LATENT NONSTRUCTURAL DIFFERENTIATION AMONG
HOMOLOGOUS CHROMOSOMES AT THE DIPLOID
LEVEL: CHROMOSOME 6B' OF
AEGILOPS LONGZSSIMA
RAMA
s.
KOTA, PATRICK E. MCGUIRE AND JAN DVORAKDepartment of Agronomy and Range Science, University of CaEi$ornaa, Davis, Calqornia 9561 6
Manuscript received February 1 1, 1986 Accepted May 1 5 , 1986
ABSTRACT
Previous work has shown that chromosome pairing at metaphase I (MI) of wheat homologous chromosomes from different inbred lines (heterohomologous chromosomes) is reduced relative to that between homologous chromosomes within an inbred line (euhomologous chromosomes). In order to determine if a potential for this phenomenon exists in diploid species closely related to the wheat B genome, MI chromosome pairing was investigated between euhomolo- gous and heterohomologous 6B" (=6Sc) chromosomes, each from a different population of Aegilops longissima Schweinf. et Muschl. ( 2 n = 2x = 1 4 ) substituted for chromosome 6B of Chinese Spring wheat (Triticum aestivum L., 2 n = 6x =
4 2 ) . Euhomologous and heterohomologous monotelodisomics, i.e., plants with one complete chromosome 6B"and a telosome of either 6B"p or 6 ~ q , were constructed in the isogenic background of Chinese Spring. Pairing at MI of the
Ae. longissima chromosomes was reduced in heterohomologous monotelodisomics compared to that in the corresponding euhomologous monotelodisomics. T h e remaining 2 0 pairs of Chinese Spring chromosomes paired equally well in the euhomologous and heterohomologous monotelodisomics. Thus, the cause of the reduced pairing must reside specifically in the Ae. longissima heterohomologues. In the hybrids between the Ae. longissima lines that contributed the substituted chromosomes, pairing between the heterohomologous chromosomes was normal and did not differ from that of the euhomologous chromosomes. These data provide evidence that a potential for reduced pairing between the heteroho- mologues is present in the diploid species, but is expressed only in the polyploid wheat genetic background. T h e reduction in heterohomologous chromosome pairing was greater in the p arm than in the q arm, exactly as in chromosome
6B of wheat. It is concluded that the reduced pairing between Ae. longissima
heterohomologues has little to do with constitutive heterochromatin. T h e value of chromosome pairing as an unequivocal means of determining the origin of genomes in polyploid plants is questioned.
N bread wheat, Triticum aestivum L. (2n = 6x = 42, genome formula
I
AABBDD), homologous chromosomes from different inbred lines (hetero- homologous chromosomes) pair at meiosis less regularly in hybrids between inbred lines than do homologous chromosomes from the same inbred line (euhomologous chromosomes). The three wheat genomes consistently differ in580 R .
s.
KOTA, P. E. MCGUIRE AND J.DVOEAK
the magnitude of difference between heterohomologous and euhomologous chromosome pairing. T h e B-genome chromosomes show the greatest differ- ences, the D-genome chromosomes show almost none and the A-genome chro- mosomes are intermediate between the B- and D-genome chromosomes (Dvo-
~ A K
and MCGUIRE 1981). T h e occurrence of this phenomenon in polyploid wheat may be related to the activity of genes that prohibit met.aphase I (MI) chromosome pairing between homoeologous chromosomes and, in a sense, treat the heterohomologous chromosomes as homoeologues. Since this phe- nomenon has not been reported elsewhere, with a possible exception of poly- ploid oats, it is not known whether it is unique to wheat or if the potential for it exists in other species.Bread wheat A- and B-genome chromosomes could be more polymorphic among inbred lines than the D-genome chromosomes because of introgressive hybridization between T. aestivum and T. turgidum L. ( 2 n = 4x = 28, genome formula AABB). Variation in the A- and B-genome chromosomes of hexaploid wheat could have been enhanced by introgression from the A and B genomes of T. turgidum to the A and B genomes of T. aestivum, whereas the D genome, which does not have a counterpart in T. turgzdum, would not have been af- fected. A similar model can be used to explain greater variation in the B genome than in the A genome if there were introgressive hybridization of the type AAB'B" X BJBr (x and y stand for differentiation of the B genomes) or AAB'B" X AABJBY as was proposed by ZOHARY and FELDMAN (1962). If modi- fication of the A and B genomes by introgression at the polyploid level is the primary reason for the observed reduced pairing of heterohomologous chro- mosomes relative to the pairing of euhomologous chromosomes, a potential for this phenomenon would not exist at the diploid level. However, if this potential does exist and, particularly, if in the wheat genetic background it is manifested by reductions in MI pairing between heterohomologous chromo- somes of a diploid species of magnitudes similar to those observed for B- genome heterohomologous chromosomes, then it would be likely that this chromosome behavior is intrinsic to a given genome. Modification of genomes by introgression would become an inconsequential issue.
This problem can be addressed if chromosomes from several populations of each diploid species related to the three genomes of wheat are introduced individually into a single genotype of wheat and if the levels of their euho- mologous and heterohomologous MI pairing are determined. This paper re- ports the pairing behavior of three homologous 6 B p chromosomes of Aegzlops
longzssima Schweinf. et Muschl. ( 2 n = 2x = 14, B%") individually substituted for chromosome 6B of Chinese Spring wheat. If the poor MI pairing of the wheat B-genome heterohomologues is an intrinsic attribute of the molecular architecture of the B genome, then the euhomologues and heterohomologues of Ae. longissima will behave like those of the wheat B genome, i.e., the euho- mologues will pair reasonably well, but heterohomologues will pair relatively poorly. However, if the poor pairing of the wheat B-genome heterohomologues
TABLE 1
Description of the chromosomal stocks
Stock Chromosome constitution
Disomic substitution 6Bq6B)-1, -2, Twenty pairs of Chinese Spring chromosomes plus a pair of 6Br chromosomes from Ae. longissima line 1 , 2 or 3 substituted for 6 8 of wheat
Twenty pairs of Chinese Spring chromosomes plus a pair of long ( p ) arm telosomes of chromosome 6B'of Ae. lon- gissima line 1 substituted for 6B of wheat
Twenty pairs of Chinese Spring chromosomes plus a pair of short (4) arm telosomes of chromosome 6 H o f Ae. lon- gissima line 2 substituted for 6B of wheat
Twenty-one pairs of Chinese Spring chromosomes plus a pair of long (p) arm telosomes of chromosome 6 P o f Ae. longissima
or -3"
Ditelosomic substitution 6B"p(6B)-l
Ditelosomic substitution 6BG(6B)-2
Ditelosomic addition" 6B/p-3
~~ ~ ~ _____ _ _ _ _ _ _ ~ ~
~ _ _ _ _ _ ~
a The 6Bf6B)-3 and 6B"p-3 stocks were developed by N. A. TULEEN from a complex chromo-
somal stock developed by FELDMAN (1975).
level, the Ae. longissima heterohomologues and euhomologues should pair equally well.
Although this phenomenon has not been reported at the diploid level, it is possible that differences between the pairing of euhomologous and heteroho- mologous chromosomes exist but that they are minor and have escaped atten- tion. To assess this possibility, the levels of chromosome pairing in several lines of Ae. longissima, which is a natural inbreeder, including those that were the sources of chromosomes in the disomic substitutions, and in hybrids among them were determined.
MATERIALS AND METHODS
Genotypes: T h e description and designation of chromosomal stocks is listed in Table
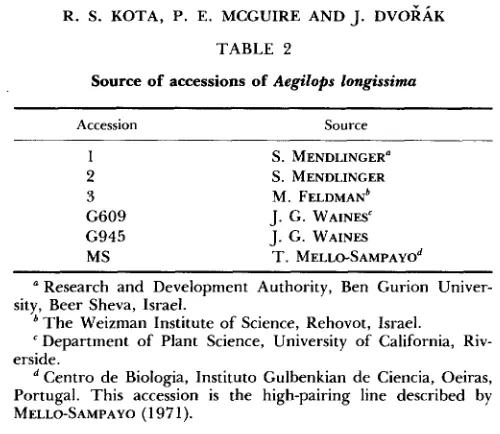
1 . T h e nomenclature of aneuploids proposed by KIMBER a n d SEARS (1968) was used. T h e development of disomic substitutions 6B76B)-1 a n d 6Bf6B)-2 a n d ditelosomic sub- stitutions 6B%(6B)-l and 6BtS(6B)-2 from accessions 1 a n d 2 of Ae. longissima was described by KOTA a n d DVOGAK (1985). Disomic substitution 6Bq6B)-3 a n d ditelosomic addition 6 B 2 - 3 were developed and supplied by N. A. TULEEN, Texas A&M University, College Station, Texas from a complex chromosomal stock developed by FELDMAN
(1975) (Table 2).
5 8 2 R. s. KOTA, P . E. MCGUIRE AND J.
DVOGK
TABLE 2Source of accessions of Aegilops longissima
Accession Source
~ ~~~
1 S. MENDLINGER" 2 S. MENDLINGER 3 M. FELDMAN~ G609 J. G. WAINES" G945 J. G. WAINES
MS T . MELLO-SAMPAYO~ Research and Development Authority, Ben Gurion Univer-
The Weizman Institute of Science, Rehovot, Israel.
Department of Plant Science, University of California, Riv- erside.
Centro de Biologia, Instituto Gulbenkian de Ciencia, Oeiras, Portugal. This accession is the high-pairing line described by MELLO-SAMPAYO (1 97 1).
sity, Beer Sheva, Israel.
Chinese Spring produce symmetrical bivalents. Thus, the Ae. longissima chromosome pair can be unequivocally distinguished from the Chinese Spring chromosomes in MI configurations.
MI pairing: Eight monotelodisomic plants from each F1 progeny were grown in a greenhouse. Immature spikes were fixed in 6:3:1 (ethanol, chloroform, acetic acid, v/ v) Carnoy's solution a n d were stored in 70% ethanol at 2-5". Squashes were made in 1 % acetocarmine. Slides were systematically scanned, and 100 pollen mother cells (PMCs) per plant were analyzed for the pairing behavior of the telosome with its homologue. T h e pairing frequency of each telosome was expressed as a percentage of the total number of cells scored in each plant. T h e analysis of variance (ANOVA) of the mean telosome pairing percentages was made on angularly transformed values using a completely randomized design with equal sample size. Comparisons among the means were made using least significant differences (LSDs).
T o assess the genetic effects of the substituted chromosome on MI pairing, the number of ring bivalents a n d univalents other than the heteromorphic pair per cell were scored in at least 30 cells per plant. Significance of variation of the means from the control was determined by ANOVAs, followed by comparisons using LSD. For the mean percentage of cells with univalents, ANOVA was conducted on angularly trans- formed values of mean percentage of cells with univalents. Statistical significance was determined as above.
T h e accessions of Ae. longissima were maintained as selfed populations, and hybrids were made among them. From the resulting F1 plants and some siblings of the parents, immature spikes were fixed in 6:3:1 Carnoy's solution and were stored in 70% ethanol at 2-5". Squashes were made in 1 % acetocarmine. At least 100 PMCs per plant were analyzed at MI for frequency of ring bivalents and univalents. T h e mean numbers of configurations were compared among the parents a n d hybrids by an ANOVA with a complete randomized design and unequal sample size. Pollen viability for parents and hybrids was measured as percentage of grains for which the cytoplasm stained with 1 %
acetocarmine.
C-banding: Root tips were cut from the seedlings of disomic substitutions and pre- treated at 2 " in distilled H 2 0 for 16 hr. They were then fixed in 45% acetic acid for 12 hr, and C-bands were revealed following a procedure described by DVOGK and
APPELS ( 1 982).
583
I
2
3
FIGURE I.-Gbanded 6E'chromosomes of DS 6B'(6B)-l, DS 6B'(6B)-2 and DS 6B'(6B>3.
ing to their size in a 1.5% agarose horizontal-slab gel. T h e DNAs were then denatured and transferred o n t o nitrocellulose (Schleicher and Schuell) according to the manufac- turer's specification. T h e blot was hybridized with a J2P-labeled probe of PTA 250.15
as described by APPELS and D V O ~ A K (1982). T h e clone PTA 250.15 is the 750-base- pair (bp) HhaI fragment of the nontranscribed spacer separating the 18s a n d 26s rRNA genes (APPELS and D V O ~ A K 1982). T h e nitrocellulose blot was then washed, dried and a u toradiographed.
Gliadin electrophoresis: Endosperm of a single seed was ground with acid-washed sand in a mortar and pestle. T h e flour was extracted with 0.2 ml of 1.5 M aqueous solution of NJdimethylformamide (KASARDA 1980). T h e extracts were electropho- resed in 6% polyacrylamide vertical-slab gels using a lactate buffer, p H system 3.1,
according to a procedure of L A U R I ~ R E and Moss6 (1 982).
RESULTS
Genetic relationships among the 6B' chromosomes: To describe the three
Ae. longissimu 6B' chromosomes involved in the study, polymorphism was in- vestigated in 1 1 C-bands, the rDNA locus (Nor-BQ) and a gliadin locus (Gli-
6B2). T h e long arms of the 6B'chromosomes in DS 6Bf6B)-l and DS 6B/(6B)- 2 were identical both in size and location of C-bands, but both differed in C- bands 2 and 3 from chromosome 6B'in DS 6B76B)-3 (Figure 1; Table 3). T h e short arms of all three chromosomes differed in their C-band patterns. Chro- mosomes 6BcI and 6BC2 differed in bands 6 and 7; 6Bc2 and 6Bc3 differed in bands 5, 6 and 7; and 6Bc2 and 6E-3 differed in band 5 (Figure 1; Table
Ribosomal RNA locus Nor-B2 on chromosome 6B of Chinese Spring in wheat is characterized by two fragments 2.7- and 2.8-kb long cleaved from the non- transcribed spacer by TuqI endonuclease (APPELS and DVO~AK 1982). In all three disomic substitutions those two fragments were absent and were replaced by a fragment 2.6-kb long in disomic substitutions DS 6B/(6B)-1 and DS 6B/(6B)-2 and 2.4-kb long in disomic substitution DS 6B/(6B)-3 (Figure 2). I t was previously shown that the Nor-B/2 locus is in the long arm of chromosome ~B'(KOTA and
DVOGAK
1985).584 R. s. KOTA, P. E. MCGUIRE AND J. D V O ~ \ , ~ K
TABLE 3
Relationships among the 6B/chromosomes from disomic s u b ~ t i t u t i ~ ~ 1, 2, and 3 of Ae. longirsirrur substituted for chromosome 6 8 of Chinese Spring
~~
Disomic substitution 6Bq6B)
A m Region 1 2 3
c4 a* a a
Gli-B? a b C
C 3 a a b
Nor-B? a a b
C-2 a a b
C-1 a a a
Centromere C-1 a a b
C 2 a a a
C-3 a a a
c4 a a a
d=S) C 5 a a b
G 6 a b b
C 7 a b a
The Cbands are designated by a number that indicates their position rela- tive to the centromere. T h e centromeric band is C-1 in each arm, whereas the telomeric band is C 4 in the p arm and C 7 in the q arm. The Cband at the
N o r - m locus was not considered since the Nor-EM locus is described molecu- larly. The N 0 r - m locus is placed relative to the centromere and the Cbands. The Gli-B? locus has not been mapped relative to Cbands. I t s position is tentative and based on the location of the wheat Gli-E2 locus ( D v o ~ ~ K and
CHEN 1984).
a Genetic stocks with the same letter are similar.
I 2 3 4 5 6 7 8 9 1 0
,3.5kb
,3.1
k b
,2.8kb
'2.6
kb
'2.5kb
'2.4
kb
'2.3 kb
-2.7
kb
-1.7kb
FIGURE 2.-Southem blots of genomic DNAs of (1) Chinese Spring, (2) amphiploid (CS X Ae.
lon&sima-2), (3) Ae. longissima-2. (4) DS 6B'(6B)-2. (5) amphiploid (CS X Ae. longissimu-3). (6) Ae.
longissimn-3, (7) DS 6B'(6B)-3. (8) amphiploid (CS X Ae. longissima-1), (9) Ae. longisSima-1 and (IO) DS 6B'(6B)-1. The 3.5- and 3.1-kb bands are from CS chromosome I B the 2.8- and 2.7-kb bands are from CS chromosome 6 B the 2.6-kb band in lanes 2, 3, 4, 8, 9 and 10 is from Ae. longissimCr
585
I 2 3 4 5
, 1
FIGURE 3.-Electrophoretic profiles of gliadins of ( I ) DS 6B'(6B)-l, (2) DTS 6B$(6B)-1 (=DTS
6B'L(6B>1), (3) DS 6B'(6B)-2, (4) DS 6B'(6B)-3. and (5) Chinese Spring. Arrows indicate the bands assigned to Ae. longhima 6B'chromosomes.
arm of chromosome
6B/,
because the electrophoretic profiles of the ditelosomic substitution 6B%(6B>1 and the disomic substitution 6B/(6B>1 are the same (Figure 3).T h e loci found on the long arm of Ae. longissima chromosome 6B'; Nor-BQ and Gli-B/2, are in the
p
arm of wheat chromosome 6B. This indicates that the long arm of Ae. longissima chromosome 6B/is homoeologous with thep
arm of wheat chromosome 6B. Since the short arm of chromosome 6B/compensates for chromosome 6B and is, thus, homoeologous with one of the arms of wheat chromosome 6B (KOTA and DVO~AK 1985), it must be homoeologous with theq arm of wheat chromosome 6B. Therefore, the long and short arms of chro- mosome 6B'will henceforth be designated 6B$ and 6@q, respectively, in accord with the meaning of
p
and q as defined by SEARS and SEARS (1979). However, thep
and q arms of the Ae. longissima chromosome 6B/may differ from wheat chromosome 6B by a pericentric inversion (KOTA andDVO~AK
1985).586 R .
s.
KOTA, P. E. MCGUIRE AND J.~ v 0 6 . i ~
TABLE 4
Chromosome pairing at MI in lines of Ae. longissima and their hybrids
Mean no
Ring bivalents Line o r hybrid No. of plants No. of cells Univalents (range) (range)
1 2 G609 G945 MS Mean
1 x 2
G609 X G945
MS X G945 1 X MS MS X 2
Mean 224 238 819 664 573 240 339 675 339 231 0.000 0.000 0.024 0.003 0.014 (0-2) (0-2) (0-2) 0.008 0.008 (0-2) 0.006 (0-2) 0.006 (0-2) 0.012 (0-2) 0.026 (0-2) 0.012 6.51 6.40 6.36 6.43 6.42 (4-7) (4-7) (3-7) (3-7) (3-7) 6.42 6.32 6.58 6.44 6.39 6.36 (4-7) (4-7) (4-7) (4-7) (3-7) 6.42
DS 6BP(6B)-2 and between DS 6Bq6B)-2 and DS 6Bq6B)-3 were 0.71. T h e similarity ratio between DS 6Bq6B)-1 and DS 6Bq6B)-3 was 0.57.
Hybrids between Ae. longissima lines: Three parents and all hybrids had at least some cells with two univalents (Table
4).
No more than two univalents were observed in any cell. No indication of structural chromosome rearrange- ments among the lines was observed. The ANOVA for the number of ring bivalents per cell and for the number of univalents per cell yielded nonsig- nificant F-values indicating the hybrids and parents did not differ in levels of MI pairing. T h e means of the mean numbers of univalents and ring bivalents of the parents did not differ from those of the hybrids. Hence, the MI pairing between Ae. longissima heterohomologues was not reduced relative to that between euhomologues. All plants, parents and hybrids had pollen viability of7 5 % or greater.
587
TABLE 5
Mean telosome pairing (percentage of cells with telosome p a i d ) at MI in the monotelodisomics of Ae. Iongissimu chromosomes 6&-X,-Z and -3
Mean no.
Chromo- Complete Maximum Mean percentage of of ring Mean percentage some chromosome Chromo- MI pairing cells in which telo- bivalents of cells with univa-
arm X telosome some no. configurations some paired“ per cell lents“,b
~~
P 1 x 1 41
+
t 2 0 ” + t l ” 83.9 a 3 x 3 42+
t 20”+
t l ”+
1’ 84.3 a2 x 1 41
+
t 20”+
t l ” 64.9 c 3 x 1 41+
t 20”+
t l ” 33.5 b 1 x 3 42+
t 20”+
t l ”+
1’ 34.6 b 2 x 3 42+
t 20”+
t l ”+
1’ 32.9 b16.9 a
15.5 b
16.6 a 16.7 a
15.6 b 15.5 b
13.2 a 95.6 b 17.7 a 11.4 a 23.6 c 92.2 b 9 2 x 2 4 1 + t 2 0 ” + t l ” 86.4 a
1 x 2 41
+
t 20”+
t l ” 83.7 a 3 x 2 41+
t 20”+
t l ” 65.7 c16.5 a 16.5 a 15.8 a
10.7 a 11.0 a
12.7 a Means followed with the same letter are not statistically significant. Means with different letters are statistically significant at the 5% level of probability.
*
Univalents derived from the heteromorphic pair of 6B‘chromosomes or the extra 6B chro- mosome in plants involving the ditelo addition 6B$-3 are not included.frequency of about 84% of cells (Table 5). Compared to the euhomologues, the telosome pairing frequency was significantly lower in the heterohomolo- gous monoteIodisomics. T h e p-arm telosome of DTS 6B“‘p6B)- 1 paired signif- icantly better with chromosome 6B“of DS 6B$(6B)-2 than it did with chro- mosome 6B‘of DS 6B76B)-3. There was no significant difference between the pairing of the p-arm telosome of DTS 6B““p6B)-3 with chromosome 6B‘from DS 6B76B)-1 and DS 6B76B)-2.
T h e q-arm telosome was derived from DS 6BP(6B)-2. In the euhomologous monotelodisomic, the telosome paired in 86.4% of cells (Table 5). T h e pairing of the telosome with its heterohomologues from DS 6Bf6B)-1 and DS 6B76B)- 3 was lower, but the difference was significant only for the monotelodisomic involving chromosome 6B‘ of DS 6B76B)-3.
588 R. s. KOTA, P. E. MCGUIRE AND J.
D V O G ~ K
longissima chromosomes was higher in both euhomologous and heterohomol- ogous monotelodisomics involving this telosome (Table 5 ) . These data indicate that one of the Chinese Spring chromosome pairs had greatly reduced MI pairing in monotelodisomics involving DTA 6B9-3. There is probably a mod- ified Chinese Spring chromosome other than 6B in DTA 6B9-3. T h e ditelo- somic addition 6B9-3 was derived from a complex derivative (N. TULEEN, personal communication), and it is possible that one of the Chinese Spring chromosomes might be a recombinant between a wheat chromosome and an
Ae. longissima chromosome. Except for this exceptional chromosome, the pair- ing behavior of the remaining 19 Chinese Spring chromosomes was similar in all monotelodisomics. Thus, reduction of pairing observed among the hetero- homologous Ae. longissima chromosomes relative to the euhomologous chro- mosomes was specific to heterohomologous chromosomes and did not occur between Chinese Spring euhomologues in the same cells.
DISCUSSION
Previous studies established that wheat B-genome chromosomes have a strong tendency to pair poorly at MI with homologous chromosomes from other wheat cultivars. These studies also showed that this tendency is primarily caused by reduced likelihood for crossing over (DvoGAK and MCGUIRE 1981; DVOGAK and APPELS 1986). T h e present study revealed that a potential of equal magnitude for reduced MI pairing between heterohomologues also exists in a diploid species, Ae. longissima, which has a genome closely related to the wheat B-genome. T h e data are admittedly limited to only one chromosome, but since the chromosome was chosen without any prior knowledge of its pairing behavior, it is probable that the other Ae. longissima chromosomes would behave in a similar manner.
Whether the potential is going to be manifested or not is determined by the physiological environment of the meiocyte, which itself is determined by the genotype. In bread wheat, the
p
arm of Ae. longissima chromosome 6Bcl failed to pair with its homologue 6B42 in 35.1% of cells, and the q arm of 6Bc2 failed to pair with chromosome 6Bcl in 16.3% of cells. If the probability of the pairing failure of a complete chromosome is a product of these two prob- abilities, the complete chromosomes 6Bcl and 6Bc2 would be expected to fail to pair in 5.7% (35.1 X 16.3) of cells. However, in the hybrid between Ae.longissiha lines 1 and 2, only 0.5% of cells had a chromosome pair failing to pair at MI. Thus, the failure of pairing of Ae. longissima 6B"chromosomes in the genetic background of wheat is ten times greater than that of all seven Ae.
longissima chromosomes in the genetic background of the diploid.
In the wheat genetic background, pairing between Ae. longissima homologues was assessed between a telosome and a complete chromosome, but in Ae.
arms of normal bibrachial chromosomes (SALLEE and KIMBER 1979; DVO~AK and MCGUIRE 1981). It is likely that the Ae. longissima chromosomes behave like wheat chromosomes in this sense. Thus, the great discrepancy between the MI pairing of Ae. longissima chromosomes in the wheat genetic background and that in Ae. longissima was probably not caused by the telocentric state of the chromosomes.
Euhomologous Ae. longissima chromosomes also pair more poorly in the genetic background of wheat than in that of the diploid. From the pairing data for the
p
arm of 6Bcl and the q arm of 6Bc.2, the expected frequency of pairing failure between euhomologous 6B" chromosomes is 2.19% (1 6.1 X13.6%) of cells, which is five times greater than that of all seven Ae. longissima euhomologues in the genetic background of the diploid. T h e level of MI pairing of euhomologous Ae. longissima 6B"chromosomes in wheat is similar to that of wheat chromosome 6B, which fails to pair in 0.8% of cells ( D v o ~ ~ K and MCGUIRE 1981).
T h e similarity in pairing behavior of chromosomes 6B"and 6B in the wheat background goes even further. In every pair of wheat 6B heterohomologues investigated, the level of MI pairing of the
p
arms was much more reduced than the q arm. T h e Ae. longissima chromosome also shows much greater reduction in the level of MI pairing in thep
arm than in the q arm. All these facts create the inescapable impression that the pairing behavior of wheat chromosome 6B is determined by its molecular architecture and that this ar- chitecture has probably not changed since the divergence of the B-genome diploid species of the Sitopsis section of Aegilops. Since there is no significant difference in the MI pairing levels between euhomologous and heterohomol- ogous chromosomes in the diploid genetic background, chromosome 6B of wheat might not show reduced heterohomologous pairing if it were incorpo- rated into Ae. longissima.D V O ~ ~ K and MCGUIRE (1981) and
ENDO
and GILL (1984) speculated that the reduced MI pairing between heterohomologues is caused by repeated se- quences of constitutive heterochromatin, which are more abundant in the B genome than in A and D genomes. Subsequent work (Dvo~AK and APPELS1982; J. D v o ~ ~ K , unpublished results), however, challenged this hypothesis. T h e present data provide additional evidence against it. If this hypothesis were true, the
p
arm of chromosome 6B'would be expected to pair better than theq arm since it is less heterochromatic than the q arm. However, the
p
arm heterohomologues pair more poorly than those of the q arm. Thus, the amount of heterochromatin has little or no relationship to the level of MI chromosome pairing.590 R. s. KOTA, P. E. MCGUIRE AND J.
DVOGAK
chromosomes in wheat is caused by their structural differentiation (defined as the rearrangement of a chromosome that alters its linear order of loci). If so, it would have to be assumed that the rearrangements involved both arms in more than two chromosomes.
T h e level of pairing of the GB'euhomologues in the nionotelodisomics, which contain two exact copies of a 6B"chromosome arm, is reduced in the wheat genetic background relative to the pairing of 6B"euhomologues in the genetic background of Ae. longissima. Thus, it seems that the physiological control of meiosis in wheat, which prevents recombination between homoeologues, may also affect homologous recombination by reducing the likelihood of crossing over between both euhomologous and heterohomologous Bcgenome chromo- somes. T h e reductions are, however, more striking for the heterohomologues that are treated as if they were homoeologues. Additionally, for some unknown reason, the B-genome chromosomes are much more affected than the A- or D- genome chromosomes ( D v o ~ ~ K and MCGUIRE 198 1).
Currently, it can only be speculated why the B-genome and the 6B' hetero- homologues are more affected by the physiological control of wheat meiosis than are the euhomologues. Of the three GB'chromosomes, 6Bcl and 6Bc.2 paired better with each other than either did with 6Bc3. Comparison of these three chromosomes in 11 C-bands, the rDNA locus and the gliadin locus revealed that chromosomes 6Bc1 and 6Bc2 were more alike. It seems reason- able to assume that these chromosome regions are not exceptional, but rep- resent the overall similarity in the nucleotide sequences of the three chromo- somes. While these arbitrarily selected regions may have nothing to do with the meiotic process, some of the nucleotide sequences on these chromosomes must play a vital role for homologous recombination. The parallel between the level of the MI pairing and the level of polymorphisni between homologues would indicate that heterozygosity in specific or all nucleotide sequences of these 6Bp chromosomes is recognized by the physiological control of meiosis, with the result that these chromosomes are treated as if they were homoeo- logues of different degrees of relatedness.
It was shown here that H-genome heterohomologous chromosomes that crossover and pair well at MI at the diploid level are unable to undergo a single crossover (and are unpaired) in a large percentage of MI cells in a polyploid genetic background. It was concluded that this striking contrast is an intrinsic attribute of the B-genome molecular architecture. From this prem- ise, it is predicted that all diploid and polyploid species with this type of molecular architecture will display a chromosome pairing behavior similar to that of the wheat B-genome chromosomes or those of Ae. longissima. Thus, it should not be surprising that the chromosomes of diploid species of Aegilops section Sitopsis, which includes Ae. longissima, do not appreciably pair with chromosomes of the B genome of wheat if the P h l gene is active (RILEY 1960; KIMBER and ATHWAL 1972), although their C-banding (DvoGAK 1983a) and other attributes (SARKAR and STEBBINS 1956; RILEY, UNRAU and CHAPMAN
Neither should it be surprising that B-genome chromosomes pair poorly in interspecific hybrids between T. aestivum and T. timopheevii (Zhuk.) Zhuk. (FELDMAN 1966; D VO ~A K and APPELS 1982). Extensive modification of all € 4 chromosome arms would have to be involved to explain the poor pairing of the B-genome chromosomes in T. aestivum X T . timopheevii hybrids by intro- gressive hybridization, as do ZOHARY and FELDMAN (1962). T h e 6Bp arm of T. aestivum paired in hybrids with two different lines of T. timopheevii ssp.
araraticum in 9.4 and 13.5% of cells (Dvo~AK and APPELS 1982). However, chromosome arms 6B9 of Ae. longissima that are homologous and pair regu- larly at the diploid level paired with a frequency as low as 32.9% of cells in the wheat genetic background; thus, the low level of pairing between the 6Bp arms of T. aestivum and T. timopheevii appears less significant. Consequently, the boundary between homology and homoeology is clearly arbitrary. T h e poor pairing between Ae. longissima heterohomologues reported here, between wheat B-genome heterohomologues ( D v o ~ ~ K and MCGUIRE 198 1 ) and be- tween B-genome homoeologues (LILIENFELD and KIHARA 1934; FELDMAN
1966; MILLER 1981), appears to be an intrinsic attribute of these chromosomes that becomes apparent in the genetic background of polyploid wheat. In view of these findings, whether introgressive hybridization played a role in modifi- cation of Triticum genomes becomes a less important or even an inconsequen- tial issue.
T h e arbitrary nature of the distinction between homology and homoeology when based on M I chromosome pairing is an important factor to consider in determinations of genome relatedness. T h e paradoxical behavior of the Ae. Eongissima chromosomes shown here demonstrated that it is unjustified to use chromosome pairing as a simple universal measure of genome relatedness. T h e inability to find evidence by MI chromosome pairing for genome differentia- tion at the diploid level does not necessarily mean that the genomes in question do not possess a latent differentiation of some degree. This situation may apply to all species of Aegilops section Sitopsis or to the diploid species of Solanum, as pointed out by DVO~AK (1983b). On the other hand, it is equally faulty to assume that observed poor o r no pairing at the polyploid level implies that genomes are greatly differentiated. This situation may apply to chromosome pairing between the B genomes of Triticum species or between the B genomes of Triticum and Aegilops section Sitopsis species.
LITERATURE CITED
APPELS, R. and J. D v o ~ ~ K , 1982
D v o ~ ~ K , J., 1983a
D v o ~ ~ K , J., 1983b
DVORAK, J. and R. APPELS, 1982
D v o ~ ~ K , J. and R. APPELS, 1986
The wheat ribosomal DNA spacer region: its structure and
The origin of wheat chromosomes 4A and 4B and their genome reallocation.
Evidence for genetic suppression of heterogenetic chromosome pairing in
Chromosome and nucleotide sequence differentiation in ge-
Investigation of homologous crossing over and sister chromatid variation in populations and among species. Theor. Appl. Genet. 63: 337-348.
Can. J. Genet. Cytol. 25: 210-214.
polyploid species of Solanum sect. Petotu. Can. J. Genet. Cytol. 25: 530-539.
592 R.
s.
KOTA, P. E. MCGUIRE AND J.D V O ~ A K
exchange in the wheat Nor-B2 locus coding for rRNA and Gli-B-BP locus coding for gliadins. Genetics 113: 1037-1056.
D v o ~ ~ K , J. and K.-C. CHEN, 1984 Distribution of nonstructural variation between wheat cultivars along chromosome arm 6Bp: evidence from the linkage map and physical map of the arm. Genetics 1 0 6 325-333.
Nonstructural chromosome differentiation among wheat cultivars, with special reference to differentiation of chromosomes in related species. Genetics
97: 391-414.
ENDO, T . R. and B. S. GILL, 1984 Somatic karyotype, heterochromatin distribution, and nature of chromosome differentiation in common wheat, Triticum aestiuum L. em. Thell. Chromosoma
FELDMAN, M., 1966 Identification of unpaired chromosomes in F, hybrids involving Triticum
FELDMAN, M., 1975 Alien addition lines of common wheat containing Triticum longissimum chro-
KASARDA, D. D., 1980 Structure and properties of y-gliadins. Ann. Technol. Agric. (Paris) 29:
A reassessment of the course of evolution of wheat. Proc.
D v o ~ A K , J. and P. E. MCGUIRE, 198 1
8 9 361-369.
aestivum and T. timopheeuii. Can. J. Genet. Cytol. 8: 144-151.
mosomes. Proc. 12th Int. Bot. Congr. 2: 506.
151-173.
KIMBER, G. and R. S. ATHWAL, 1972
Natl. Acad. Sci. USA 69: 912-915.
KIMBER, G. and E. R. SEARS, 1968
KOTA, R. S. and J. D v o ~ ~ K , 1985
L A U R I ~ E , M. and J. Mo.%b, 1982
acid pH. Anal. Biochem. 122: 20-25.
LILIENFELD, F. and AND H. KIHARA, 1934 Timophemi Zhuk. Cytologia 6 87-122.
Aegilops longissima. Genet. Iber. 23: 1-9.
genome relationships in the Triticeae. Z. Pflanzenzuecht. 87: 69-78.
Nomenclature for the description of aneuploids in the Triti-
A rapid technique for substituting alien chromosomes into
Polyacrylamide gel-urea electrophoresis of cereal proteins at
Genomanalyse bei Triticum und Aegilops. V. Triticum
Promotion of homoeologus pairing in hybrids of Triticum aestiuum X
Chromosome pairing of intergeneric amphiploids as a means of assessing
ceae. pp. 468-472. In: Proceedings of 3rd International Wheat Genetics Symposium, Canberra.
Triticum aestiuum and determining their homoeology. Can. J. Genet. Cytol. 27: 549-558.
MELLO-SAMPAYO, T., 197 1
MILLER, T . E., 1981
RILEY, R., 1960 The diploidization of polyploid wheat. Heredity 15: 407-429.
RILEY, R., J. UNRAU and V. CHAPMAN, 1958
SALLEE, P. J. and G. KIMBER, 1979
SARKAR, P. and G. L. STEBBINS, 1956
SEARS, E. R., 1972
SEARS, E. R. and L. M. S. SEARS, 1979
ZOHARY, D. and M. FELDMAN, 1962
Evidence on the origin of the B genome of wheat.
An analysis of the pairing of wheat telocentric chromosomes.
Morphological evidence concerning the origin of the B
Reduced proximal crossing-over in telocentric chromosomes of wheat. Genet.
The telocentric chromosomes of common wheat. pp.
Hybridization between amphiploids and the evolution of
Communicating editor: M. T. CLECC J. Hered. 49: 91-98.
pp. 408-419. In: Proceedings of the 5th International Wheat Genetics Symposium, New Delhi.
genome in wheat. Am. J. Bot. 43: 297-304.
Iber. 24: 233-239.
389-407. In: Proceedings of the 5th International Wheat Genetics Symposium, New Delhi.