3515
Introduction
Invagination of epithelial sheets is an important process during gastrulation of many organisms and the formation of tissues such as the vertebrate lung, gut, insect trachea and the salivary glands. During invagination, the cells specified to be particular tissues break down their cell adhesion with the rest of the epithelium, reorganize their cytoskeleton and apically constrict to ingress into the embryo (Schöck and Perrimon, 2002). The process of invagination has been extensively studied during ventral furrow formation in Drosophilaembryos. During this process, cells close to the ventral midline constrict apically to produce a shallow groove, which is deepened by cell shortening along the apicobasal axis (Leptin and Grunewald, 1990). Prior to apical constriction, nonmuscle myosin II and
βHspectrin are translocated to the apical side of the ventral furrow cells. Disruption of this accumulation prevents ventral furrow formation, suggesting that actin-myosin contractility is required for apical constriction (Nikolaidou and Barrett, 2004; Thomas and Kiehart, 1994; Young et al., 1991). In addition to the cytoskeletal changes, ventral furrow formation requires coordination between the cell cycle and invagination. Normally, ventral furrow cells delay mitosis until after they are inside the embryo. As shown by tribbles mutants, premature entry into mitosis before invagination prevents ventral furrow formation (Grosshans and Wieschaus, 2000; Mata et al., 2000; Seher and Leptin, 2000). As invagination is a fundamental process used repeatedly during organogenesis, we are interested in understanding whether the process of invagination and its genetic control are similar in other epithelial tissues.
The embryonic salivary glands of Drosophila provide another good system to study epithelial invagination. The
salivary glands are derived from a disc of columnar, postmitotic epithelial cells in parasegment 2 of the Drosophila embryo known as the salivary placodes (Fig. 1B,O). The salivary placodes are specified at stage 10 by three positive regulators – the homeotic gene Sex combs reduced, extradenticle and homothorax (Henderson and Andrew, 2000; Panzer et al., 1992). The dorsal and ventral boundaries of the salivary placodes are determined by the decapentaplegic and Egfr signaling pathways, respectively (Abrams et al., 2003; Panzer et al., 1992). Following their specification, the salivary placodes invaginate into the embryo to form the tubular salivary glands. The process of invagination begins with a wave of apical constriction and basal movement of nuclei that progresses from the dorsal posterior cells to the dorsal anterior cells and finally to the ventral cells of the salivary placodes. The order of invagination follows the apical constriction wave, beginning with the dorsal posterior cells, followed by the dorsal anterior cells and then the ventral cells (Myat and Andrew, 2000a; Zhou et al., 2001). This sequential internalization of the cells results in a narrow tubular gland where the first invaginated cells form the distal tip of the gland and the last cells to ingress into the embryo form the proximal part of the salivary gland.
We are just beginning to understand the genetic control of salivary invagination. Interestingly, at least one of the signaling pathways used during ventral furrow formation is also involved in salivary invagination. Signaling by folded gastrulation(fog) activates RhoGEF2, a RhoGTPase exchange factor, in the ventral furrow and the salivary placodes. Both fog and RhoGEF2 are necessary for the invagination of the ventral furrow and the salivary glands (Hacker and Perrimon, 1998; Epithelial invagination is necessary for formation of many
tubular organs, one of which is the Drosophilaembryonic salivary gland. We show that actin reorganization and control of endocycle entry are crucial for normal invagination of the salivary placodes. Embryos mutant for
Tec29, the Drosophila Tec family tyrosine kinase, showed delayed invagination of the salivary placodes. This invagination delay was partly the result of an accumulation of G-actin in the salivary placodes, indicating that Tec29is necessary for maintaining the equilibrium between G- and F-actin during invagination of the salivary placodes. Furthermore, normal invagination of the salivary placodes
appears to require the proper timing of the endocycle in these cells; Tec29 must delay DNA endoreplication in the salivary placode cells until they have invaginated into the embryo. Taken together, these results show that Tec29
regulates both the actin cytoskeleton and the cell cycle to facilitate the morphogenesis of the embryonic salivary glands. We suggest that apical constriction of the actin cytoskeleton may provide a temporal cue ensuring that endoreplication does not begin until the cells have finished invagination.
Key words: Tec kinase, Actin, Endocycle, Btk29A
Summary
Tec29 controls actin remodeling and endoreplication during
invagination of the Drosophila embryonic salivary glands
Vidya Chandrasekaran and Steven K. Beckendorf*
Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720, USA
*Author for correspondence (e-mail: beckendo@berkeley.edu)
Accepted 2 June 2005
Development 132, 3515-3524
Published by The Company of Biologists 2005 doi:10.1242/dev.01926
Research article
De
Nikolaidou and Barrett, 2004). In the ventral furrow, RhoGEF2 causes the apical myosin II localization that is necessary for invagination of the ventral furrow (Nikolaidou and Barrett, 2004). It is therefore possible that RhoGEF2 has a similar function in the salivary glands, thereby facilitating apical constriction of the placode cells. In addition to the fog–RhoGEF2signaling pathway, the apical constriction of the salivary placodes cells requires fork head, a winged helix transcription factor; in its absence, the salivary primordium fails to invaginate (Myat and Andrew, 2000b; Weigel et al., 1989).
Once the salivary placode cells invaginate into the embryo, they enter a modified cell cycle known as the endoreplication cycle or endocycle, in which the cells alternate between G and S phase without cell division, leading to an increase in ploidy. The salivary gland cells are the first cells to enter the endocycle in the embryo, and endoreplication in the gland reliably progresses from the distal tip of the gland to the proximal part during stages 12-14 (Smith and Orr-Weaver, 1991). Thus, the wave of endoreplication in the invaginated glands follows the same order as the preceding wave of apical constriction and invagination. This endoreplication is first of many in the salivary cells, leading to the giant polytene chromosomes present in mature larvae.
The early events in salivary morphogenesis, including the localized initiation of invagination, the orderly progression of invagination to other placode cells and the beginning of endoreplication, appear to be carefully coordinated. Thus far, however, the mechanisms coordinating these processes have remained elusive.
Here we find that the Tec29 (Btk29A – FlyBase) tyrosine kinase is necessary to coordinate two essential processes: actin cytoskeletal organization and regulation of the cell cycle. Tec29 is a member of the Tec family of non receptor tyrosine kinases, which includes BTK, TEC, ITK, ETK and TXK (Gregory et al., 1987, Mano, 1999). Mutations in BTK are known to cause X-linked agammaglobulinemia in humans and X-linked immunodeficiency in mice. The human disorder results from absence of mature B lymphocytes, whereas mice with the immunodeficiency have abnormal B cells (Maas and Hendriks, 2001; Satterthwaite and Witte, 2000). Other Tec kinases regulate many processes during development of lymphocytes, including cell cycle, cell death, cell adhesion and migration (Mano, 1999; Takesono et al., 2002). By contrast, the DrosophilaTec kinase Tec29has only been linked to the actin cytoskeleton during Drosophilaembryogenesis and oogenesis (Guarnieri et al., 1998; Roulier et al., 1998; Tateno et al., 2000; Thomas and Wieschaus, 2004). Tec29 is needed for actin filament reorganization and bundling of actin during early cellularization and dorsal closure in embryos, as well as in the ring canals of the ovary. Our data show that lack of Tec29 caused a delay in invagination of the salivary glands because of a shift in the equilibrium between F-actin and G-actin, and because of premature endoreplication in the salivary placode cells. Thus, like ventral furrow formation, invagination of the salivary placodes requires both the reorganization of the actin-myosin cytoskeleton and a cell cycle delay.
Materials and methods
Drosophila stocks
Tec29K00206/CyO and Tec29K05610/CyO are two P-element alleles of
Tec29 that lack any Tec29 expression in embryos and behave as embryonic null alleles (Roulier et al., 1998). Tec29e482/CyOhas a stop codon in the kinase domain and is predicted to lack all catalytic activity (Guarnieri et al., 1998). Src64PI/TM3,a P-element insertion allele of Src64 was a gift from M. Simon (Dodson et al., 1998). Src42AE1/CyO, a null allele of Src42A, was from Adachi-Yamada (Tateno et al., 2000). UAS-CycE was a gift from P. O’Farrell. All the remaining stocks were obtained from the Bloomington Stock Center. w1118flies were used as wild type controls for all experiments.
Immunocytochemistry
Embryo fixation and immunocytochemistry were performed as described previously (Chandrasekaran and Beckendorf, 2003). The following primary antibodies were used in this study: rat anti-CREB (1:5000) (Andrew et al., 1997), rabbit FKH (1:1000), rabbit
anti-β-galactosidase (1:1000, Vector Laboratories), mouse anti-TEC29 (1:10), mouse anti-CRUMBS (1:100) and guinea pig anti-SCRIBBLE (1:1000, D. Bilder), mouse anti-ENGRAILED (1:10, DSHB), mouse anti-actin (1:100,), rat anti-DEADRINGER (1:15000, D. Andrew) and mouse anti-CYCLIN E (1:50, H. Richardson). Embryos were then incubated with the appropriate secondary antibodies that were either biotinylated (1:200, Vector Laboratories) or Alexa-conjugated (1:500, Molecular Probes). The biotinylated secondary antibodies were detected using the Vectastain ABC kit (Vector Labs), followed by incubation with 0.5 mg/ml diaminobenzidine and 0.06% hydrogen peroxide. The embryos were then cleared with methyl salicylate and photographed using Nomarski optics on the Leica DMRB microscope. The fluorescent embryos were cleared in 70% glycerol in PBS containing 2% n-propyl gallate (Sigma) and visualized using the Zeiss 510 confocal microscope.
In situ hybridization
Whole-mount in situ hybridization was performed as described by Tautz and Pfeifle (Tautz and Pfeifle, 1989) with modifications (Harland, 1991) using antisense digoxigenin-labeled probes. The signal was visualized using nitro blue tetrazolium and BCIP as substrates for alkaline phosphatase. Following in situ hybridization, the embryos were immunostained for β-galactosidase as described above in the immunocytochemistry protocol. The embryos were rinsed and cleared in 50% glycerol followed by 70% glycerol and photographed using Nomarski optics on the Leica DMRB microscope.
BrdU labeling of embryos
BrdU labeling and detection were performed using the protocol described by Shermoen (Shermoen, 2000). Briefly, embryos were dechorionated and incubated in n-octane (Sigma) for 5 minutes, followed by incubation in 1 mg/ml BrdU in PBS for 40 minutes. Then the embryos were fixed in a 1:1:2 mixture of PBS, 10% formaldehyde and heptane, followed by devitellenization with methanol. The embryos were stored in methanol at –20°C. Embryos were rehydrated and immunostained with rabbit anti-FKH as well as rabbit-anti-β -galactosidase, followed by a fluorescent-conjugated secondary antibody as described above. Following the immunostaining, embryos were treated with 2.2 N HCl containing 0.1% triton twice for 15 minutes each followed by neutralization in 0.1 M sodium borate. The embryos were then incubated with mouse anti-BrdU overnight at 4°C. The BrdU labeling in the nuclei was detected using a fluorescent-conjugated secondary antibody and embryos were processed and imaged as described above.
Results
Tec29 is expressed in the Drosophila embryonic salivary primordium
Although previous studies described Tec29RNA and protein
De
Tec29 is necessary for salivary invagination
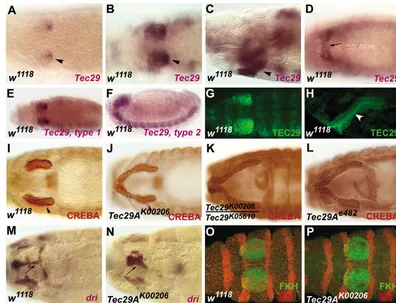
expression in embryos (Katzen et al., 1990; Vincent et al., 1989; Wadsworth et al., 1990), the details of salivary gland expression were not reported. We find that Tec29 RNA expression begins at stage 11 (5.5-7.5 hours) in the dorsal posterior part of the salivary placodes, colocalizing with the site of initial invagination (Fig. 1A). By stage 12, all salivary placode cells express Tec29 (Fig. 1B). Tec29 continues to be expressed in the invaginating salivary glands at stage 12 (Fig. 1C) and later during embryogenesis (data not shown). Then as the placodes invaginate, Tec29 expression expands into the more ventral cells that will become the salivary ducts (Fig. 1D) and is weakly expressed in the salivary ducts of older embryos (data not shown). A similar expression pattern was observed for TEC29 protein (Fig. 1G,H). TEC29 is localized to the apical membrane in the salivary gland cells (Fig. 1H).
Most Tec family kinases have an N-terminal pleckstrin homology (PH) domain, followed by Tec homology (TH), SH2, SH3 and kinase domains. In Drosophila, there are two isoforms of Tec29RNA. The longer form (type 2) is similar to the vertebrate Tec kinases and includes the PH domain, whereas the shorter type 1 isoform lacks the PH and part of the TH domain at its N terminus (Baba et al., 1999a). We observed that the longer isoform of Tec29 is expressed only in the nervous system, whereas the shorter isoform is expressed in the epidermis and at high levels in the salivary glands (Fig. 1E,F). Thus, TEC29 function in the salivary glands apparently does not require a PH domain.
Embryos mutant for Tec29 have long salivary glands
Expression in the salivary glands suggested that Tec29 might be important for salivary gland morphogenesis. Embryos mutant for a P-element allele of Tec29, Tec29K00206 had abnormally long salivary glands that did not fully invaginate and were still connected to the surface (Fig. 1I,J). The phenotype was fully penetrant, and identical phenotype was observed in embryos mutant for at least two other Tec29alleles, Tec29K05610 and Tec29e482, as well as Tec29K00206/Tec29K05610 heterozygotes (Fig. 1K,L).
A possible explanation for this phenotype was that the mutant salivary placodes had more cells than wild-type placodes, resulting in long glands. However, cell counts did not support this idea. Tec29 salivary placodes had 113±36 cells compared with 117±39 in wild type. In addition, location of the placodes is normal. As shown by co-staining for ENGRAILED, the placodes respect both the normal AP boundaries at the edges of parasegment 2 (Fig. 1O,P) and the DV boundaries that separate them from the dorsal epidermis and the more ventral salivary duct cells (data not shown). In addition, staining for the mitotic marker phosphohistone H3 produced no evidence of extra mitoses, either in the placodes or at later stages. Thus, the long glands did not result from the recruitment of extra cells into the primordium or from the production of extra cells during development.
[image:3.612.164.560.73.376.2]The salivary ducts were also defective in Tec29 mutant embryos (compare Fig. 1M,N). As evidenced by staining for duct markers such as dead ringer, duct cell fate appeared to be
Fig. 1.Tec29is expressed in embryonic salivary gland and has a salivary gland
phenotype. In all the figures, anterior is towards the left. (A-D) Tec29mRNA is expressed in the salivary placodes at stage 11 and in the invaginating salivary glands (arrowhead). Tec29is also expressed in the salivary ducts from stage 12 onwards (D, arrow). (E,F) The shorter isoform of Tec29, type 1, is expressed in the salivary primordium (E), while the longer isoform, type 2, is expressed only in the nervous system (F).
(G,H) Immunostaining for TEC29 protein shows that it is expressed in the salivary placodes and is apically localized in the invaginating gland (white arrowhead). (I-L) Immunostaining with anti-CREB antibody showed that multiple alleles of Tec29,Tec29K00206, Tec29K00206/Tec29K05610and Tec29e482have long salivary
glands compared with w1118 embryos (I). (M,N) The salivary ducts in Tec29K00206, as visualized by dead ringer (dri), do not go through normal morphogenesis (arrows). (O,P) Double labeling for FKH and EN show that the salivary placodes at stage 11 are normal and respect their AP boundary in Tec29K00206embryos.
De
normally specified in these embryos, but the duct cells did not undergo normal morphogenesis (Fig. 1N). Staining for a lumenal marker, CRUMBS, showed that tubular ducts were not formed in these embryos (data not shown).
Tec29is necessary for the invagination of salivary glands
As the long salivary glands in Tec29mutants were not due to extra cells and there were uninvaginated cells in the salivary glands of Tec29 embryos, we reasoned that the detailed analysis of salivary placode invagination in Tec29 embryos might explain the salivary gland phenotype. In wild-type embryos, the progress of salivary invagination can be
monitored relative to germ band retraction, as both processes occur during stage 12 of embryogenesis.
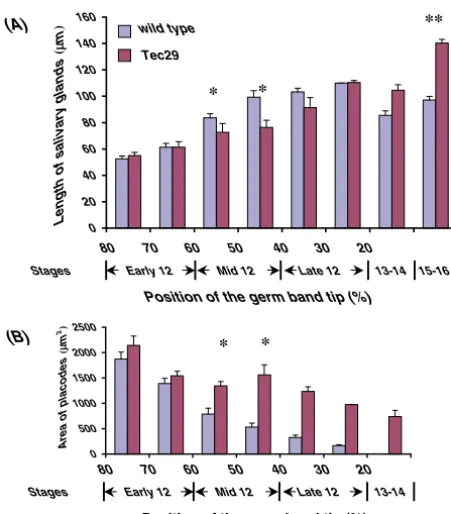
In wild-type embryos, there was an inverse relationship between the area of the placodes left on the surface and the length of the invaginated gland, such that by end of stage 12 there were no placodes left on the surface (Fig. 2B). The length of the glands increased as the embryos progress through stage 12, but then decreased at later stages (Fig. 2A). As germ band retraction appeared to be normal in Tec29 embryos, similar analyses of salivary glands were performed in these embryos. The salivary placodes began invagination on schedule and at early stage 12 looked similar to wild type (Fig. 2A,B). During mid stage 12, when there was a linear increase in the length of the invaginated gland in wild type, Tec29 embryos stalled; the glands did not increase in length and the placode area did not decrease. By late stage 12, the length of the invaginated salivary glands in Tec29 embryos was comparable with wild type, but by stage 15 the salivary glands in Tec29 embryos were significantly longer than wild type (Fig. 2A). Despite this, there were still many placode cells remaining on the surface at late stage 12 and even at stage 13-14 (Fig. 2B). Thus, in Tec29embryos there was an arrest in the salivary gland invagination process at mid stage 12, followed by a late increase in gland length. These results indicate that the delay in invagination precedes the lengthening of the gland in Tec29 mutants and is the main cause of the Tec29salivary gland phenotype.
There is a paradox in understanding the Tec29phenotype. Although fewer cells invaginate, the mutant glands eventually become much longer than in wild-type embryos. As the increase in the salivary gland length occurred late in Tec29 embryos, we guessed that it might come from stretching them during head involution in older embryos. To test this possibility, Tec29 embryos were immunostained either for CRUMBS, which outlines the apical ends of epithelial cells or for SCRIBBLE, a septate junction protein that is localized to the lateral margins of epithelial cells, more basal to CRUMBS (Bilder and Perrimon, 2000; Schöck and Perrimon, 2002; Tepass et al., 1990). Both markers highlight the salivary gland cells, producing nearly isodiametric outlines in wild-type embryos at stage 15 (Fig. 3A,B,E). In Tec29 embryos stained for either SCRIBBLE or CRUMBS, the distal region of the salivary glands looked normal, but the cells in the proximal region of the glands were elongated in the AP direction (Fig. 3C,D,F). These results show that the arrest of invagination coupled with the anterior movement of the epidermis during head involution caused stretching of the mutant salivary glands, leading to the observed phenotype of long salivary glands.
Stretching of the glands in Tec29mutants suggests that they were tethered at both ends. The anterior tether probably results from the invagination defect. As the placodes do not completely invaginate in Tec29 embryos, the anterior part of the gland appears to remain anchored to the moving ectoderm. To permit stretching, the posterior part of the gland must also be tethered, probably by attachment to tissues close to the distal tip of the gland, maybe the anterior midgut. This posterior attachment may be part of normal salivary morphogenesis as the posterior part of the Tec29-mutant glands are located normally and have the same apical outlines as wild-type glands.
(A)
(B)
0 500 1000 1500 2000 2500
Area of placodes
(
μm 2)
* *
Stages
80 70 60 50 40 30 20
Early 12 Mid 12 Late 12 13-14
Position of the germ band tip (%)
0 20 40 60 80 100 120 140 160
Length of salivary glands
(
μ
m
)
80 70 60 50 40 30 20
Early 12 Mid 12 Late 12 13-14 15-16 Stages
Position of the germ band tip (%)
* *
** wild type
[image:4.612.52.278.235.492.2]Tec29
Fig. 2.Tec29embryos show delays in invagination of the salivary placodes. (A) Changes in the length of the salivary gland (μm) as the placode cells invaginate during stage 12 of embryogenesis and at stages 13 through 16. Progression through stage 12 was monitored by the position of the tip of the germ band (as percent embryo length measured from the posterior end of the embryo). The bars show the mean length of the salivary glands in embryos whose germ band tip lies in the indicated intervals. Seventy-five wild-type salivary glands and 46 Tec29K00206salivary glands from embryos at different stages were measured. As the germ band has finished retraction by the end of stage 12, the later stages are indicated only by stage numbers, not position. In w1118embryos (blue bars), the length of the salivary glands gradually increases during stage 12 and then decreases slightly at stages 13-16. In Tec29K00206embryos (red bars), there is no increase in length of gland observed at mid stage 12 (*); however, the length of the gland increases and exceeds w1118at stage 15 (**). (B) This graph shows the area of the salivary placodes on the surface measured in the same embryos used for measurement in A. Although there is a decrease in the surface area of the placodes in w1118 embryos (blue bars), in Tec29K00206embryos (red bars) the area of the placodes does not decrease such that at stage 13-14, one-third of the placode is still on the surface. Uninvaginated cells remain on the surface of Tec29K00206embryos at stage 15-16; however, these cells have moved more anteriorly in the head and are hard to quantitate.
De
Tec29 is necessary for salivary invagination
Apical actin cytoskeleton is disorganized in Tec29 embryos
Previous studies have shown that Tec29 can reorganize the actin cytoskeleton in the ovary and during the cellularization of Drosophilaembryos (Djagaeva et al., 2005; Roulier et al., 1998; Thomas and Wieschaus, 2004). Therefore, we examined whether the actin distribution was normal in the placodes of Tec29embryos. Staining with phalloidin to visualize F actin or with an α-spectrin antibody did not reveal any gross abnormalities (Fig. 4A,B; data not shown). However, use of an actin monoclonal antibody that detects both F and G actin showed that in Tec29 embryos, actin appeared to be disorganized at the apical end of the placode cells (Fig. 4C,D), but looked similar to wild type on the basolateral surface (data not shown). The disorganization of actin occurred early in stage 12 in the ventral cells of the placodes and preceded the delay in invagination observed in these cells. In addition, we found that there were genetic interactions between Tec29and the actin-binding proteins, profilin and cofilin. The Drosophila profilin homolog, chickadee (chic), is important for promoting actin polymerization, thereby increasing F-actin in the ovary, embryo and imaginal discs, whereas the Drosophila cofilin
homolog twinstar (tsr), promotes depolymerization, thus limiting actin filament growth and increasing G-actin (Cooley et al., 1992; Gunsalus et al., 1995). Embryos mutant for either chicor tsralone had normal salivary glands. However, Tec29 chic double mutants showed an enhancement of the Tec29 salivary gland phenotype. The salivary glands in 80% of Tec29 chicdouble mutants showed more severe invagination defects with large placodes left on the surface (Fig. 5B,E,G). The remaining 20% of the embryos had salivary glands similar to Tec29. By contrast, 30% of the Tec29 tsrdouble mutants had salivary glands that invaginated normally or nearly so, indicating a partial suppression of the Tec29mutant phenotype (Fig. 5C,F,G). These genetic interactions show that in Tec29 mutants there is shift from F-actin to G-actin on the apical surface of the salivary placode cells. They also suggest that the apparent disorganization seen with the actin antibody results from increases in G- relative to F-actin. The partial rescue of the Tec29 salivary gland phenotype by tsrindicates that the shift in the balance between G-actin and F-actin in the salivary placodes caused the invagination delay in Tec29 mutant salivary placodes. Therefore, Tec29was necessary to facilitate the formation or maintenance of F-actin at the apical surface of the salivary placodes cells, and this localization of actin was crucial for normal invagination.
Endoreplication is aberrant in Tec29 embryos
[image:5.612.68.268.70.375.2]In at least two cases, the Drosophila ventral furrow and the paraxial mesoderm in Xenopus, the cell cycle must also be regulated to allow normal invagination, (Grosshans and Wieschaus, 2000; Leise and Mueller, 2004; Mata et al., 2000; Seher and Leptin, 2000). This prompted us to examine the cell cycle control in the salivary placodes of Tec29 mutants.
Fig. 3.The salivary glands at stage 15 are stretched in Tec29mutant embryos. (A-D) SCRIBBLE and FKH staining in the salivary glands show that the cells are isodiametric in w1118but are stretched along the axis of the glands in Tec29K00206embryos (arrowheads).
[image:5.612.336.559.74.275.2](E,F) Staining with CRUMBS and FKH also shows that the proximal cells of the salivary glands are stretched in Tec29embryos (arrows). Because the salivary gland cells are wedge shaped with their apical surface facing the lumen, CRUMBS, being more apical, produces a smaller outline of the cells than does SCRIBBLE.
Fig. 4.Actin is disorganized in the salivary placodes of Tec29K00206 embryos. All the panels show optical sections of embryos focused on the apical surface of the salivary placodes. In all the panels, the circle outlines the salivary placodes. (A,B) The apical staining for α -spectrin looks normal in Tec29K00206embryos. (C,D) Actin disorganization on the apical surface of the cells in Tec29K00206 embryos is evident at early stage 12 when the embryos are stained with a monoclonal antibody against ACTIN, which detects both G-actin and F-G-actin.
De
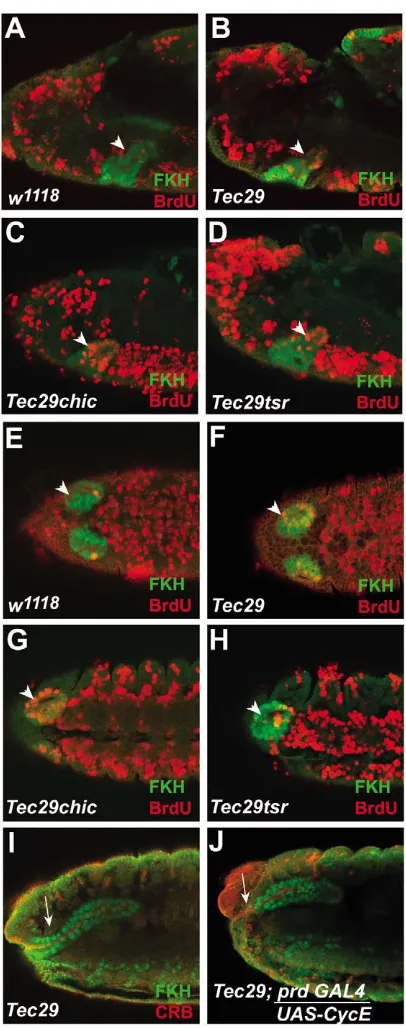
Though the salivary placode cells are postmitotic, they do regulate a specialized cell cycle, the endoreplication cycle (endocycle), during their invagination. Therefore, we sought to understand if the control of the endocycle was necessary for salivary invagination and whether the endocycle was normal in Tec29embryos. The salivary glands in Drosophilaembryos are the first cells in the embryo to leave the mitotic cycle and enter endoreplication (Smith and Orr-Weaver, 1991). Endoreplication begins in these cells immediately following their invagination into the embryo at stage 12 (Fig. 6A) (Smith and Orr-Weaver, 1991). In wild-type salivary glands, endoreplication proceeds as a wave from the distal tip of the gland, progressing proximally until all the cells are included. It is rare for cells to enter endoreplication while still on the surface of the embryo (Fig. 6E). Following one round of endoreplication, the salivary gland cells enter G phase and do not resume replication until larval stages. During the early parts of invagination (early to mid stage 12), we found few BrdU-labeled, S-phase nuclei in wild-type glands (Fig. 6A). However, in Tec29 embryos, the coordinated progression of salivary gland endoreplication disappeared. Though the salivary gland cells began endoreplication on schedule, endoreplicating cells were not confined to the distal tip. Isolated cells in the rest of the gland incorporated BrdU and eventually many cells that were still on the surface became labeled (Fig. 6B,F). Thus, in Tec29embryos the synchronized wave of endoreplication in the salivary glands is disrupted, resulting in uncoordinated endoreplication throughout the placodes and invaginating glands.
Rescuing endoreplication can rescue the salivary gland phenotype of Tec29 embryos
To see whether premature endoreplication contributes to the invagination defects in Tec29 embryos, we delayed endoreplication in Tec29 embryos. Previous studies have shown that, although pulses of cyclin E are necessary for endoreplication, continuous expression can block endoreplication (Knoblich et al., 1994; Weiss et al., 1998).
When we used paired-GAL4 to express UAS-cyclin E in the salivary primordium, we found ectopic expression in the salivary placodes beginning at stage 11 and continuing to be expressed in the salivary glands in older embryos. Although expression of prd-GAL4 in the gland is patchy, cells that express cyclin E have a corresponding absence of BrdU labeling, indicating that ectopic cyclin E efficiently blocked endoreplication (data not shown). Continuous expression of cyclin E did not alter wild-type salivary gland invagination; however, continuous cyclin E caused a partial or complete suppression of the mutant phenotype in 69% of the Tec29 homozygous embryos (Fig. 6I,J). These results indicate that synchronized endoreplication is necessary for invagination of the salivary glands and that Tec29controls the progression of the endoreplication wave in the salivary glands.
The effects of Tec29 on endoreplication may be due its effects on actin cytoskeleton
[image:6.612.243.559.73.410.2]As Tec29 has effects on both actin cytoskeleton and endoreplication, we wanted to understand if Tec29 independently affects these processes. Hence, we examined endoreplication in Tec29 chic and Tec29 tsr double mutants. Though Tec29 tsrmutants show a suppression of the salivary gland phenotype, there was no clear decrease in the number or extent of the endoreplication defects in these embryos (Fig. 6D,H). However, Tec29 chicdouble mutants showed a marked enhancement of the endoreplication defects observed in Tec29 alone. There were more endoreplicating cells in embryos
Fig. 5. Tec29 phenotype in the salivary glands is enhanced by chickadee and suppressed by twinstar. (A-C) More cells in the salivary glands of Tec29K00206chic221double mutants (B) are on the surface at stage 14 compared with
Tec29K00206alone (A). However, Tec29K00206 tsrK05633double mutants can rescue the Tec29K00206phenotype in the salivary glands (C). (D-F) The actin disorganization in Tec29K00206embryos is increased by chic221and rescued by tsrK05633(circle). (G) The graphical representation of the enhancement and suppression by chic221and tsrK05633,
respectively. At stage 14, a third of the salivary placodes is left on the surface in Tec29K00206 embryos (759±36 μm2, n=30), whereas it is
almost doubled in Tec29K00206chic221embryos (1304±45 μm2, n=30) as well as in
Tec29K00206Src42AE1embryos (1278±43 μm2,
n=30) and it is halved in Tec29K00206tsrK05633 (330±59 μm2, n=30).
De
Tec29 is necessary for salivary invagination
mutant for both Tec29 and chicthan in Tec29 mutants alone (Fig. 6C,G). This effect on endoreplication was observed at
early stage 12, prior to the onset of invagination defects. These results suggest that the defects in actin remodeling can affect the endoreplication cycle in the salivary glands.
There is a genetic interaction between Tec29 and Src family kinases in the salivary glands
Src family kinases have been shown to activate Tec kinases in vertebrates (Takesono et al., 2002). In addition, the Drosophila Src-family kinases, Src42Aand Src64, genetically interact with Tec29 either in the embryo or in the ovary (Djagaeva et al., 2005; Guarnieri et al., 1998; Tateno et al., 2000; Thomas and Wieschaus, 2004). We found that Tec and Src kinases also interacted genetically in the salivary glands. Though Src42Aby itself did not show salivary placode invagination defect, mutations in Src42A enhanced the Tec29 salivary gland phenotype (Fig. 7B). In Tec29 Src42A double mutants, the invagination defects are more severe than in Tec29 single mutants. In 70% of the double mutant embryos fewer salivary gland cells invaginate, resulting in large placodes that remain on the surface (Fig. 7B and Fig. 5G). In addition, more salivary gland cells prematurely entered endoreplication in these double mutants than in Tec29alone (Fig. 7G,H). These Tec29 Src42A embryos also showed disorganization of actin in the salivary placodes, but it is unclear if the disorganization is more severe than Tec29alone (Fig. 7E,F).
There were also genetic interactions between Tec29and the other Src kinase, Src64. Similar to Src42A mutants, Src64 mutant embryos did not show any salivary gland abnormalities. However, many of the Tec29 Src64 double mutant embryos showed gross abnormalities such as lack of head segments, making it difficult to evaluate the salivary gland phenotype. Of the Tec29 Src64double mutant embryos that looked normal in outline, 16% showed more severe salivary gland phenotypes than Tec29 alone (Fig. 7C). The Tec29-Src64 interaction is most clearly shown with heterozygous Tec29 embryos. The salivary glands are normal in Tec29/+ Src64/+ embryos. However, about one-third of Tec29/+ Src64/Src64 embryos show Tec29-like invagination defects (Fig. 7D). These results show that there is a strong genetic interaction between Src64 and Tec29 in the salivary glands. The lack of salivary gland invagination defects in embryos mutant for either Src kinase and the enhancement of the null mutant Tec29 phenotype by Src mutations suggest that Tec29 and the Src kinases may function in a parallel pathways to promote salivary gland invagination.
Discussion
[image:7.612.66.269.68.582.2]Salivary placode cells must constrict apically and move their nuclei basally in order to invaginate into the embryo (Myat and Andrew, 2000a; Myat and Andrew, 2000b). Therefore, the process of salivary gland invagination is expected to require actin-myosin contractility. We show that Tec29, a non receptor tyrosine kinase, is responsible for regulating the actin reorganization in the salivary placodes prior to invagination. Lack of Tec29 in the salivary placodes resulted in a shift of actin in the salivary placodes from F-actin to more G-actin, leading to incomplete invagination of the salivary placodes cells. This change in the balance between F-actin and G-actin was observed only on the apical surfaces of these cells, suggesting that the delayed invagination was due to aberrant
Fig. 6. Tec29is necessary to delay endoreplication in the salivary glands. There are more BrdU-labeled nuclei in the invaginated gland labeled with FKH antibody (green) at early stage 12 in Tec29K00206 compared with w1118(A,B, arrowhead) and also ventrally in the salivary placodes at mid stage 12 (arrowhead, compare E with F). In addition, there are more BrdU labeled nuclei in Tec29K00206chic221 embryos compared with Tec29K00206alone at early stage 12 (C) and at mid stage 12 (G). BrdU labeling in Tec29K00206tsrK05633is similar to Tec29K00206embryos (D,H). (I,J) Delaying endoreplication by driving UAS-CycEusing prd-GAL4in Tec29K00206embryos rescues the long salivary gland phenotype of Tec29K00206embryos (arrows).
De
apical constriction of the salivary placodes cells in Tec29 embryos. Increasing actin depolymerization further in Tec29 mutants by a mutation in chic increased the salivary invagination delay, whereas promoting actin polymerization in Tec29 mutants by mutating tsr partially rescued the invagination delay. Thus, our results provide the first evidence that actin reorganization is necessary for salivary gland invagination.
The shift in the equilibrium towards more G-actin in Tec29 mutants might be due either to decreased polymerization of actin or decreased stability of F-actin. Tec kinases affect both actin polymerization and actin crosslinking in vertebrates and in Drosophila (Finkelstein and Schwartzberg, 2004). ITK-deficient mice show impaired actin polymerization in response to T-cell activation (Labno et al., 2003). In addition, both BTK and ITK interact with WASP, a protein that can activate the Arp2/3 complex and promote new actin filament formation (Baba et al., 1999b; Bunnell et al., 1996; Guinamarda et al., 1998). During early Drosophilaoogenesis, accumulation of G-actin has been observed in the cortex of Tec29mutants, leading to defective fusome formation (Djagaeva et al., 2005). In addition, Tec29has been suggested to affect actin crosslinking by phosphorylating KELCH, an actin-bundling protein in the ring canals of the ovary. The phosphorylation of KELCH is necessary to decrease actin crosslinking, thereby allowing expansion of the ring canals (Kelso et al., 2002). It is therefore possible that in the salivary placodes, Tec29might be needed for new F-actin formation or bundling of the actin filaments to promote invagination.
Besides salivary gland invagination, Tec29 affects actin organization during two other processes in Drosophila embryogenesis: dorsal closure and cellularization. Tec29 in conjunction with Src42Ais necessary for dorsal closure and lack of both these kinases result in a dorsal open phenotype. So, during dorsal closure as with salivary invagination, there is a positive interaction between Tec29 and Src42A. However,
Src42is the main player during dorsal closure, whereas Tec29 is more important for salivary invagination. Interestingly, there is a reduction of F-actin observed at the leading edge of the dorsal epidermal cells in these double mutants, causing delayed dorsal closure (Tateno et al., 2000). Thus, the lack of these tyrosine kinases causes changes in actin dynamics that result in delayed migration of both the salivary placodes and during dorsal closure. Similarly, the process of basal closure during cellularization resembles apical constriction prior to invagination and requires an actin-myosin based contraction at the base of the cellularization front. In embryos arising from Tec29 germline clones, the actin microfilament ring does not contract, resulting in membrane invagination of varying depths and impairment of basal closure during late cellularization (Thomas and Wieschaus, 2004). It is possible that, similar to the salivary invagination and dorsal closure, the absence of the contractile ring during cellularization is due to decreased F-actin and/or increased G-F-actin. In general, Tec29appears to be needed for regulation of actin during periods of extensive and rapid reorganization of the actin cytoskeleton as observed during migration and contraction of cells.
[image:8.612.108.500.72.272.2]Although Tec kinases are known to alter the actin cytoskeleton in many systems, we are the first to show a relationship between Tec29 and the endocycle. Our data support a previous observation that the salivary placodes in wild-type embryos enter endoreplication only after they invaginate into the embryo (Smith and Orr-Weaver, 1991). In Tec29 mutants, however, the wave of endoreplication is disrupted, such that the ventral cells in the placodes initiate endoreplication prior to invagination. As a result, these cells fail to invaginate on schedule, resulting in the long salivary glands. Therefore, delaying endoreplication appears to be necessary to allow invagination, and coordinating the two events is crucial for normal development of the salivary glands. Similar coordination between the cell cycle and morphogenesis is observed during ventral furrow formation in Drosophila
Fig. 7. Tec29interacts with Src42and Src64in the salivary glands. (A-D) The invagination defects in Tec29K00206embryos (A) are enhanced by Src42AE1(B). In addition, phenotypes of both homozygous and heterozygous Tec29K00206embryos are enhanced by Src64PI(C,D, arrow). (E-H) Disorganization of actin observed in Tec29K00206Src42AE1double mutants is not obviously more severe than Tec29K00206alone (E,F, circle); however, there are more cells labeled with BrdU in the Tec29K00206Src42AE1compared with Tec29K00206(arrowhead, compare H with G).
De
Tec29 is necessary for salivary invagination
embryos and in the paraxial mesoderm in Xenopusembryos. In the ventral furrow and the paraxial mesoderm, the cell cycle delay is established by maintaining the Cdks in their phosphorylated form. In the ventral furrow, this is accomplished by tribbles inhibiting the Drosophila Cdc25 homolog string, a protein that dephosphorylates and activates Cdks (Grosshans and Wieschaus, 2000; Mata et al., 2000; Seher and Leptin, 2000). In the paraxial mesoderm, the localized phosphorylation of Cdks by Wee2 is sufficient to prevent the cells from entering mitosis prior to invagination (Leise and Mueller, 2004). In both these cases, as in the salivary gland, the coordination between cell cycle and invagination is crucial for normal morphogenesis of the embryo. An important difference is that unlike the salivary glands, it is the mitotic cycle that is regulated with invagination of the ventral furrow and the paraxial mesoderm. Our study provides the first indication of a link between the endocycle and morphogenesis, and suggests that coordination of endoreplication with invagination is crucial for normal development.
As shown in the ventral furrow and the paraxial mesoderm, mitosis and invagination use the same cytoskeletal components and require opposing levels of cell adhesion, making these two processes incompatible with each other (Grosshans and Wieschaus, 2000; Leise and Mueller, 2004; Mata et al., 2000; Seher and Leptin, 2000). However, a normal endocycle in flies does not involve nuclear membrane breakdown or other processes that might require the same cytoskeletal components as invagination (Edgar and Orr-Weaver, 2001). Thus, a normal endocycle might not interfere with invagination. But this may not be a normal endocycle. Unlike the other endocycling cells in the embryo, which enter the endocycle from G1, it has been suggested that the salivary placodes may enter the endocycle from G2 (Smith and Orr-Weaver, 1991). It is therefore possible that the endocycle in the salivary placodes retains some aspects of mitosis that would interfere with invagination. If so, this endocycle would be similar to those in some endocycling mammalian cells that enter the endoreplication cycle after G2 during early M phase (Edgar and Orr-Weaver, 2001). Perhaps because this first salivary gland endocycle is unusual, it must be delayed until after the salivary cells are safely inside the embryo.
Our data indicate that Tec29 is necessary for actin remodeling during apical constriction in the salivary placodes and for endocycle progression as the glands invaginate. In addition, we found that manipulating the actin cytoskeleton in Tec29 mutants can have effects on endoreplication in these cells. When the actin defects were enhanced by eliminating chicin Tec29embryos, there was also an enhancement of the endoreplication defects of Tec29, suggesting that the actin cytoskeleton and endoreplication cycle are linked. We propose the following model to explain how these two events might be coordinated in the salivary placodes. The actin remodeling during apical constriction and endoreplication follow each other such that the first cells to apically constrict are the first cells to invaginate and endoreplicate, and the remaining cells follow in sequence. These processes might be causally linked; the apical constriction wave might trigger the wave of endoreplication. This coupling and a lag between the two events would ensure that endoreplication does not occur prematurely, while the cells are still on the surface of the
embryo. With this model, Tec29 would then regulate the endocycle indirectly, rather than independently regulating both actin and endoreplication. Disruption of the apical constriction wave in Tec29mutants would lead to subsequent disruption of the endoreplication wave. An invagination delay due to inadequate actin polymerization would be compounded by placode cells endoreplicating prior to invagination. This model also explains the ability of cyclin E to partially rescue the Tec29 phenotype. Cyclin E overexpression would delay endoreplication and relieve some of the effects of inadequate actin polymerization. Studies in progress to identify TEC29 targets are likely to provide further insight into its effects on endoreplication and invagination, and should enable us to better understand the link between these processes.
We are grateful to Deborah Andrew, David Bilder, Mike Simon, Pat O’Farrell, T. Adachi-Yamada and the Bloomington Stock Center for providing the fly stocks and the antibodies used in this study. We would like to thank Eileen Beall, Mark Stern, Tereza Kolesnikov and Katherine Harris for their advice and critical reading of the manuscript. This work was supported by NIH grant DE12519 to S.K.B.
References
Abrams, E. W., Vining, M. S. and Andrew, D. J.(2003). Constructing an
organ: the Drosophilasalivary gland as a model for tube formation. Trends
Cell Biol.13, 247-254.
Andrew, D. J., Baig, A., Bhanot, P., Smolik, S. M. and Henderson, K. D.
(1997). The Drosophila dCREB-A gene is required for dorsal/ventral
patterning of the larval cuticle. Development12, 181-193.
Baba, K., Takeshita, A., Majima, K., Ueda, R., Kondo, S., Juni, N. and
Yamamoto, D.(1999a). The Drosophila Bruton’s tyrosine kinase (Btk)
homolog is required for adult survival and male genital formation. Mol. Cell. Biol. 19, 4405-4413.
Baba, Y., Nonoyama, S., Matsushita, M., Yamadori, T., Hashimoto, S., Imai, K., Arai, S., Kunikata, T., Kurimoto, M., Kurosaki, T. et al.
(1999b). Involvement of wiskott-aldrich syndrome protein in B-cell
cytoplasmic tyrosine kinase pathway. Blood 93, 2003-2012.
Bilder, D. and Perrimon, N. (2000). Localization of apical epithelial
determinants by the basolateral PDZ protein Scribble. Nature403, 676-680.
Bunnell, S. C., Henry, P. A., Kolluri, R., Kirchhausen, T., Rickles, R. J.
and Berg, L. J.(1996). Identification of Itk/Tsk Src homology 3 domain
ligands. J. Biol. Chem. 271, 25646-25656.
Chandrasekaran, V. and Beckendorf, S. K.(2003). senselessis necessary
for the survival of embryonic salivary glands in Drosophila. Development
130, 4719-4728.
Cooley, L., Verheyen, E. and Ayers, K.(1992). chickadeeencodes a profilin
required for intercellular cytoplasm transport during Drosophilaoogenesis.
Cell69, 173-184.
Djagaeva, I., Doronkin, S. and Beckendorf, S. K.(2005). Src64is involved
in fusome development, cytokinesis and in karyosome formation during Drosophilaoogenesis (in press).
Dodson, G. S., Guarnieri, D. J. and Simon, M. A.(1998). Src64is required
for ovarian ring canal morphogenesis during Drosophila oogenesis.
Development125, 2883-2892.
Edgar, B. A. and Orr-Weaver, T. L.(2001). Endoreplication cell cycles: more
for less. Cell105, 297-306.
Finkelstein, L. D. and Schwartzberg, P. L.(2004). Tec kinases: shaping
T-cell activation through actin. Trends Cell Biol. 14, 443-451.
Gregory, R. J., Kammermeyer, K. L., Vincent, W. S., 3rd and Wadsworth,
S. G.(1987). Primary sequence and developmental expression of a novel
Drosophila melanogastersrc gene. Mol. Cell. Biol. 7, 2119-2127.
Grosshans, J. and Wieschaus, E. (2000). A genetic link between
morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell101, 523-531.
Guarnieri, D. J., Dodson, G. S. and Simon, M. A.(1998). SRC64 regulates
the localization of a Tec-family kinase required for Drosophilaring canal
growth. Mol. Cell. 1, 831-840.
Guinamarda, R., Aspenströmb, P., Fougereaua, M., Chavriera, P. and
De
Guillemota, J.(1998). Tyrosine phosphorylation of the Wiskott-Aldrich
Syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Lett. 434,
431-436.
Gunsalus, K. C., Bonaccorsi, S., Williams, E., Verni, F., Gatti, M. and
Goldberg, M. L.(1995). Mutations in twinstar, a Drosophilagene encoding
a cofilin/ADF homologue, result in defects in centrosome migration and
cytokinesis.J. Cell Biol. 131, 1243-1259.
Hacker, U. and Perrimon, N. (1998). DRhoGEF2 encodes a member of the
Dbl family of oncogenes and controls cell shape chages during gastrulation
in Drosophila. Genes Dev. 12, 274-284.
Harland, R. M.(1991). In situ hybridization: an improved whole-mount
method for Xenopus embryos. Methods Cell Biol. 36, 685-695.
Henderson, K. D. and Andrew, D. J.(2000). Regulation and function of Scr,
exd, and hthin the Drosophilasalivary gland. Dev. Biol. 217, 362-374.
Katzen, A. L., Kornberg, T. and Bishop, J. M.(1990). Diverse expression
of dsrc29A, a gene related to src, during the life cycle of Drosophila melanogaster. Development 110, 1169-1183.
Kelso, R. J., Hudson, A. M. and Cooley, L.(2002). Drosophila Kelch
regulates actin organization viaSrc64-dependent tyrosine phosphorylation.
J. Cell Biol. 156, 703-713.
Knoblich, J. A., Sauer, K., Jones, L., Richardson, H., Saint, R. and Lehner,
C. F.(1994). Cyclin E controls S phase progression and its down-regulation
during Drosophila embryogenesis is required for the arrest of cell
proliferation. Cell 77, 107-120.
Labno, C. M., Lewis, C. M., You, D., Leung, D. W., Takesono, A., Kamberos, N., Seth, A., Finkelstein, L. D., Rosen, M. K., Schwartzberg,
P. L. and Burkhardt, J. K. (2003). Itk functions to control actin
polymerization at the immune synapse through localized activation of Cdc42
and WASP. Curr. Biol. 13, 1619-1624.
Leise, W. F., 3rd and Mueller, P. R.(2004). Inhibition of the cell cycle is
required for convergent extension of the paraxial mesoderm during Xenopus
neurulation. Development 131, 1703-1715.
Leptin, M. and Grunewald, B.(1990). Cell shape changes during gastrulation
in Drosophila. Development 110, 73-84.
Maas, A. and Hendriks, R. W.(2001). Role of Bruton’s tyrosine kinase in B
cell development. Dev. Immunol. 8, 171-181.
Mano, H.(1999). Tec family of protein-tyrosine kinases: an overview of their
structure and function. Cytokine Growth Factor Rev. 10, 267-280.
Mata, J., Curado, S., Ephrussi, A. and Rorth, P. (2000). Tribbles
coordinates mitosis and morphogenesis in Drosophila by regulating
string/CDC25 proteolysis. Cell101, 511-522.
Myat, M. M. and Andrew, D. J.(2000a). Organ shape in the Drosophila
salivary gland is controlled by regulated, sequential internalization of the
primordia. Development127, 679-691.
Myat, M. M. and Andrew, D. J.(2000b). Fork headprevents apoptosis and
promotes cell shape change during formation of the Drosophila salivary
glands. Development 127, 4217-4226.
Nikolaidou, K. K. and Barrett, K.(2004). A Rho GTPase signaling pathway
is used reiteratively in epithelial folding and potentially selects the outcome
of Rho activation. Curr. Biol. 14, 1822-1826.
Panzer, S., Weigel, D. and Beckendorf, S. K.(1992). Organogenesis in
Drosophila melanogaster: embryonic salivary gland determination is controlled by homeotic and dorsoventral patterning genes. Development
114, 49-57.
Roulier, E. M., Panzer, S. and Beckendorf, S. K.(1998). The Tec29tyrosine
kinase is required during Drosophila embryogenesis and interacts with
Src64 in ring canal development. Mol. Cell1, 819-829.
Satterthwaite, A. B. and Witte, O. N.(2000). The role of Bruton’s tyrosine
kinase in B-cell development and function: a genetic perspective. Immunol. Rev. 175, 120-127.
Schöck, F. and Perrimon, N. (2002). Molecular mechanisms of epithelial
morphogenesis.Annu. Rev. Cell Dev Biol. 18, 463-493.
Seher, T. C. and Leptin, M. (2000). Tribbles, a cell-cycle brake that
coordinates proliferation and morphogenesis during Drosophila
gastrulation. Curr. Biol. 10, 623-629.
Shermoen, A. W. (2000). BrdU labeling of chromosomes. In Drosophila
Protocols(ed. W. Sullivan, M. Ashburner and R. S. Hawley), pp. 57-65. New York: Cold Spring Harbor Press.
Smith, A. V. and Orr-Weaver, T. L.(1991). The regulation of the cell cycle
during Drosophilaembryogenesis: the transition to polyteny. Development
112, 997-1008.
Takesono, A., Finkelstein, L. D. and Schwartzberg, P. L.(2002). Beyond
calcium: new signaling pathways for Tec family kinases. J. Cell Sci. 115,
3039-3048.
Tateno, M., Nishida, Y. and Adachi-Yamada, T.(2000). Regulation of JNK
by Src during Drosophiladevelopment. Science287, 324-327.
Tautz, D. and Pfeifle, C. (1989). A non-radioactive in situ hybridization
method for the localization of specific RNAs in Drosophilaembryos reveals
translational control of the segmentation gene hunchback. Chromosoma 98,
81-85.
Tepass, U., Theres, C. and Knust, E.(1990). crumbsencodes an EGF-like
protein expressed on apical membranes of Drosophilaepithelial cells and
required for organization of epithelia. Cell. 61, 787-799.
Thomas, G. H. and Kiehart, D. P.(1994). Beta heavy-spectrin has a restricted
tissue and subcellular distribution during Drosophila embryogenesis.
Development120, 2039-2050.
Thomas, J. H. and Wieschaus, E.(2004). Src64and Tec29are required for
microfilament contraction during Drosophilacellularization. Development
131, 863-871.
Vincent, W. S., 3rd, Gregory, R. J. and Wadsworth, S. C. (1989).
Embryonic expression of a Drosophila src gene: alternate forms of the
protein are expressed in segmental stripes and in the nervous system. Genes Dev. 3, 334-347.
Wadsworth, S. C., Muckenthaler, F. A. and Vincent, W. S., 3rd(1990).
Differential expression of alternate forms of a Drosophilasrc protein during
embryonic and larval tissue differentiation. Dev. Biol. 138, 296-312.
Weigel, D., Bellen, H., Jürgens, G. and Jäckle, H. (1989). Primordium
specific requirement of the homeotic gene fork headin the developing gut
of the Drosophilaembryo. Roux’s Arch. Dev. Biol. 198, 201-210.
Weiss, A., Herzig, A., Jacobs, H. and Lehner, C. F.(1998). Continuous
Cyclin E expression inhibits progression through endoreduplication cycles
in Drosophila. Curr. Biol. 8, 239-242.
Young, P. E., Pesacreta, T. C. and Kiehart, D. P.(1991). Dynamic changes
in the distribution of cytoplasmic myosin during Drosophilaembryogenesis.
Development. 111, 1-14.
Zhou, B., Bagri, A. and Beckendorf, S. K. (2001). Salivary gland
determination in Drosophila: a salivary-specific, fork head enhancer
integrates spatial pattern and allows fork head autoregulation. Dev. Biol. 237,
54-67.