~ phenotype. The vertical axis shows the mean number of vitellogenic eggs seen per ov;~ry. The odd columns show
the results for o d J 2 females also heterozy- gous for the indicated Sxl allele (only nlutant superscripts are shown. fill7 is .SxP‘..””’).
The even columns. denoted by
”+.”
show the results for the OVO”’ fenlale siblings that received the txllancer chronlosome. The stippled box shows the range of the means seen i n crosses of tnales to 14 of 15 different wild-type strains (see Table 2). See Table 3 for full genotypes.l,l~,l,..
---
”“- “”- ””_ ~ ” ” “ ” ” ” ” ” ” ~ ” ” “ ” ” ~“”II-m-m-m”
I“‘ + f L s + 7 8 0 + M t l + t m 7 + 1 9 + f r t l + t r a 3 +
1 4
8
E G 6 G S
P
E
R
o 4 V A
R
Y
2
sX/ ALLELE TESTED
heterozygous for O U O ~ ) ~ and either SxlJqnl or Sxl/’”’ have about two eggs per ovary as do the control sibling females (Figures 4 and 5 , Table 3). T h e general rule with respect to the interaction between O U O ‘ ) ~ and Sxl appears to be that decreasing the functional dose of Sxl in the soma suppresses the O U O ’ ) ~ phenotype.
T h e correlation between Sxl allele cell line depend- ence and suppression of O U O ” ~ is not strict (Table 4),
but the exceptions can be explained. Two somatic line dependent Sxl alleles, Sxl*“’ and S x l J y , clearly fail to suppress O U O ~ ’ ~ . These results are not surprising, as SxlJ‘”’ is a very weak allele and S x l f y is an allele with some unusual properties (see DISCUSSION). T w o other somatic line dependent Sxl mutations, SxlfHL2 and SxlJ7”’, significantly suppress O I J O ~ ’ ~ as compared to control siblings but are at the high end of the ouonZ/
+
phenotypic range (see Tables 2 and 3). T h e final exception, Sx1‘“*’, is treated in detail in the DISCUS-We have not observed any dominant interactions between recessive alleles of ouo and Sxl (data not shown). T h e remaining dominant alleles of ovo, OUO”’
and O V O ‘ ) ~ , may be suppressed by at least some of the
same alleles of Sxl that suppress ouon2 (data not shown), but these interactions are more difficult to score due to the extreme nature of the OUO”’ mutation and the
weak nature of the OUO‘)’ mutation.
The OVO” mutations act synergistically with stf6“: A number of candidates for positive regulators of Sxl
SION.
have been advanced. These regulators have in com- mon genetic interactions with S x l , but there are be- lieved to be differences in the way they function. T h e
sis-a, sis-b loci and maternal da product are believed to be required for Sxl activation and are not believed to be required once Sxl is “on” (CLINE 1986; 1988a, b). T h e activity of the snf locus is likely to be important for maintaining Sxl expression following activation, but an additional role in activation cannot be ruled o u t (CLINE, 1988b; OLIVER, PERRIMON and MAHOW- ALD 1988; STEINMANN-ZWICKY 1988). T h e da and snf loci also differ in the tissues that require expression of those genes. T h e da locus is required in the mater- nal germ line for activation of somatic Sxl function in the next generation, but the absence of da in the zygotic germ line is of no consequence to that gener- ation (CRONMILLER and CLINE 1987). T h e snf locus is probably required for female sexual identity in the zygotic germ line (WIESCHAUS, AUDIT and MASSON
198 1 ; PERRIMON and GANS 1983).
T h e positive regulators of Sxl show different inter- actions with ouo mutations. T h e ouol”, ouon2 or O U O ” ~ mutations act synergistically with snf162’ (Figure 6). Females heterozygous for ouoD2 and snf’62’ never have vitellogenic eggs and occasionally have no germ cells. Even more dramatic is the interaction between OUO”’
544 B. Oliver, D. Pauli and A. P. Mahowald
TABLE 3
The w o D Z phenotype is suppressed by some Sxl alleles
Genotype o f female” Eggs/ovaryb Statistics‘
Parent Progeny Mean No. T I = P
Sxlfg’ oc p t g v l F M 7 c
cm S x l p ’ ctlFM3
y Sxlp-’ oc v f / F M 3
y cm Of ( 1 )Sxl ’”“/Binsc
y Sxl““/FM7
Sxl SxPfi7~’wt‘ et v ;
D p ( l ; 3 ) s n ” “ ’ f T M 3 , Sb
y cm S x l f 7‘:2/Bins~
y cm Sxlf‘1‘.2fBinsc
cm SxlJ‘ v/Binsc
y cm Sxlp“/Binsc
y cv .Sxl/’”’ v f l F M 7 c
Sxlfi“’IFM3
ovo”’/Sxl
ovo”2fSxl
ov0”2/Sxl
o v o ” ~ / S x l
ov0”2/Sxl
ov0”2/Sxl
ov0”2/Sxl; S X l + / +
0 v o ” ~ f S x l
O V O ” ~ / S X l
ovo’)2 f S x l
o v o ” ~ / S x l
ovo1)2 f S x l
ovoD2 f S x l
o v o ” ~ / S x l o ~ o ” ~ f F M 7 c
ovo”z/FM3
ovo”z/FM3
ovo”’/Binsc
ouoD2 f FM 7 0 v o ~ ) ~ / F M 7
ovo”2/Sxl; T M 3 / +
ovaDZ fBinsc
ovoD2 fBinsc
ovo”Z/Binsc
ovo”’/Binsc
ovo1)2 fBinsc
o ~ o ” ~ f F M 7 c
ovo”z/FM3 5.5 1.3 6.1 0.8 6.0 2.3 4.8 1.3 8.1 2.1 14.6 3.5 0.2 5.3 10.1 6.3 3.4 1 .8 3.6 1.8 0.2 0.3 3.1 3.1 1.5 2.5 2.2 2.0 75 60 99 64 91 79 81 110 64 59 67 41 81 81 81 75 70 68 75 71 58 64 66 66 79 54 73 58 0.521 0.7 15 0.368 0.504 0.629 0.730 0.827 0.315 0.318 0.252
0.07 1
0.059
0.207
0.157
co.0 1
c o . 0 1
cco.01 cco.01 co.01 cco.01 cco.01 co.01 <0.0Id <o.05d NS‘ NS NS NS
a Females of the indicated genotype were crossed to ovaDZ uz4 Y males. Progeny genotypes were assigned based on the balancer phenotype.
‘ T I is the maximum distance between cumulative distributions of eggs/ovary for the Sxl females us. the balancer females, sI(x) and &).
The T I statistic approximates the area of a distribution that is exclusive to one population such that TI = 1 .O means that no individuals of a given number of eggslovary in one population are found in the second population and T I = 0.0 means that the populations are identical. The P = 0.05 or P = 0.01 significance levels for T I for each distribution pair is equal to 1.52 or 1.63 multiplied by the square root of (nl
+
n2)/(nlnp), where n l and np are the number of ovaries scored in the experimental and control siblings. Number of ovaries scored and the mean number of egg chambers/ovary at stage 10 or greater.
Significant when compared to the control siblings, but within the normal phenotypic range for ovoDz/+. NS indicates that the experimental and control classes are not significantly different at P = 0.05.
Sxlf’, or the trans-allelic combinations of ovo mutations shown in Figure 1. Females trans-heterozygous for 0voD3 and snf“” have no eggs, or rarely one egg (Figure 6 ) . This is a dramatic interaction as ovoD3/+ females overlap wild-type in terms of egg production
(5.7 vitellogenic eggs/ovary). There is no evidence
that snf1621 has a maternal effect on the ovoD pheno- types (data not shown). T h e da, sis-a, and sis-b loci have no effect on ovoD2 (data not shown).
Given that snf is a positive regulator of Sxl (CLINE 198813; OLIVER, PERRIMON and MAHOWALD 1988; STEINMANN-ZWICKY 1988) one might assume that the snf interaction with ouo is due to reduced Sxl function in the germ line. If this were the case the female germ line specific SxlfS4’ and Sxlf’” mutations might be
expected to interact with ovoD synergistically, like snf. Germ line dependent Sxlf’ mutations have no effect on ovoD2. Further, since the interaction of snf with ovo is synergistic, not suppressive like somatic line depend- ent Sxl mutations, it is unlikely that the interaction between ovo and snf is mediated by the soma. These data suggest that the snf interaction with ovo may be independent of Sxl expression in the germ line or somatic line. This suggestion highlights a potential problem with the previous interpretation of the inter- action between Sxl and snf. At 1 8 O , 2 0 ° , 22’ and 29’ (but not at 2 5 ” ) it has been shown that snf’62’/Sx1- females are sterile (OLIVER, PERRIMON and MAHOW-
The ovo Locus 545
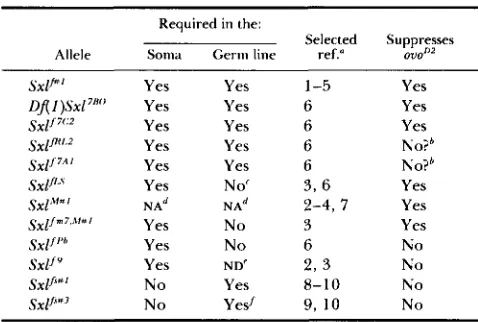
TABLE 4
Summary of Sxl suppression of ouoDz
Required in the:
Allele Soma Germ line ref." 07J0n2
Selected Suppresses
Sxlr"' Yes Yes 1-5 Yes
of(
I )SXl7'"' Yes Yes 6 Yessx1/7':2 Yes Yes 6 Yes
s x p 2 Yes Yes 6 No?'
s x 1 / 7 * ~ Yes Yes 6 No?'
s x p Yes No' 3 , 6 Yes
Sxl""' NA" NA" 2 - 4 , 7 Yes s x p m 7 . i l l t t l Yes No 3 Yes
SXlf''h Yes No 6 No
SXlfY Yes ND' 2 , 3 No
SXl/"' No Yes 8-10 N o
s X p 3 N o Yesf 9, IO N o
" 1, SANCHEZ and NOTHICER (1982); 2, CLINE (1985); 3, CLINE
(1988a); 4, STEINMANN-ZWICKY, SCHMID and NOTHICER (1989); 5,
SCHUPRACH (1985); 6 , SALZ, CLINE and SCHEDL (1987); 7, STEIN- MANN-ZWICKY (1988); 8 , PERRIMON et al. (1986); 9, J. D. MOLHER, personal communication; 10, B. OLIVER, unpublished results.
'
Results in significant suppression of ovonZ when compared to controls, but falls within the normal wild-type range for ovoD2/+females.
' Based on complementation with Sxlf'*'.
" Not applicable. I Not done.
/ N o t analyzed in clones, but 2 X 2 A flies homozygous for this nlutarion are somatically female with a transformed germ line.
it seemed likely that the genetic interaction occurred in germ cells. This may be wrong. At 29", many snf'"'/Sxlf"', snf162'/Df( I ) S X E ~ ~ ' , and snf'"'/Sxlfm7~ M#'
females are sterile, while most snf'62'/Sxlf" and ~ n f ' ~ ~ ' / S x l f S # ~ females are fertile (Table 5). T h e inter- action between snf and Sxl, at least at 29O, is associated with reduced doses of somatic Sxl function.
DISCUSSION
Mosaic experiments and analysis of the somatic line dependent sterility caused by sex determination mu- tants provide evidence that communication between the somatic and germ lines is important for prolifer- ation and differentiation of germ cells (DOBZHANSKY and BRIDGES 1928; DOBZHANSKY 193 1 ; NOVITSKI
1951; MULLER 1955; BROWN and KING 1961, 1962; STERN and SHERWOOD 1962; VAN DEUSEN 1976; GEHRING, WIESCHAUS and HOLLIGER 1976; MARSH and WEISCHAUS 1978; LAUGB and KING 1979; SZABAD and FAJSZI 1982; CLINE 1984; SCHUPBACH 1982;
1985; STEINMANN-ZWICKY, SCHMID, and NOTHIGER 1989; this study). More specifically the general rule appears to be that a 2X:2A germ cell which survives can become partially transformed to male sexual iden- tity in a 1X:2A male or in a 2X:2A fly genetically transformed to male (SCHUPBACH 1982, 1985; CLINE 1984; NOTHIGER et al. 1989; STEINMANN-ZWICKY, SCHMID, and NOTHIGER 1989; B. OLIVER, unpub- lished data). T h e converse does not hold. A surviving
1X:2A germ cell attempts to enter a spermatogenic pathway in 2X:2A flies or in 1X:2A flies transformed to female by constitutive expression of a female tra mRNA (STEINMANN-ZWICKY, SCHMID and NOTHIGER 1989; B. OLIVER, unpublished data). These observa- tions suggest that the somatic sexual identity can at least partially influence the sexual differentiation of the germ line.
Although the nature of the somatic signal required for germ line sex determination is not clear, Sxl is involved in the response to that signal (STEINMANN- ZWICKY, SCHMID and NOTHIGER 1989). In transplan- tation experiments 2X:2A Sxl- germ cells have a spermatocyte-like morphology in both 2X:2A flies and
1X:2A flies (SCHUPBACH 1985; STEINMANN-ZWICKY, SCHMID and NOTHIGER 1989). Even more striking is the fact that constitutive expression of Sxl female function in 2X:2A germ cells (via SxlM#') can block the otherwise masculinizing influence of a 1X:2A soma (STEINMANN-ZWICKY, SCHMID and NOTHIGER 1989). These results suggest that Sxl expression in the germ line responds to some soma/germ line signal transduction mechanism. We suggest that ovo is in- volved in this process.
ovo and germ line sex: Mutations in ovo may switch germ line sexual identity. Expression of ovo is required for female germ line viability, but some combinations of ovo alleles allow the survival of 2X:2A germ cells. These germ cells are strikingly similar to early sper- matocytes and can not be morphologically distin- guished from the germ cells found in homozygous snf"*I or Sxlfi females. T h e mutant combinations sug- gest that extreme reduction, but not the elimination of ovo function, results in germ line sex transforma- tion. T h e absence of ovo function results in female germ cell death. T h e cause of ovo- germ cell death is unknown, but we suggest that this is also due to defective sex determination or differentiation in germ cells. Possible causes of ovo germ cell death include mismatched germ line and somatic line sexual identity and/or dosage compensation (see ovo and Somatic Line Sex). T h e sex specific lethality and the sex transfor- mation phenotypes suggest that ovo is involved in germ line sex determination.
Females double mutant for both the loss of function allele O V O ~ ' ~ ~ ' and Sxlf#' or Sxlfi*' are phenotypically
546 B. Oliver, D. Pauli and A. P. Mahowdd
FIGURE C,.”ovo‘) nlutations and .snf’“” interact synergistically. Ovaries o f females Irans-hetero/\go~Is fo r ouo” nlutations and snf’”” are shown. Note the ; h e n c e of any vitellogenic egg chambers. Genotypes are: ( A ) snf‘“” v2f/snf’hz’ v”
.
Note t h a t large ovarian tunmrs cancstentl through entire owrioles. (B) ovo‘” v2‘/snf’“z‘ u”. No vitellogenic egg chambers are present. (C) A FA47/snf’”2’ v2‘ sibling of the female shown i n (H). <)ne vitellogenic egg chanlber is present ( a r r o d w d ) . (D) ova')' v”/snf’“” u”
.
No vitellogcnic egg chambers are present. (E)ouo”’ vZ‘/.~nf’“Z‘ u”. (F) The germ cells of an ovo’)’ u2‘/snf’hz’ v” female shown at higher n1;lgnification. Tllese cells are similar to the ovarian tunlor cells and early spermatocytes shown i n Figure 1. B ; I ~ = 100 pn1 ( A , H. C . D, E). Bar = 1 0 pni (F).
females die, while the germ cells of S x l - females are viable. We suggest that the germ cells of ouo- females are dead before female Sxl gene product is required and therefore can not be rescued by the constitutive activity of S x l n d a f . If ovo and Sxl are part of a common pathway in germ cells the ovo gene product is probably upstream.
T h e synergistic interactions between snff6” and ovo‘’ mutations also suggest that ouo is involved in germ line sex determination, as snf is known to be
The ovo Locus 547
TABLE 5
The snf'"' interaction with Sxl may depend on somatic Sxl expression
No. Percent Ovarian E'etnale progeny" scored sterileb phenotype'
y N ,~xlJ'"' v f/snf"" vzi 82 4 Wild-type
cm S X P ' ct/snfih2' v Z 4 17 65 Male, >I5
<yxp*'lSnf'"Z' v z 4 72 1 Wild-type
nurse cells defective eggs SxP' oc ptg v/snfih2' v Z i 40 25 Defective eggs y cm ~f(I)Sxi7'J''/snf'h" uz' 94 71 Male, >I5
nurse cells defective eggs
wild-type
c m Lyxy"7. .AI*' ct vlsnf'"' v Z i 85 83 Defective eggs,
a All female parents were ~ n f " ~ ' vZ4/FM3. All progeny were
grown and scored at 29".
'
Females that produced no larvae after 11 days were scored as sterile.' Sterile females were dissected following the 1 I-day fertility test. Individual females often exhibit more than one of the phenotypes; the relative portion of the sterile females showing a given phenotype varied in different experiments. See OLIVER, PERRIMON and MA- HOWALD ( 1 988) and STEINMANN-ZWICKY (1988) for full descriptions of Sxllsnfsterility phenotypes.
possible that snf acts upstream of o w . Only one allele of snf exists and it is not a complete loss of function allele (COLLIN and KING 1981). It remains possible that the absence of any snfe activity would result in female germ cell death. Indeed the complete absence of female germ cells has been observed in interactions between snf and Sxl (STEINMANN-ZWICKY 1988), even though neither of these genes alone result in such a phenotype.
T h e female germ line phenotype (i.e., partial sex transformation) caused by snf'"' and Sxlrs mutations is similar. One might expect that o m D mutations would interact with snf and Sxlfi in a similar manner. Inter- estingly, female sterile alleles of Sxl not only fail to interact with ovoD but also fail to interact with snf1621. Sxlfi/snf females are fertile under conditions where Sxl-lsnf females are sterile. Because both snf and Sxl have germ line dependent phenotypes, it was sug- gested that the snf'62'/Sx1- interaction leading to ste- rility occurs in germ cells (OLIVER, PERRIMON and MAHOWALD 1988; STEINMANN-ZWICKY 1988). Data presented here imply that this interaction is mediated by somatic line/germ line communication. Regardless of the cause of Sxllsnf female sterility, it is not sur- prising that Sxlr. alleles fail to interact with ovoD given that Sxlf" alleles also fail to interact with snf"".
ovo and somatic line sex: Some ovoD/+ germ cells clearly survive to adulthood, but in mitotic recombi- nation studies the frequency with which wild-type germ line recombinants are recovered from ovoD/+ females falls dramatically during development (PER-
RIMON 1984). This is consistent with reduced viability
as compared to wild-type germ cells. It has been suggested that o m D , by antagonizing the
ova+
gene product, makes the 2X:2A germ line more male-like resulting in both cell death and defective oogenesis (OLIVER, PERRIMON and MAHOWALD 1987). Reducing the dose of somatic Sxl or downstream sex determi- nation genes involved in somatic sexual identity and differentiation might allow for the greater survival and better development of ovoD2 germ cells because of compensatory changes in both somatic and germ line sexual identity. [Similar suppression of ovoD2 has been observed with some alleles of the somatic line dependent tra-2, dsx, and ix loci (B. OLIVER, unpub- lished data). T h e cause of the allele specificity is un- known.] T h e phenotypic consequence of this interac- tion would be the production of more eggs. These compensatory changes in somatic sexual identity, if they exist, must be subliminal, as 2X:2A flies hetero- zygous for any of the tested somatic sex determination genes are phenotypically wild-type females.T h e suppression of O V O ~ ' ~ ~ ' germ cell lethality by
Sxlf" in double homozygotes supports the idea that changing the somatic sexual identity increases the viability of ovo- 2X:2A germ cells. However, the find- ing that a second ovo-, allele lzl', is not suppressed by Sxlf#' tempers this conclusion. T h e cause of this allele specificity is unclear, but could be due the molecular nature of ovo mutations. Mutations in the ovo locus show evidence of complexity both in terms of seem- ingly unrelated genetic defects ( i . e . , ovo, lozenge-like and shavenbaby phenotypes) and in term of comple- mentation (OLIVER, PERRIMON and MAHOWALD 1987; M~VEL-NINIO, MARIOL and GANS 1989). In particular, lzlG and OUO"~~" have the same recessive female germ line phenotype, but lzl" has a semi-dominant cold sensitive eye phenotype which shows some indications of neomorphic gene activity. T h e lozenge-like eye phe- notype of many ovo alleles is due to the insertion of a gypsy transposable element into the ovo locus ( M ~ v E L -
548 B. Oliver, D. Pauli and A. P. Mahowald
certainly be required to clarify the cause of ovo- female germ cell death.
The suppression of o w D 2 by SxZ provides the best evidence that ovo responds to signals from the soma: Egg formation requires germ line Sxl expression (CLINE 1983b; SCHUPBACH 1985; PERRIMON et al.
1986; SALZ, CLINE and SCHEDL 1987; STEINMANN- ZWICKY, SCHMID and NOTHIGER 1989). Sxl also has a somatic line dependent effect on fertility, as suggested by the somatic line dependent germ line sex transfor- mation of 2X:2A Sxl flies (CLINE 1984) and possibly by the interaction between Sxl and snf’”’ (this study). Some 2X:2A flies homozygous for S ~ l f ” ~ , ~ ” can sur- vive to adulthood and develop as sterile somatic males with germ cells that have a male sexual identity (CLINE
1984). This is probably due to defective somatic Sxl expression since 2X:2A germ cells homozygous for ~ ~ l f ~ ~ * ~ ~ ’ can form fully functional eggs in mosaics (CLINE 198313; also cited in CLINE 1984). It would appear that, in the absence of the female specifying functions of Sxl in the soma, germ line development switches to male.
T h e genetic character of Sxl mutations associated with the ability of an allele to suppress ovo is somatic line dependence. For example, lowering the dose of Sxlf”79M#’ or other somatic line dependent Sxl alleles, such as Sxlfls, suppress ovoD2. Mutant alleles required in both the soma and germ line such as
Of(
I )Sxl 780, Sxlf#’, or Sxlf7“’ also suppress ovoD2, while the germ line specific Sxl@ alleles d o not interact. T h e finding that deletions of Sxl are good suppressors of ovoD2 while weak Sxl alleles such as S x l f P b have no effect on ovoD2 are consistent with the idea that Sxl mediated suppression of ovoDz is due to reduced Sxl+ dose. It is unlikely that the allele specificity is due to the genetic background of the Sxl chromosomes, since many of the alleles that interact with ovoD2 are derived from the weak allele S x l f P b that fails to interact with ovoD2 and duplications of S x l + eliminate S ~ l f “ ~ ~ Mar’ suppres-sion of ovoD2. T w o alleles of Sxl interact with ovoD2 in ways which require further explanation.
There is one strong allele of S x l , S x l f 9 , that is defective for somatic line Sxl function but fails to interact with ovoD2. This result is easily explained and was indeed expected. T h e S x l f 9 allele is lethal to homozygous females, but is viable and results in full female development in somatic clones, leading to the suggestion that Sxlf’ is defective for initiation of Sxl function but not for stable expression of Sxl function (CLINE 1988a). Because Sxl+ appears to be autoregu- lated (CLINE 1984), a ovoD2
+/+
Sxlf’ double heter- ozygote is probably functionally homozygous for wild- type Sxl activity.T h e SxlM#’ allele suppresses ovoD2. SxlM*’ is able to express female Sxl functions when da+ is at subthresh- old levels for S x l + activation and is lethal to 1X:2A
flies, suggesting that SxlM#’ is constitutive for female function (CLINE 1978, 1979, 1980). If SxlM7’ 1s ‘ con- stitutive for the female Sxl gene activity one might expect that this allele would have no effect on the ovoD2 phenotype. However, Sxl‘#’ also acts in the germ line as a constitutive producer of “ S x P activity. This constitutive expression results in the suppression of the masculinizing influence of a male soma on 2X:2A germ cells in pole cell transplants (STEINMANN-
ZWICKY, SCHMID, and NOTHIGER 1989) and in the
rescue of the female sterility of snf”” (STEINMANN-
ZWICKY 1988). If ovo is upstream of Sxl in the germ
line sex determination pathway, constitutive expres- sion of downstream female S x l + gene product could be expected to suppress the ovoD2 phenotype. This does not contradict the previous argument explaining the failure of SxlM#’ to rescue ovo- germ cells, since in the case of ovoD2 heterozygotes the germ cells develop to stages that normally express S x l .
Conclusions: T h e ovo locus appears to be involved in germ line sex determination. Mutations in ovo can cause arrested oogenesis, female germ cell death or the production of “ovarian tumors,” depending on the allelic combination. It has been suggested that Sxl or snfgerm cells showing an ovarian tumor morphology have been partially transformed to male. T h e finding that ovoD and snf’”’ mutations interact in a synergistic manner suggests that ovo and snf are involved in a common pathway or in synthetic pathways that would both be required for proper sex determination in the germ line. Because most ovo mutations result in sex specific germ cell death while Sxl causes the produc- tion of “ovarian tumors,” ovo is likely to act upstream or independently of Sxl and not downstream as pre- viously suggested. T h e allele specific suppression of ovoD2 by Sxl suggests that ovo is involved in a sex- specific germ line process that is connected in some manner to the interpretation of sex determining sig- nals provided by the soma.
We thank T. CLINE, J. D. MOHLER, and H. SALZ for providing many of the stocks used in this study. We are indebted to J. D. MOHLER for helping to spark our interest in “ovarian tumor” loci as candidates for sex determination genes. We also thank K . MAIER for scanning electron microscopy. The Stanford statistics consulta- tion service provided advice on data handling. We are grateful to B. BAKER, T. GORALSKI, R. NACOSHI and H. SALZ for extensive comments on the manuscript. This work was supported by grants from the National Institutes o f Health, U.S.A (HD-17608 to A.P.M. and T32-HD07104 to B . 0 ) and the Swiss National Foundation for Scientific Research (to D.P.).
L I T E R A T U R E C I T E D
BAKER, B. S., 1989 Sex in flies: the splice of life. Nature 340:
BAKER, B. S., and J. M. BELOTE, 1983 Sex determination and dosage compensation in Drosophila rnelanogaster Annu. Rev. Genet. 17: 345-393.
BAKER, B. S., and K. A. RIDGE, 1980 Sex and the single cell. 1.