Original Article
Heart formation and left-right asymmetry in separated
right and left embryos of a newt
KAZUHIRO TAKANO
1, YUZURU ITO
2, SHUICHI OBATA
3, TSUTOMU OINUMA
4, SHINJI KOMAZAKI
1,
HIROAKI NAKAMURA
1and MAKOTO ASASHIMA
2,5,6,*
1Department of Anatomy, Faculty of Medicine, Saitama Medical University, Iruma, Saitama, 2International Cooperative Research
Program (ICORP), Japan Science and Technology Agency (JST), Department of Life Science (Biology), Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, 3Division of Biology, College of Liberal Arts and Sciences, Kitasato University, Sagamihara,
Kanagawa, 4Department of Anatomy Ultrastructural Cell Biology, Faculty of Medicine, University of Miyazaki, Kiyotake, Miyazaki, 5National Institute of Advanced Industrial Sciences and Technology (AIST), Tsukuba, Ibaraki and 6Department of Life Science (Biology),
Graduate School of Arts and Sciences, The University of Tokyo, Meguro-ku, Tokyo, Japan.
ABSTRACT During vertebrate cardiac development, the heart tube formed by fusion of right and left presumptive cardiac mesoderms (PCMs) undergoes looping toward the right, resulting in an asymmetrical heart. Here, we examined the right and left PCMs with regard to heart-tube looping using right- and left-half newt embryos (Cynops pyrrhogaster ). In the half embryos, the rightward (normal) loop of the heart tube was formed from the left PCM, irrespective of the timing of its separation, while the leftward (reversed) loop of the heart tube was formed from the right PCM, separated by stage 18. In addition, the direction of the leftward loop was inverted to the rightward direction in right-half embryos bisected after stage 18. Incision or resection of the embryonic caudal region implicated interactions between the right and left sides of this region as crucial for inverting the direction of the heart-tube loop from leftward to rightward in the right-half embryos. In situ hybridization of CyNodal (Cynops nodal-related gene) suggested that the inversion of heart looping in the right-half embryos has no association with the CyNodal expression pattern. Based on these findings, we propose a mechanism for the rightward looping underlying normal amphibian cardiac development.
KEY WORDS: Cynops pyrrhogaster, cardiac development, heart rudiment, cardiac looping, newt
Introduction
The external morphology of metazoans is symmetric, but the shape and arrangement of their internal organs are often asym-metric. Asymmetry appears earlier in the heart than in any other organ during embryonic development. The vertebrate heart is formed by fusion of the symmetrically distributed right and left presumptive cardiac mesoderms (PCMs). The right and left PCMs migrate to the thorax of the embryo, where they fuse to form a heart tube. The heart tube then undergoes looping toward the right, resulting in the formation of atria and ventricles (reviewed by Manner, 2000).
Mechanisms by which this heart tube directionality is deter-mined have been extensively studied. Most of these studies investigated the direction of heart-tube loops formed after fusion of the right and left PCMs (reviewed by Levin, 2005; Ramsdell,
*Address correspondence to: Makoto Asashima. Department of Life Science (Biology), Graduate School of Arts and Sciences, The University of Tokyo, 3-8-1 Komaba, Meguro-ku, Tokyo 153-8902, Japan. Fax: +81-3-5454-4330. e-mail: asashi@bio.c.u-tokyo.ac.jp
0214-6282/2007/$30.00 © UBC Press
Printed in Spain
www.intjdevbiol.com
2005). In contrast, Spemann and Falkenberg (1919) demon-strated that when a two-cell newt embryo was ligated along the first cleavage plane, two complete embryos developed from each blastomere. In each of these two separated embryos, a heart was formed, with the heart-tube loops directed rightward (normal direction) in the left-half embryos (n=2) and half of the loops directed leftward (reversed) in the right-half embryos (n=2). Hoyle et al. (1992) demonstrated in chick that when the right and left PCMs were exchanged between donor and host embryos at the neurula stage, double-right-sided embryos (two areas of PCM of the same right sidedness) formed heart-tube loops directed leftward in almost 50% of cases. These findings suggested that any intrinsic difference in heart-tube loop formation between the
right and left PCMs was derived from the right and left sides of an embryo, raising the question as to how both PCMs cannot fail to form a rightward loop after fusion in spite of their different properties.
The present study therefore sought to address the following questions using separated right- and left-half embryos of newt. Can separated right and left PCM form normal heart tubes, respectively? What direction will be taken by the looping of each heart tube, if it is assumed that each PCM formed an original heart tube? Finally, what influences do the individual right and left PCMs exert on the rightward looping of the heart tube formed after fusion of the PCMs?
Recent studies have also implicated asymmetrically expressed genes in the determination of embryonic left-right asymmetry, including Nodal (Levin et al., 1995; Collignon et al., 1996; Lowe et al., 1996; Lohr et al., 1997; Patel et al., 1999; Mogi et al., 2003; Toyoizumi et al., 2005), Pitx2 (Campione et al., 1999; Patel et al., 1999; Campione et al., 2001; Yu et al., 2001; Mogi et al., 2003; Toyoizumi et al., 2005), BMP (Ramsdell and Yost, 1999; Branford et al., 2000; Monsoro-Burq and Le Douarin, 2000; Monsoro-Burq and Le Douarin, 2001) and SnR (Issac et al., 1997, Patel et al., 1999). Of these genes, Nodal, which is expressed only in the left side of the embryo, is important in determining embryonic left-right asymmetry across many species (Levin et al., 1995; Collignon et al., 1996; Lowe et al., 1996; Lohr et al., 1997; Patel et al., 1999; Mogi et al., 2003; Toyoizumi et al., 2005). Therefore, in the present study, we additionally analyzed the relationship between the heart-tube loop direction in right- and left-half embryos and the expression of CyNodal, a nodal-related gene of newt (Ito et al., 2006), therein.
Results
Looping of the heart tube in ligated embryos
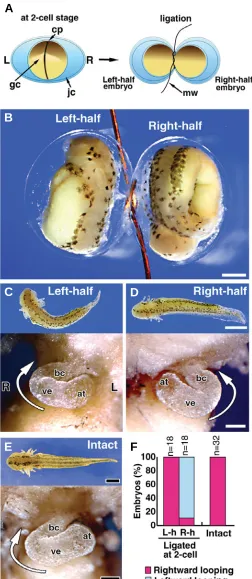
First, we revisited the twinning experiments of Spemann and Falkenberg (1919) using two-cell stage newt embryos and ex-tended on their findings. Stage 2 (two-cell) embryos were ligated along the cleavage furrow together with the jelly capsule to obtain two blastomeres (Fig. 1A). The symmetrically divided embryos underwent development at the same pace as normal embryos (Fig. 1B). At stage 37, both right- and left-half embryos showed normal and similar external morphology (Fig. 1C, D, upper), although each half embryo was shorter in length than intact normal embryos (Fig. 1E, upper). The rightward loops of the heart tubes were formed in 100% of left-half embryos (Fig. 1C, lower, F), identical to intact embryos (Fig. 1E, lower). In contrast, the leftward loops of the heart tubes were formed in 89% of right-half embryos (Fig. 1D, lower, F). All of the hearts formed in the right-and left-half embryos had normal external morphology compared
B
C
D
E
F
A
with intact embryos, with the exception of the loop direction. These results indicate that each of the right- and left-half embryos separated at first cleavage could form looping heart tubes, but that the loop direction assumed a mirror image relationship to each other. This series of experiments did not, however, provide information about the developmental potential of right and left PCMs, since these structures were not apparent at the two-cell stage. We therefore adopted another experimental approach to address this issue.
Looping of the heart tube in bisected embryos
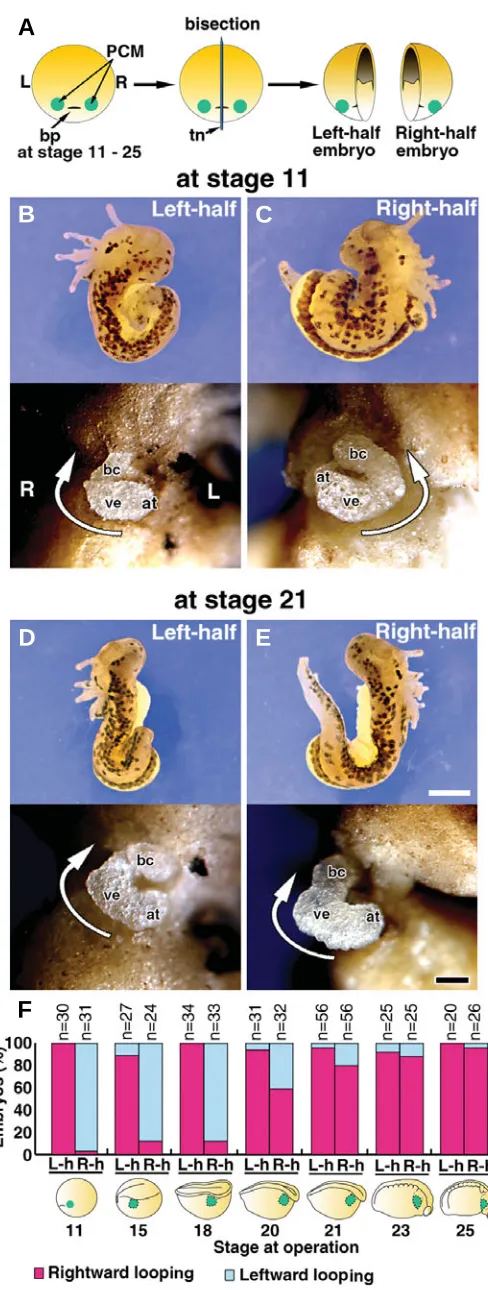
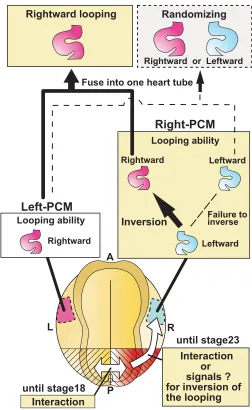
To investigate the ability of right and left PCMs to form the heart-tube loops, we bisected embryos through the center of the blastopore at several stages from the appearance of PCM (stage 11) to immediately before fusion of the left and right PCMs (stage 25) (Fig. 2A, F, lower). The right- and left-half embryos were incubated until stages 35-37 when the loops of the heart tubes became evident. The external morphology of these half embryos differed from that of normal embryos, in that they possessed the complete structure of either the right or left side of an intact embryo, but not both, with the cut plane serving as the border. The left-half embryo lacked structures such as the right eye, balancer, gill and epidermis, whereas the right-half embryo lacked the structures seen on the left side alike (Fig. 2B-E, upper). However, the hearts formed in these half embryos were normal in external morphology. In the left-half embryos bisected at stage 11-25, rightward loops of the heart tubes were formed in 89-100% of cases (Fig. 2B, D, F). In the right-half embryos bisected at stage 11-18, leftward loops of the heart tubes were formed in 88-97% of specimens (Fig. 2C, F). These results were identical to those obtained in the ligation experiment on the two-cell embryos (Fig. 1); however, when embryos were bisected at stage 20-23, the direction of the heart-tube loop gradually shifted from leftward to rightward in the right-half embryos and became almost right (>88% of cases) after stage 23 (Fig. 2E, F).
Effects of the embryonic caudal region on looping of the heart tube
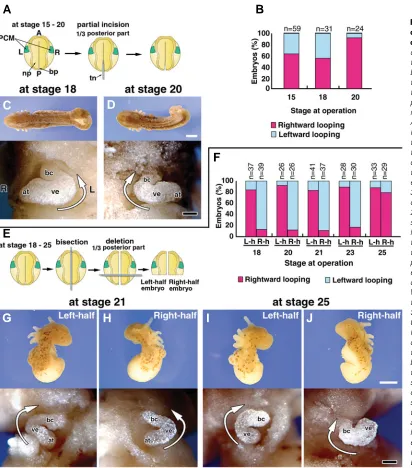
We next examined the effects of the embryonic caudal region (which contains equivalent regions to the node of mammalian and avian embryos) on formation of the heart-tube loop. To disturb interactions between the left and right sides of the caudal region, the caudal one-third (1/3 of the posterior part) of the embryo was incised along the median sagittal plane (Fig. 3A). These experi-mented embryos had normal external morphology, except for a
Fig. 2. Hearts formed in bisected embryos.(A)Schematic representa-tion of the bisecrepresenta-tion experiment. bp, blastopore; PCM, presumptive cardiac mesoderm; tn, tungsten needle; L, left; R, right. (B-E) Right- and left-half embryos (stage 37) formed from an embryo bisected at the early gastrula (stage 11) (B,C) or late neurula stage (stage 21) (D,E). The right-and left-half embryos at stage 37 viewed from the dorsal side (upper pictures) and the heart tube viewed from the ventral side (lower pictures). Portions of the body wall and the pericardium were removed to expose the heart. bc, bulbus cordis; at, atrium; ve, ventricle. White arrow indicates the direction of the heart-tube loop. Scale bar: 0.5 mm (upper pictures), 0.1 mm (lower pictures). (F) Graphic representation of the relationship be-tween the timing of bisection and the direction taken by each heart-tube loop formed in the bisected embryos. L-h, left-half embryo; R-h, right-half embryo. PCM is denoted in green in the illustration.
B
C
D
E
bifurcated tail at stage 36 (Fig. 3C, D, upper). It should be noted that the right and left PCM were fused in this experiment. In the embryos incised at stages 15 and 18, approximately one-half (55-63%) of the loops were rightward while the remainders were leftward (Fig. 3B, C). This indicated that the embryos incised at these stages had lost the ability to form a normal rightward loop of heart tube, resulting in the loops twisting in random directions. If the caudal regions were incised at stage 20, heart-tube loops showed the normal direction (rightward) in 92% of cases (Fig. 3B, D). These results supported the observation that heart-tube loops were directed rightward in the right-half embryos bisected after stage 20 (Fig. 2F). Together, the findings suggested that interac-tions in the caudal region from stage 15 to 18 are crucial for
determining the direction of the heart-tube loop formed by PCM fusion, possibly the change in direction of the looping of heart tube derived from the right PCM.
We next resected the caudal one-third of right- and left-half embryos to block signals from the caudal region to the PCM and investigated the directions of heart-tube looping (Fig. 3E). In the resected embryos incubated until stage 36, the external morphol-ogy was identical to that of the bisected embryos shown in Fig. 2, except for the absence of a tail (Fig. 3G-J, upper). The loops of the heart tubes were rightward in >83% of tailless/left-half embryos operated on between stages 18 and 25, (Fig. 3F, G, I). In the tailless/right-half embryos operated by stage 23, the heart-tube loops were leftward in >83% of cases (Fig. 3F, H). However, the
G
B
C
D
E
F
H
I
J
A
prior to PCM fusion. Cytoskeletal organization (Latacha et al., 2005; Taber, 2006) and adhesion properties of heart tube cells (Garcia-Castro et al., 2000) play important roles in the looping of the developing heart tube and it seems likely that such factors cannot function properly unless both right and left PCMs can form a rightward loop at their fusion.
We also disturbed the interactions between right and left parts of the caudal area (equivalent to the nodal area of mammalian and avian embryos) to elucidate the involvement of this region in the inversion of heart-tube looping direction observed in the right-half embryos. In these experiments, loops of heart tubes were formed with random directions when the caudal region was separated by approximately stage 18. This is probably because the perturbed interactions between the right and left caudal regions hampered inversion of the looping direction (from leftward to rightward) of heart tube formed from the right PCM. In mammalian and avian embryos, signal transduction within the nodal area, such as nodal flow by monocilia (Nonaka et al., 2002; McGrath et al., 2003; Tanaka et al., 2005) and Ca2+ signal transduction (McGrath et al., 2003; Raya et al., 2004), is important in determining the left-right asymmetry of various organs including the heart. The present results thus indicated that interactions between the right and left
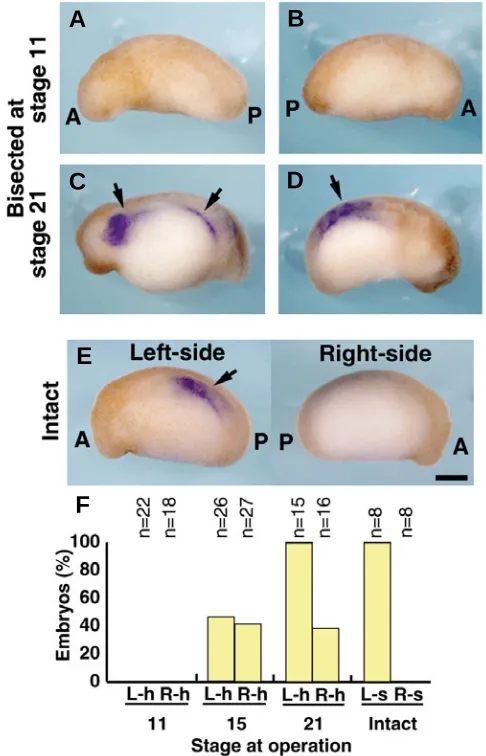
Fig. 4. CyNodal expression in the separated right- and left-half embryos. (A, B) CyNodal expression was not detected in either the left-(A) or right- (B) half embryos at stage 23 that developed from an embryo bisected at stage 11. (C, D) CyNodal expression (arrows) was observed, however, in those left- (C) and right- (D) half embryos at stage 23 that developed from embryos bisected at stage 21. The images depict the left side of the left-half embryo (A,C) and the right side of the right-half embryo (B,D). (E) CyNodal expression (arrow) on the left side of an intact normal embryo at stage 23. (F)Graphic representation of the percentage of CyNodal expression at stage 23-25 developed from bisected embryos. CyNodal expression was absent on the cut side (right side of the left-half embryos and left side of the right-half embryos) of all half embryos. A, anterior; P, posterior; L-h, left-half embryo; R-h, right-half embryo; L-s, left side, R-s, right side. Scale bar: 0.5 mm.
direction of the loop was inverted rightward in 79% of the tailless/ right-half embryos operated on at stage 25 (Fig. 3F, J). These results indicate that the caudal region has an important role until stage 23 in the inversion of the looping direction in heart tube formed from the right PCM in right-half embryos.
CyNodal expression in bisected embryos
To investigate the relationship between the looping direction of the heart tube and the expression of CyNodal in the lateral plate mesoderm, we performed whole-mount in situ hybridization on the right- and left-half embryos at stages 23-25. In the embryos bisected at stage 11, neither the right- nor the left-half embryos showed CyNodal expression in the lateral plate mesoderm (Fig. 4A, B, F). Among the embryos bisected at stage 15, CyNodal expression was detected in 46% and 41% of the left- and right-half embryos, respectively (Fig. 4F). In the embryos bisected at stage 21, CyNodal was expressed in all left-half embryos, but only in 38% of the right-half embryos (Fig. 4C, D, F). In intact embryos at stages 23-25, CyNodal expression localized in the left lateral plate mesoderm, but not in the right side of the body (Fig. 4E, F).
Discussion
Previous experimental findings including the ligation of two-cell newt embryos (Spemann and Falkenberg, 1919) and the transplantation of PCMs into chick embryos (Hoyle et al., 1992) suggested that any intrinsic differences in loop formation between the right and left PCMs were derived from the right and left sides of an embryo, respectively. Halving experiments involving the ligation and bisection of early newt embryos in the present study strongly supported this possibility. We clearly demonstrated that heart tube derived from the right PCM looped toward the left, while heart tube derived from the left PCM looped toward the right. In our bisected embryos, right and left heart tubes were formed from right and left PCMs, respectively. The loops of these heart tubes were normally oriented rightward in left-half embryos irrespective of the developmental stage of the operation, but were reversed in right-half embryos bisected at stage 11-18. These results were identical to those obtained in the earlier ligation experiments on two-cell embryos, supporting that PCMs derived from the right and left sides of an embryo formed heart-tube loops with a mirror image orientation. However, during the later stages (18-21), the right-side PCM must receive a signal to alter the direction of the heart-tube looping from leftward to rightward, as both right and left PCMs possess the ability to form a rightward looping heart tube
B
C
D
E
sides of the caudal region are important in determining the rightward looping of heart tube in amphibian embryos, as sug-gested for mammalian embryos (Nonaka et al., 2002; McGrath et al., 2003; Tanaka et al., 2005) and avian embryos (Raya et al., 2004).
The mechanism of determination of left-right asymmetry in vertebrates, various signals from the right and left sides of the node or its equivalent area to the anterior part of the embryo appears to involve induction of the expression of Nodal on the left side with concomitant suppression of Nodal expression on the right side (Levin et al., 1995; Collignon et al., 1996; Lowe et al., 1996; Lohr et al., 1997). This is followed by the restricted expression of transcription factors, such as Pitx2 on only the left side of the embryo (Campione et al., 1999; Patel et al., 1999; Campione et al., 2001; Yu et al., 2001; Mogi et al., 2003; Toyoizumi et al., 2005), BMP on the right side alone (Ramsdell et al., 1999; Branford et al., 2000; Monsoro-Burq and Le Douarin, 2000; Monsoro-Burq and Le Douarin, 2001) and SnR (Issac et al., 1997; Patel et al., 1999). It has been suggested that these factors are also important in determining the rightward direction of heart-tube looping in mammalian, avian and amphibian embryos. Based on these previous reports, we evaluated the roles of the caudal region in this determination by resection in right- and left-half embryos. The caudal region was indispensable until around stage 23 for inverting the direction of the heart-tube loop in the separated right-half embryo, implicating the right caudal region, possibly signaling interactions exerted on the anterior right PCM until around stage 23, in determining the direction of the looping of heart tube (Fig. 5).
We additionally examined the involvement of Nodal-related signaling in the heart-tube loop inversion in the separated right-half embryo using bisection experiments. In normal embryos, CyNodal (a nodal-related gene in newts) expression was de-tected from stage 21 to 25 only on the left side of the embryo and was not seen on the right side (Ito et al., 2006). However, in stage-23-25 embryos bisected at stage 15 or 21, CyNodal was ex-pressed in 41% and 38% of the right-half embryos, respectively. Midline structures such as the notochord and the floor plate of neural tube suppress the expression of Nodal on the right sides of embryos (Danos and Yost, 1996; Lohr et al., 1997; Meno et al., 1998). To check for notochord formation in the bisected embryos, we incubated the right-half embryos (bisected at stage 21) until stage 36 when the midline structures are obvious and then prepared the specimens for histological observation. Notochord and a neural tube were clearly formed in 55% of the right-half embryos (n=20, data not shown). Thus, the CyNodal expression rate (38%) in right-half embryos (bisected at stage 21) was approximately equal to the percentage of right-half embryos that did not form notochord (45%). This result is similar to that reported by Lohr et al. (1997) that Xnr-1 (Xenopus nodal-related 1) expression was also seen in the right lateral plate mesoderm of embryos from which the midline structures (including the notochord) had been removed. Although CyNodal expression was noted in 41% and 38% of our right-half embryos separated at stages 15 and 21, respectively, inversion of the heart-tube loop direction was noted in 13% and 80% of the right-half embryos separated at stages 15 and 21, respectively. Furthermore, the CyNodal expression rates were 0% and 100% in the left-half embryos separated at stages 11 and 21, respectively and nearly
A
Fuse into one heart tube
Looping ability
all of the loops of heart tube formed from these embryos were rightward (100% and 96%, respectively). Thus, CyNodal expres-sion in the half embryos was not correlated to the percentage of the looping direction of heart tube.
In conclusion, our study revealed that (1) the PCMs in the right-and left-half embryos independently formed heart-tube loops, (2) the looping directions of the heart tubes from the two halves were reverse when the embryos were separated at early stages and (3) the right PCM and the left PCM fused after both PCMs had gained the capacity to form heart-tube looping in the same direction (rightward looping). However, there are several questions that remain unanswered. How does each PCM loop in opposite directions (rightward looping of the left PCM, leftward looping of the right PCM) in the early stages and how does the fusion of the left PCM looping to the rightward and the right PCM looping to the leftward result in a random looping direction. More extensive study will be needed in the future to address these important issues in cardiac development.
Materials and Methods
Embryos
In this study we used newt (Cynops pyrrhogaster) embryos, as fertilized newt eggs have large diameters (2 mm) allowing easy separa-tion of the right and left halves. Embryos were obtained by injecting human chorionic gonadotropin (100 U) (Gestron, Denka Seiyaku Co., Kanagawa, Japan) into mature female newts twice, one day apart. The embryos were disinfected by immersion in 70% ethanol for 30 seconds and then washed gently with Steinberg’s solution (58 mM NaCl, 0.67 mM KCl, 0.34 mM Ca(NO3)2, 0.83 mM MgCl2, 3 mM N-2-hydroxyethylpiperazine-N’-ethansulfonic acid [HEPES] and 100 mg/l kanamycin sulfate, pH 7.2). The jelly capsule of each embryo was removed with fine forceps. The embryos were subsequently transferred into plastic dishes coated with 3% agarose and filled with Steinberg’s solution. The fertilization membrane of each embryo was removed with fine forceps. The stage of embryonic development was rated in accor-dance with the criteria of Okada and Ichikawa (1947).
Ligation experiment
This experiment was carried out using a modification of the method reported by Spemann and Falkenberg (1919). We used two-cell embryos in which the first cleavage furrow passed the center of the grey crescent and had two blastomeres of the same size. Using a fine enamel-copper wire (0.1 mm in diameter, Nilaco Corp., Tokyo, Japan), each two-cell embryo was ligated along the first cleavage furrow, together with the jelly capsule, into two separate blastomeres. The blastomere located left of the grey crescent was deemed the left-half embryo and that located to the right deemed the right-half embryo. The ligated embryo was incubated until stages 35-37 at 20°C and the heart-tube loop direction was con-firmed. To obtain accurate data, the following cases were excluded from the evaluation: embryos which failed to undergo equivalent separation, incomplete separations, morphologically incomplete embryos and em-bryos that failed to form a heart tube.
Bisection, caudal incision and caudal resection
Embryos were operated using fine tungsten needles under the follow-ing three sets of conditions. In experiment 1, embryos from the early gastrula to tail bud stages (stages 11-25) were bisected along the median sagittal plane and were separated into right- and left-half embryos. In experiment 2, the caudal one-third (1/3 of the posterior part) of embryos was incised along the median sagittal plane at the late gastrula to tailbud stages (stages 15-20). In experiment 3, the embryos at the mid neurula to tail bud stages (stages 18-25) were bisected along the median sagittal
plane, followed by resection of the caudal one-third (1/3 of the posterior part) of each half embryo. The operated embryos were incubated at 20°C until stages 35-37 and the direction of the heart-tube loop was then confirmed. To obtain accurate data, morphologically incomplete embryos and embryos which failed to form a heart tube were excluded from the evaluations.
Whole-mount in situ hybridization
According to the method described elsewhere (Ito et al., 2006), bisected embryos that developed to stages 23-25 were subjected to whole-mount in situ hybridization, using an antisense RNA probe for CyNodal (Cynopus nodal-related gene). We recently showed that CyNodal mRNA expressed in the left lateral plate mesoderm at stages 21-25 (Ito et al., 2006).
Acknowledgements
This work was partly supported by grants from the International Cooperative Research Program (ICORP) of Japan Science and Technol-ogy Agency (JST) to M.A., the National Institute of Advanced Industrial Sciences and Technology (AIST) to M.A., the Life Science Foundation of Japan to K.T. and by a Grant-in-Aid for Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) to S.O.
References
BRANFORD, W.W., ESSNER, J.J. and YOST, H.J. (2000). Regulation of gut and heart left–right asymmetry by context-dependent interactions between Xeno-pus lefty and BMP4 signaling. Dev. Biol. 223: 291-306.
CAMPIONE, M., ROS, M.A., ICARDO, J.M., PIEDRA, E., CHRISTOFFELS, V.M., SCHWEICKERT, A., BLUM, M., FRANCO, D. and MOORMAN, A.F. (2001). Pitx2 expression defines a left cardiac lineage of cells: evidence for atrial and ventricular molecular isomerism in the iv/iv mice. Dev. Biol. 231: 252-264. CAMPIONE, M., STEINBEISSER, H., SCHWEICKERT, A., DEISSLER, K., VAN
BEBBER, F., LOWE, L.A., NOWOTSCHIN, S., VIEBAHN, C., HAFFTER, P., KUEHN, M.R. and BLUM, M. (1999). The homeobox gene Pitx2: mediator of asymmetric left–right signaling in vertebrate heart and gut looping. Develop-ment 126: 1225-1234.
COLLIGNON, J., VARLET, I. and ROBERTSON, E. (1996). Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381: 155–158.
DANOS, M. and YOST, H. (1996). Role of notochord in specification of cardiac left– right orientation in zebrafish and Xenopus. Dev. Biol. 177: 96–103. GARCÍA-CASTRO, M. I., VIELMETTER, E. and BRONNER-FRASER, M. (2000).
N-Cadherin, a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science 288: 1047-1051.
HOYLE, C., BROWN, N. A. and WOLPERT, L. (1992). Development of left/right handedness in the chick heart. Development 115: 1071–1078.
ISAAC, A., SARGENT, M.S. and COOKE, J. (1997). Control of vertebrate left–right asymmetry by a snail-related zinc finger gene. Science 275: 1301-1304. ITO, Y., OINUMA, T., TAKANO, K., KOMOZAKI, S., OBATA, S. and ASASHIMA,
M. (2006). CyNodal, the Japanese newt nodal-related gene, is expressed in the left side of the lateral plate mesoderm and diencephalon. Gene Expr. Patterns. 6: 294-298.
LATACHA, K.S., RÉMOND, M.C., RAMASUBRAMANIAN, A., CHEN, A.Y., ELSON, E.L. and TABER, L.A. (2005). Role of actin polymerization in bending of the early heart tube. Dev. Dyn. 233: 1272-1286.
LEVIN, M. (2005). Left-right asymmetry in embryonic development: a comprehen-sive review. Mech. Dev. 122: 3–25.
LEVIN, M., JOHNSON, R., STERN, C., KUEHN, M. and TABIN, C. (1995). A molecular pathway determining left–right asymmetry in chick embryogenesis. Cell 82: 803–814.
LOWE, L.A., SUPP, D.M., SAMPATH, K., YOKOYAMA, T., WRIGHT, C.V., POT-TER, S.S., OVERBEEK, P. and KUEHN, M.R. (1996). Conserved left–right asymmetry of nodal expression and alterations in murine situs inversus. Nature 381: 158–161.
MÄNNER, J. (2000). Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 259: 248–262.
MCGRATH, J., SOMLO, S., MAKOVA, S., TIAN, X. and BRUECKNER, M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114: 61-73.
MENO, C., SHIMONO, A., SAIJOH, Y., YASHIRO, K., MOCHIDA, K., OHISHI, S., NOJI, S., KONDOH, H. and HAMADA, H. (1998). Lefty-1 is required for left–right determination as a regulator of lefty-2 and nodal. Cell 94: 287–297. MOGI, K., GOTO, M., OHNO, E., AZUMI, Y., TAKEUCHI, S. and TOYOIZUMI, R.
(2003). Xenopus neurula left-right asymmetry is respecified by microinjecting TGF-beta5 protein. Int. J. Dev. Biol. 47: 15-29.
MONSORO-BURQ, A.H. and LE DOUARIN, N. (2000). Left–right asymmetry in BMP4 signalling pathway during chick gastrulation. Mech. Dev. 97: 105-108. MONSORO-BURQ, A.H. and LE DOUARIN, N. (2001). BMP4 plays a key role in
left–right patterning in chick embryos by maintaining sonic hedgehog asymme-try. Mol. Cell 7: 789-799.
NONAKA, S., SHIRATORI, H., SAIJOH, Y. and HAMADA, H. (2002). Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418: 96-99.
OKADA, Y. and ICHIKAWA, M. (1947). Normal table of Triturus pyrrhogaster. Jpn. J. Exp. Morphol. 3: 1-6.
PATEL, K., ISAAC, A. and COOKE, J. (1999). Nodal signalling and the roles of the transcription factors SnR and Pitx2 in vertebrate left-right asymmetry. Curr. Biol. 9: 609-612.
RAMSDELL, A.F. (2005). Left-right asymmetry and congenital cardiac defects:
Getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 288: 1-20.
RAMSDELL, A.F. and YOST, H.J. (1999). Cardiac looping and the left– right axis: antagonism of left-sided Vg1 activity by a right-sided ALK2-dependent BMP pathway. Development 126: 5195-5205.
RAYA, A., KAWAKAMI, Y., RODRIGUEZ-ESTEBAN, C., IBANES, M., RASSKIN-GUTMAN, D., RODRIGUEZ-LEON, J., BUSCHER, D., FEIJO, J.A. and IZPISUA BELMONTE, J.C. (2004). Notch activity acts as a sensor for extracellular calcium during vertebrate left–right determination. Nature 427: 121–128. SPEMANN, H. and FALKENBERG, H. (1919). Uber asymmetrische Entwicklung
und Situs inversus viscerum bei Zwillingen und Doppelbildungen. Arch. Entwick. Org. 45: 371-422.
TABER, L. A. (2006). Biophysical mechanisms of cardiac looping. Int. J. Dev. Biol. 50: 323-332.
TANAKA, Y., OKADA, Y. and HIROKAWA, N. (2005). FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 435: 172-177.
TOYOIZUMI, R., OGASAWARA, T., TAKEUCHI, S. and MOGI, K. (2005). Xenopus nodal related-1 is indispensable only for left-right axis determination. Int. J. Dev. Biol. 49: 923-938.
YU, X., ST AMAND, T.R., WANG, S., LI, G., ZHANG, Y., HU, Y.P., NGUYEN, L., QIU, M.S. and CHEN, Y.P. (2001). Differential expression and functional analysis of Pitx2 isoforms in regulation of heart looping in the chick. Develop-ment 128: 1005-1013.
The International Journal of Developmental Biology
UBC Press - Editorial Service University of the Basque Country E-48940 Leioa, (Vizcaya) SPAIN
ORDER BY
Web: http://www.intjdevbiol.com
E-mail: ijdb@ehu.es
(include the information indicated above)FAX:
+34-94-601-3266
Preface
by E.M. De Robertis and J. Aréchaga
HERITAGE OF THE 1924 ARTICLE BY HANS SPEMANN AND HILDE MANGOLD
Introducing the Spemann-Mangold organizer: experiments and insights that generated a key concept in developmental biology
by K. Sander and P. Faessler
Induction of embryonic primordia by implantation of organizers from a different species Reprint of the original 1924 article by Hans Spemann and Hilde Mangold, translated into English by Viktor Hamburger
Developmental biology of amphibians after Hans Spemann in Germany by H. Grunz
Spemann´s heritage in Finnish developmental biology by L. Saxén
Spemann´s influence on Japanese developmental biology by M. Asashima and T. Okada
Contribution of the Belgian school of embryology to the concept of neural induction by the organizer
by H. Alexandre
Contrasting influences of the organizer and induction concepts on the scientific activity of French embryologists
by J.-C. Beetschen and A.-M. Duprat
Consequences of the Spemann-Mangold organizer concept for embryological research in Russia: personal impressions
by A.T. Mikhailov and N.A. Gorgolyuk
The organizer concept and modern embryology: Anglo-American perspectives by T. Horder
THE ORGANIZER CONCEPT: OVERVIEWS AND THEORETICAL APPROACHES
Evolution of the organizer and the chordate body plan by J. Gerhart
Continuity and change: paradigm shifts in neural induction by S. Gilbert
Formation and maintenance of the organizer among vertebrates by K. Joubin and C.D. Stern
Organizer and axes formation as a self-organizing process by H. Meinhardt
ORGANIZER RESEARCH TODAY
Molecular mechanisms of cell-cell signalling by the Spemann-Mangold organizer by E.M. De Robertis, O. Wessely, M. Oelgeschläger, B. Brizuela, E. Pera, J. Larraín, J. Abreu and D. Bachiller
Formation of a functional morphogen gradient by a passive process in tissue from the early Xenopus embryo
by N. McDowell, J.B. Gurdon and D.J. Grainger
A study of Xlim1 function in the Spemann-Mangold organizer
by L. Kodjabachian, A.A. Karavanov, H. Hikasa, N.A. Hukriede, T. Aoki, M. Taira and I.B. Dawid
Making mesoderm - upstream and downstream of Xbra by J. Smith
Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway
by J.B. Wallingford, K.M. Vogeli and R.M. Harland
Generation of the germ layers along the animal-vegetal axis in Xenopus laevis by H. Yasuo and P. Lemaire
Dickkopf1 and the Spemann-Mangold head organizer by C. Niehrs, O. Kazansakaya, W.Wu, and A. Glinka
Siamois cooperates with TGFβββββ signals to induce the complete function of the Spemann-Mangold organizer
by M.J. Engleka and D. S. Kessler
ORDER FORM
I would like to order ____ cop(y/ies) of the Int. J. Dev. Biol. Special Issue “The Spemann-Mangold Organizer” (Vol. 45, Nº 1)
at US$ 70 or Euro 70 per copy (including post and packaging). Total to be charged: __________US$ / Euro (please specify currency)
Organizer
Edited by Eddy M. De Robertis and Juan Aréchaga
The Spemann-Mangold organizer: the control of fate specification and morphogenetic rearrangements during gastrulation in Xenopus
by T. Bouwmeester
Functional analysis of the Xenopus frizzled 7 protein domains using chimeric receptors by R.K. Swain, A. Medina and H. Steinbeisser
Fox (forkhead) genes are involved in the dorso-ventral patterning of the Xenopus
mesoderm
by H. El-Hodiri, N. Bhatia-Dey, K. Kenyon, K. Ault, M. Dirksen and M. Jamrich
In vitro induction systems for analyses of amphibian organogenesis and body patterning
by T. Ariizumi and M. Asashima The avian organizer
by T. Boettger, H. Knoetgen, L. Wittler and M. Kessel Nodal signaling and the zebrafish organizer
by A.F. Schier and W.S. Talbot
The role of the homeodomain protein Bozozok in Zebrafish axis formation by L. Solnica-Krezel and W. Driever
Role of the anterior visceral endoderm in restricting posterior signals in the mouse embryo
by A. Perea-Gomez, M. Rhinn and S.-L. Ang
Roles of Sox factors in neural determination: conserved signaling in evolution? by Y. Sasai
Getting your head around Hex and Hesx1 : forebrain formation in mouse by J.P. Martínez Barbera, R.S.P. Beddington
The role of Otx2 in organizing the anterior patterning in mouse by A. Simeone and D. Acampora
Defects of the body plan of mutant embryos lacking Lim1 , Otx2 or Hnf3βββββ activity by S.J. Kinder, T.E. Tsang, S.-L. Ang, R.R. Behringer and P.P.L. Tam
Otx2 and Hnf3βββββ genetically interact in anterior patterning by O. Jin, K. Harpal, S.L. Ang and J. Rossant The isthmic organizer and brain regionalization
by S. Martínez