Development 140, 4375-4385 (2013) doi:10.1242/dev.097733 © 2013. Published by The Company of Biologists Ltd
INTRODUCTION
A variety of craniofacial organs and tissues, such as the Meckel’s cartilage, maxillary and mandible bone, trigeminal ganglion and dentin-producing odontoblasts, derive from craniofacial neural crest cells (Chai et al., 2000; Chung et al., 2009). Despite originating from the same progenitor population, craniofacial bone and tooth exhibit distinct developmental, morphological and histological characteristics (Lumsden, 1988; D’Souza et al., 1999; Chai et al., 2000; Zhang et al., 2005; James et al., 2006). It is well established that multiple signaling pathways, including Wnt, TGFβ/BMP, Hh and FGF signaling, are involved in regulating every step of tooth development (Thesleff and Mikkola, 2002; Tummers and Thesleff, 2009), but the mechanisms that specify and ensure the odontogenic fate in dental mesenchyme remain largely unknown.
FGF signaling has been implicated in regulating tooth development at several distinct steps. FGF signaling might be involved in the specification of odontogenic fate in both dental epithelial and dental mesenchyme, as evidenced by Fgf8expression in the presumptive dental epithelium and its induction of Pitx2and
Pax9, the earliest molecular markers of the dental epithelium and mesenchyme, respectively, to determine the tooth-forming site (Neubüser et al., 1997; Trumpp et al., 1999; St Amand et al., 2000). At the bud stage, epithelial FGF4 and FGF8 are likely to activate
Fgf3in the dental mesenchyme through the mediation of Msx1and
Runx2, and FGF3 in turn, possibly together with FGF10, acts back on the dental epithelium to induce/maintain Shhexpression in the enamel knot (Bei and Maas, 1998; Kettunen et al., 2000; Aberg et
al., 2004). At the cap stage, expression of several FGFs in the enamel knot stimulates cell proliferation in the dental epithelium, leading to epithelial folding and cusp patterning (Jernvall et al., 1994; Jernvall and Thesleff, 2000). Furthermore, releasing FGF signaling from suppression by Sprouty factors leads to tooth formation in the diastema region, indicating a potential role for FGF signaling in the regulation of odontogenic fate (Klein et al., 2006; Li et al., 2011b).
The essential role of canonical Wnt (Wnt/β-catenin) signaling in tooth development has been well documented (Liu and Millar, 2010). Many Wnt ligands are expressed in the developing tooth, predominantly in the epithelial component, with WNT5A, a non-canonical Wnt, in the mesenchyme (Dassule and McMahon, 1998; Sarkar and Sharpe, 1999). These Wnt ligands appear to act in both intra- and intertissue manners to regulate tooth development. Epithelial deletion of Catnb (Ctnnb1 – Mouse Genome Informatics), the gene encoding β-catenin, or Gpr177(Wls – Mouse Genome Informatics), the product of which is required for secretion of Wnts, leads to an arrest of tooth development at the bud or early cap stage (Liu et al., 2008; Zhu et al., 2013). A similar developmental defect was also observed in mice lacking Catnbin the dental mesenchyme (Chen et al., 2009). Conversely, constitutive activation of β-catenin signaling in oral epithelium induces ectopic tooth formation (Järvinen et al., 2006; Liu et al., 2008). Although β -catenin signaling activity is present in the dental mesenchyme of the E12.5 incisor (Fujimori et al., 2010), such activity has never been reported in the incisor mesenchyme beyond E12.5 and was not detected in developing molar mesenchyme using several Wnt/β -catenin signaling reporter mouse lines, including BATGAL,
TOPGALand TCF/Lef-lacZmice (Liu et al., 2008), suggesting that Wnt/β-catenin activity is maintained at a very low level, if any, in the dental mesenchyme. Elevated Wnt/β-catenin signaling results in the formation of bone-like tissues in the dental pulp (Chen et al., 2009; Li et al., 2011a). Thus, a finely tuned level of Wnt/β-catenin signaling is essential for proper tooth development.
1Department of Cell and Molecular Biology, Tulane University, New Orleans, LA 70118, USA. 2College of Life Science, Fujian Normal University, Fuzhou, Fujian Province 350108, P.R. China.
*Author for correspondence (ychen@tulane.edu)
Accepted 19 August 2013 SUMMARY
Odontoblasts and osteoblasts develop from multipotent craniofacial neural crest cells during tooth and jawbone development, but the mechanisms that specify and sustain their respective fates remain largely unknown. In this study we used early mouse molar and incisor tooth germs that possess distinct tooth-forming capability after dissociation and reaggregation in vitroto investigate the mechanism that sustains odontogenic fate of dental mesenchyme during tooth development. We found that after dissociation and reaggregation, incisor, but not molar, mesenchyme exhibits a strong osteogenic potency associated with robustly elevated β-catenin signaling activity in a cell-autonomous manner, leading to failed tooth formation in the reaggregates. Application of FGF3 to incisor reaggregates inhibits β-catenin signaling activity and rescues tooth formation. The lack of FGF retention on the cell surface of incisor mesenchyme appears to account for the differential osteogenic potency between incisor and molar, which can be further attributed to the differential expression of syndecan 1 and NDST genes. We further demonstrate that FGF signaling inhibits intracellular β -catenin signaling by activating the PI3K/Akt pathway to regulate the subcellular localization of active GSK3βin dental mesenchymal cells. Our results reveal a novel function for FGF signaling in ensuring the proper fate of dental mesenchyme by regulating β-catenin signaling activity during tooth development.
KEY WORDS: β-catenin signaling, FGF, Tooth development
FGF signaling sustains the odontogenic fate of dental
mesenchyme by suppressing
β
-catenin signaling
Chao Liu1, Shuping Gu1, Cheng Sun1, Wenduo Ye1, Zhongchen Song1, Yanding Zhang2and YiPing Chen1,2,*
In this study, we investigated the mechanisms underlying our previous finding that early molar and incisor tooth germs exhibit distinct tooth-forming capability after dissociation and reaggregation in vitro(Song et al., 2006).
MATERIALS AND METHODS Animals
BATGAL mice (Maretto et al., 2003) were obtained from Jackson Laboratories and were crossed onto the CD-1 background. All wild-type mice were CD-1 background and purchased from Charles River. Animals and procedures used in this study were approved by the Institutional Animal Care and Use Committee of Tulane University.
Tissue recombination, organ culture, bead implantation and subrenal culture
Embryonic day (E) 13.5 or E14.5 embryos were collected from timed pregnant mice. To prepare tooth reaggregates, mandibular incisor or molar germs from one litter of embryos were isolated and pooled, respectively, then treated with 0.25% trypsin in 1 mM EDTA at 37°C for 5 minutes, and then dispersed into a single-cell suspension by mechanical aspiration with a micropipette. About 1×106cells from either the incisor or molar pool were
added to a 1.5-ml Eppendorf tube, centrifuged at 3000 rpm (550 g) for 5 minutes, and incubated at 37°C and 5% CO2for 1 hour to allow the
formation of a firm cell pellet. Cell pellets were removed from Eppendorf tubes, placed in Trowell type organ culture in DMEM supplemented with 20% FBS overnight prior to being subjected to subrenal culture as described previously (Zhang et al., 2003; Song et al., 2006).
To prepare tooth reaggregates with exchanged dental epithelial cells, isolated incisor and molar germs were treated with 2 mg/ml dispase at 37°C for 30 minutes, washed with DMEM containing 20% FBS, and then dental epithelia were separated from dental mesenchyme with the aid of fine forceps. Incisor mesenchyme was pooled together with molar epithelia and vice versa, and pooled dental tissues were further treated with 0.25% trypsin in 1 mM EDTA at 37°C for 2 minutes to generate the single-cell suspension and tooth reaggregates as described above.
Protein-soaked bead preparation and implantation in tooth reaggregates or intact dental mesenchyme were performed as reported previously (Li et al., 2011b). Affi-Gel Blue agarose beads or heparin beads (100-200 μm in diameter; Bio-Rad) were used as carriers for FGF8 (0.5 mg/ml), FGF4 (0.4 mg/ml), FGF3 (0.5 mg/ml), FGF9 (0.5 mg/ml), FGF10 (0.5 mg/ml), DKK1 (0.4 mg/ml) and WNT10B (0.1 mg/ml) (all from R&D Systems).
For the heparinase treatment experiment, E14.5 BATGALmolar germs were isolated and treated with dispase, and the epithelia were removed, as described above. The remaining dental mesenchyme was dispersed into single-cell suspension and pelleted. Cell pellet containing ~1×106cells was
resuspended in 0.5 ml heparinase buffer (New England Biolabs). The final concentration of heparinases was adjusted as follows: heparinase I (150 unit/ml), heparinase II (10 unit/ml) and heparinase III (40 unit/ml). The cell suspension was incubated at 37°C for 1 hour before reaggregation and organ culture.
Histology, in situhybridization and X-Gal staining
Samples for histological analysis were harvested and fixed in 4% paraformaldehyde (PFA)/PBS. Ossified samples were subjected to Decalcifier I (Leica Biosystems) for demineralization for a week, and then dehydrated through graded ethanol, cleared with xylene, embedded in paraffin, and sectioned at 10 µm for standard Hematoxylin/Eosin (H&E) staining (Presnell and Schreibman, 1997).
For in situhybridization, samples were harvested in ice-cold PBS and fixed in 4% PFA/PBS at 4°C overnight prior to dehydration through graded ethanol and embedding in paraffin. Samples were sectioned at 10 μm and subjected to non-radioactive in situhybridization as described (Yu et al., 2005b). At least three samples were used for each probe.
For whole-mount X-Gal staining, samples were fixed with 4% PFA/PBS at room temperature for 20 minutes and then stained for β-galactosidase activity according to a standard procedure (Chai et al., 2000). For section X-Gal staining, samples were fixed in 4% PFA/PBS at 4°C overnight, passed
through 15% and 30% sucrose series, embedded in O.C.T. compound (Tissue-Tek) and cryosectioned at 10 μm. Sections were then subjected to standard X-Gal staining (Chai et al., 2000).
Immunostaining and immunoblotting
For immunohistochemical staining, samples were fixed in Z-Fix (Anatech) at room temperature for 2 hours, dehydrated with 15% and 30% sucrose series, embedded in O.C.T. and cryosectioned at 10 μm. Immunohistochemical staining was conducted according to the manufacturer’s instruction using the following antibodies: mouse monoclonal anti-FGF3 antibody (Santa Cruz), rat anti-mouse syndecan 1 antibody (BD Pharmingen), rat monoclonal antibodies against heparan chondroitin sulfate, heparan dermatan sulfate and heparan keratan sulfate, respectively (Antibodies-online), biotinylated rabbit anti-mouse antibody (Vector Laboratories), horseradish peroxidase-coupled goat anti-rabbit IgG (Sigma), and Alexa Fluor 488 goat anti-rat antibody (Molecular Probes).
For immunocytochemical staining, lower incisor and molar germs were treated with dispase and epithelia removed. Dental mesenchyme was collected and treated with trypsin to make a single-cell suspension as described above. Suspended dental mesenchymal cells were placed onto cell culture dishes and cultured in DMEM supplemented with 20% FBS for 6-12 hours, and then fixed with Z-Fix for 20 minutes. Immunocytofluorescence was performed using primary antibodies against
β-catenin (Millipore), GSK3βY216(Abcam) and GSK3βser9(Abcam). Alexa
Fluor 488 and Alexa Fluor 546 goat anti-rabbit IgG (Molecular Probes) were used as the secondary antibodies.
Immunoblotting was performed as described previously (Iwata et al., 2006). Rabbit polyclonal antibodies against P-AktSer473and total Akt (Cell
Signaling) and mouse monoclonal antibodies against β-actin and FGF3 (Santa Cruz) were used as primary antibodies. IRDye 800cw goat anti-rabbit IgG and IRDye 800cw donkey anti-mouse IgG were used as secondary antibodies (Li-Cor).
Quantitative RT-PCR
For quantitative (q) RT-PCR analysis, samples were subjected to RNA extraction using the RNAqueous-4PCR Kit (Ambion). The high capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used for cDNA synthesis. qPCR was carried out on the 7500 Fast Real-Time PCR System (Applied Biosystems) with gene-specific primers and SYBR Green. Values were normalized to Gapdhusing the 2–ΔΔCtmethod. Data from at least three
independent experiments or samples for each gene were used for analysis.
RESULTS
Incisor mesenchymal cells adopt an osteogenic fate in tooth germ reaggregates
We reported previously that E13.5 mouse molar germ, after dissociation and reaggregation, is able to form a well differentiated tooth organ, whereas incisor germ fails (Song et al., 2006) (Fig. 1A,B). We followed up on this observation to investigate the underlying mechanism. We first tested if failed tooth formation in incisor reaggregates results from a loss of odontogenic competence in the dental epithelial cells after dispersion by exchanging dissociated epithelial cells and mesenchymal cells between E13.5 incisor and molar germ. Reaggregates composed of incisor epithelial and molar mesenchymal cells formed teeth (n=9/10), but reaggregates constituted by incisor mesenchymal and molar epithelial cells failed (n=0/10) and generated bony structures and keratinized cysts after 2 weeks in subrenal culture (Fig. 1C,D). These observations indicate that the incisor mesenchyme loses its odontogenic capability to instruct dispersed dental epithelial cells to form teeth.
Gene expression assays demonstrate the expression of mesenchymal odontogenic markers, including Pax9, Bmp4 and
reaggregates after 3 days in culture (Fig. 1E-J). By contrast, osteogenic markers, including Runx2, osteocalcin (Bglap– Mouse Genome Informatics) and osterix (Sp7 – Mouse Genome Informatics), were activated in mesenchymal cells of incisor reaggregates but not in molar reaggregates (Fig. 1K-N; data not shown), which was further confirmed by qPCR assay (Fig. 1Q). These expression patterns persisted in incisor and molar reaggregates after 5 days in subrenal culture (data not shown). The retained expression of Pitx2, a dental epithelial molecular marker, in the reorganized dental epithelial masses in both incisor and molar reaggregates indicates their odontogenic fate (Fig. 1O,P). These results suggest a deviation of odontogenic fate and the rapid adoption of osteogenic fate in the incisor mesenchyme after dissociation and reaggregation.
Wnt/β-catenin signaling is robustly activated in mesenchymal cells of incisor reaggregates
We next investigated the molecular basis for the adoption of osteogenic fate in mesenchymal cells of incisor reaggregates. Wnt/β-catenin signaling plays a crucial role in promoting osteogenic fate and the maturation of osteoblasts (Clément-Lacroix et al., 2005; Gaur et al., 2005). We suspected that dissociation and reaggregation
of incisor tooth germ led to the activation of β-catenin signaling and subsequent osteogenesis. β-catenin signaling activity is restricted to the dental epithelium of E13.5 incisor and molar, as detected by the BATGALtransgenic reporter (Fig. 2A,B). Using the BATGAL
reporter mice, we found that after 3 days in subrenal culture, robust
BATGAL activity could be detected in both epithelial and mesenchymal cells in E13.5 incisor reaggregates (Fig. 2D;
n=14/14). By contrast, BATGALactivity was detected only in the reorganized dental epithelial structures in molar reaggregates (Fig. 2C; n=20/20). However, in molar reaggregates after 5 days in subrenal culture, BATGAL activity was detected in some mesenchymal cells that were not associated with the forming tooth but also expressed osteocalcin (Fig. 2E,F), indicating an association of active β-catenin signaling with osteogenic fate.
We examined whether molar epithelial cells played an inhibitory role in suppressing β-catenin signaling in molar reaggregates. E13.5
[image:3.612.53.415.60.480.2]BATGALincisor and molar mesenchyme without dental epithelium were dissociated and reaggregated. Robust BATGALactivity was detected in the incisor but not in the molar mesenchymal reaggregate as early as 2 hours in organ culture (Fig. 2E,F), suggesting the existence of a mechanism to inhibit β-catenin signaling in the molar mesenchyme.
Fig. 1. Adoption of osteogenic fate by mesenchymal cells in E13.5 incisor reaggregates. (A-D) H&E staining of mouse tooth reaggregates after 2 weeks in subrenal culture shows tooth formation (arrows) in E13.5 molar reaggregate (A) and in the reaggregate of E13.5 molar mesenchymal cells and incisor epithelial cells (C). Tooth formation failed in E13.5 incisor reaggregate (B) and reaggregate of E13.5 incisor mesenchymal cells and molar epithelial cells (D). (B,D) Arrows point to keratinized cysts. (E-N) In situ
hybridization of tooth reaggregates after 3 days in subrenal culture shows expression of Pax9, Bmp4and Msx1but absence of Runx2and osteocalcin expression in E13.5 molar reaggregates (E,G,I,K,M), and opposite expression patterns of these genes in E13.5 incisor reaggregates (F,H,J,L,N). Arrows point to gene expression sites. (O,P) Pitx2 expression is seen in the reorganized epithelial structures in E13.5 molar (O) and incisor (P) reaggregates. (Q) Real-time RT-PCR results show dramatically elevated expression of osteogenic markers in incisor (In) reaggregates as compared with molar (Mol) reaggregates after 3 days in subrenal culture. **P<0.01, ***P<0.001 (Student's t-test); error bars indicate s.d. Dashed lines encircle epithelial structures. Scale bars: 200 μm.
FGF3 sustains odontogenic fate and rescues tooth formation in incisor reaggregates by suppressing β-catenin signaling
We next investigated the mechanism that leads to the differential activation of β-catenin signaling in the incisor and molar mesenchyme after dissociation and reaggregation. Since Wnt ligands are expressed predominantly in the dental epithelium and the expression of several Wnt antagonists was found at much higher levels in incisor than in molar mesenchymal cell reaggregates after 12 hours in organ culture (Fig. 2I), we reasoned that β-catenin signaling is activated in a cell-autonomous manner in incisor mesenchymal reaggregates. Since FGF signaling may be involved in odontogenic specification (Neubüser et al., 1997; Trumpp et al., 1999; Kettunen et al., 2000; St Amand et al., 2000; Mandler and Neubüser, 2001), and Fgf3is expressed in the molar mesenchyme but at an extremely low level in incisor mesenchyme at E13.5 as determined by in situhybridization and qRT-PCR (Fig. 3A,B,I), we tested if FGF3 could sustain the odontogenic fate by implanting FGF3-soaked beads into E13.5 incisor germ reaggregates. Surprisingly, tooth formation was observed in FGF3-supplemented incisor reaggregates (48%, n=11/23) after 2 weeks in subrenal culture (Fig. 3D). As a negative control, BSA-soaked beads failed to have any such effect (n=0/20; Fig. 3C). In addition, we found that exogenously applied FGF3 did not affect tooth formation in molar reaggregates (n=11/11; data not shown). Consistent with the observation that loss of odontogenic fate is accompanied by rapid osteogenesis in the incisor mesenchyme, FGF3-soaked beads inhibited osteogenesis, as assessed by the dramatically reduced
expression of Runx2 and osterix in incisor mesenchymal cell reaggregates as compared with the BSA controls (Fig. 3E-H).
Because Runx2and osterix are the direct targets of β-catenin signaling and their expression is inhibited by FGF3 in incisor reaggregates, we hypothesized that FGF signaling might prevent osteogenesis by inhibiting β-catenin activity in the dental mesenchyme. We applied FGF3-soaked beads to isolated E13.5 incisor mesenchyme and found an inhibition of BATGALactivity after 12 hours in organ culture, as compared with BSA controls (Fig. 4A,B). As several other FGFs are also expressed in the early developing tooth, we further tested whether they have a similar inhibitory effect by applying FGF4-, FGF8-, FGF9- and FGF10-soaked beads to E13.5 incisor mesenchyme explants. FGF4 and FGF8, but not FGF9 and FGF10, were able to inhibit BATGAL
activity (Fig. 4C-F; data not shown).
[image:4.612.55.491.58.328.2]The fact that DKK1-soaked beads were not able to inhibit ectopic activation of β-catenin signaling in incisor mesenchyme explants suggests a ligand-independent activation through an intracellular regulatory mechanism (Fig. 4F). Although exogenously applied WNT10B, one of the canonical Wnts expressed in the dental epithelium, was able to induce BATGALexpression in E10.5 limb mesenchyme without epithelium, at which time BATGALactivity is restricted in the apical ectodermal ridge (Noda et al., 2012), WNT10B-soaked beads failed to induce BATGALexpression in E13.5 molar mesenchyme (Fig. 4G-I). These observations further support the existence of an intracellular repressive mechanism in the dental mesenchyme. Indeed, at the cellular level, in contrast to E13.5 molar mesenchymal cells in which β-catenin exhibited Fig. 2. Ectopic activation of β-catenin signaling in mesenchymal cells of incisor reaggregates.(A,B) X-Gal staining shows restricted BATGAL reporter activity in the dental epithelium of E13.5 molar (A) and incisor (B). (C,D) BATGALactivity is detected only in dental epithelial cells (arrows) of E13.5 molar reaggregate (C) but is activated in both epithelial and mesenchymal cells of incisor reaggregate (D) after 3 days in subrenal culture. (E,F) BATGALactivity is detected in some mesenchymal cells in the peripheral region (arrow) of molar reaggregates after 5 days in culture (E), where osteocalcin is also expressed (F). (G,H) Robust BATGALactivity is detected in E13.5 incisor mesenchymal cell reaggregate (H) but not in molar mesenchymal cell reaggregate (G) after 2 hours in organ culture. (I) Comparison of expression levels of Wnt antagonists, as measured by real-time RT-PCR, in incisor mesenchyme reaggregates and molar mesenchyme reaggregates after 12 hours in organ culture. **P<0.01 (Student's t-test); error bars indicate s.d. Dashed lines circle epithelial structures. DE, dental epithelium; DM, dental mesenchyme. Scale bars: 200 μm.
cytoplasmic localization (Fig. 4J-J⬙; 93%, from seven independent experiments), E13.5 incisor mesenchymal cells showed intense nuclear accumulation of β-catenin (Fig. 4K-K⬙; 88.1%, from seven independent experiments). However, application of FGF3 (250 ng/ml) to cell culture prevented the nuclear accumulation of β -catenin in incisor mesenchymal cells (Fig. 4L-L⬙; 54.8%, from three independent experiments; P<0.005 compared with untreated group), indicating that FGF3 inhibits β-catenin signaling by regulating its subcellular localization. It is interesting to note that the nuclear size of incisor mesenchymal cells is much smaller than that of molar mesenchymal cells in cell culture, but becomes enlarged after FGF3 treatment (Fig. 4J⬘,K⬘,L⬘). Although the biological importance of the changes in nuclear size is unknown, these observations suggest an involvement of FGF and β-catenin signaling in the regulation of nuclear size.
Differential expression of syndecan 1 and NDST genes is associated with distinct FGF3 retention capability between the incisor and molar mesenchyme
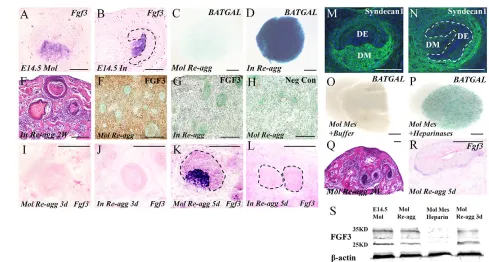
[image:5.612.52.269.55.450.2]Whereas Fgf3expression was not detectable by in situhybridization in E13.5 incisor (Fig. 3), its expression level in the incisor mesenchyme at E14.5 became comparable to that in E14.5 molar mesenchyme (Fig. 5A,B). However, despite Fgf3expression, teeth still failed to form in E14.5 incisor germ reaggregates (Fig. 5E). Interestingly, similar to E13.5 incisor mesenchymal reaggregates, E14.5 incisor mesenchymal reaggregates also showed significantly Fig. 3. FGF3 rescues tooth formation and inhibits rapid osteogenesis
in incisor reaggregates. (A,B) Fgf3expression is not detectable in the incisor (A) but is seen in the molar mesenchyme (B). (C,D) FGF3-soaked beads but not BSA-soaked beads rescue tooth formation in E13.5 incisor reaggregates after 2 weeks in subrenal culture. (E-H) FGF3 beads but not BSA beads inhibit Runx2and osterix expression in E13.5 incisor
[image:5.612.308.561.59.433.2]mesenchymal cell reaggregates after 3 days in culture. (I) Real-time RT-PCR results show relative levels of Fgf3expression in E13.5 incisor and molar germs. **P<0.01 (Student’s t-test); error bars indicate s.d. B, bead. Scale bars: 200 μm.
Fig. 4. FGF signaling inhibits β-catenin signaling in dental
mesenchymal cells. (A-F) FGF3 (B), FGF4 (C) and FGF8 (D) soaked beads prevent ectopic activation of Wnt/β-catenin signaling in isolated E13.5 BATGALincisor mesenchyme, but BSA (A), FGF10 (E) and DKK1 (F) soaked beads fail to do so. Note that Wnt/β-catenin activity is not inhibited by FGF3, FGF4 or FGF8 in the symphystic portion (asterisk) of Meckel’s cartilage. Red dashed line encircles the implanted beads.
(G-I) WNT10B- but not BSA-soaked beads induce BATGALexpression in E10.5 limb bud mesenchyme (G,H), but WNT10B beads cannot induce BATGAL activity in E13.5 molar mesenchyme after 12 hours in organ culture (I). (J-L⬙) Immunocytochemical staining shows localization of β-catenin in the cytoplasm of E13.5 molar mesenchymal cells (J-J⬙), and nuclear accumulation of β-catenin in E13.5 incisor mesenchymal cells (K-K⬙) after 12 hours in cell culture. Addition of FGF3 to cell culture prevents nuclear accumulation of β-catenin in incisor mesenchymal cells (L-L⬙; arrows point to cells with nuclear localization of β-catenin).
elevated BATGALactivity after 2 hours in culture, as compared with E14.5 molar mesenchymal reaggregates (Fig. 5C,D). We wondered whether the activation of β-catenin signaling and failure of tooth formation result from a lack of de novo Fgf3expression in incisor germ reaggregates. In situhybridization showed that Fgf3expression was lost and was never re-established in E14.5 incisor reaggregates after 3 and 5 days in culture (Fig. 5J,L). However, de novo Fgf3
expression was not detected in E14.5 molar reaggregates until 5 days in culture (Fig. 5I,K), suggesting that the rapid activation of β-catenin signaling in incisor reaggregates is not a consequence of failed de novosynthesis of FGF3. Since both E14.5 incisor and molar germs express Fgf3, we next examined whether incisor and molar mesenchymal cells have different capabilities in retaining FGF3 protein in reaggregates. Immunohistochemical studies revealed the presence of FGF3 in the mesenchymal compartment of E14.5 molar reaggregates after 2 days in culture when de novoactivation of Fgf3
had not yet begun (Fig. 5F). By contrast, no retained FGF3 was detected in E14.5 incisor reaggregates (Fig. 5G).
Heparan sulfate proteoglycans (HSPGs) play crucial roles in the transport and reception of secreted factors, and are known to promote FGF signaling by enriching FGF ligands, preventing them from degrading, and facilitating their binding to receptors (Lin, 2004; Häcker et al., 2005). We asked whether there is a differential expression of HSPGs in the incisor and molar that could account for the distinct FGF retention capability. We performed
immunohistochemical staining on E14.5 incisor and molar teeth to examine a number of HSPGs, including heparan chondroitin sulfate, heparan keratan sulfate and heparan dermatan sulfate, as well as syndecan 1, which has the highest expression level among several syndecans in the developing tooth (Thesleff et al., 1988; Vainio et al., 1989; Vainio et al., 1991; Vainio and Thesleff, 1992; Bai et al., 1994). Although heparan chondroitin sulfate, heparan keratan sulfate and heparan dermatan sulfate are among the richest HSPGs in the developing embryo, none was expressed in incisor or molar germs (data not shown). However, syndecan 1 was found to be highly expressed in the molar mesenchyme, but was absent or expressed at a very low level in the incisor mesenchyme despite its expression in the surrounding tissues (Fig. 5M,N). These results suggest that the higher level of syndecan 1 in the molar mesenchyme protects FGF3 from degradation by enzyme treatment and from diffusing into suspension during dissociation and reaggregation.
To determine whether HSPGs play a role in suppressing β-catenin signaling by facilitating FGF signaling in dental mesenchymal cells, we treated dissociated E14.5 BATGALmolar mesenchymal cells with heparinases before reaggregation. An elevated BATGAL
[image:6.612.53.542.55.317.2]activity was detected in heparinase-treated molar mesenchymal reaggregates (n=11/11) after 12 hours in culture, as compared with control reaggregates (n=2/10) (Fig. 5O,P). Moreover, heparinase-treated molar mesenchymal cells failed to form a tooth (n=0/8) after reaggregation with dissociated molar epithelial cells (Fig. 5Q), as Fig. 5. Failed FGF3 retention is associated with ectopic activation of β-catenin signaling in incisor reaggregates. (A,B) In situhybridization shows Fgf3expression in the mesenchyme of E14.5 molar (A) and incisor (B). (C,D) X-Gal staining shows elevated Wnt/β-catenin signaling in E14.5 BATGALincisor mesenchymal cell reaggregate (D) but not in E14.5 molar mesenchymal cell reaggregate (C) after 2 hours in culture. (E) H&E staining shows lack of tooth formation in an E14.5 incisor reaggregate. (F-H) Immunohistochemical staining shows retention of FGF3 in E14.5 molar reaggregate (F) but not in E14.5 incisor reaggregate (G) and lack of positive signaling in the negative control of molar reaggregate (H). (I-L) In situhybridization shows lack of Fgf3 expression in E14.5 molar reaggregate after 3 days in subrenal culture (I) and in E14.5 incisor reaggregate after 3 days (J) and 5 days (L) in subrenal culture. However, Fgf3expression was detected in mesenchymal cells immediately adjacent to a reorganized epithelial structure in an E14.5 molar reaggregate after 5 days in culture (K). (M,N) Immunohistochemical staining shows differential expression of syndecan 1 in E14.5 molar (M) and incisor (N). (O,P) X-Gal staining shows activation of β-catenin signaling in E14.5 BATGALmolar mesenchymal cell reaggregate after treatment with heparinases (P) but not in control (O). (Q,R) E14.5 molar reaggregates failed to form tooth (Q) and did not express Fgf3after heparinase treatment. (S) Western blotting shows the presence of FGF3 protein in intact E14.5 molar germ (lane 1) and retention of FGF3 in E14.5 molar reaggregates after 1 hour (lane 2) and 3 days (lane 4) in culture but not in heparinase-treated reaggregates after 1 hour in culture. Dashed lines demarcate dental epithelial structures. Scale bars: 200 μm.
compared with controls (n=7/8; data not shown). Such reaggregates also failed to express Fgf3after 5 days in culture (Fig. 5R). Western blotting further confirmed that heparinase-treated molar reaggregates could not retain FGF3 even after 1 hour in culture (Fig. 5S), consistent with failed tooth formation in such reaggregates (Fig. 5Q). The fact that FGF3 signals through FGFR1 and FGFR2 (Powers et al., 2000) and that both receptors are expressed in an overlapping pattern in the dental mesenchyme of E13.5 and E14.5 incisor and molar (Fig. 6A-H), further supports the functional importance of FGF3 retention in tooth formation.
N-deacetylase/N-sulfotransferases (NDSTs) are the speed-limiting enzymes for heparan sulfate modification after HSPGs are synthesized. We further examined the expression patterns of Ndst1-4in the developing incisor and molar. With the exception of Ndst3, all of the other three NDST genes were expressed in both incisor and molar germs with distinct patterns (Fig. 6; data not shown). In the developing incisor, the expression of Ndst1 and Ndst2was restricted in the epithelium at E12.5 and E13.5, but was expanded into the mesenchyme slightly at E14.5 (Fig. 6). By contrast, in the developing molar, Ndst1was expressed in the epithelium and mesenchyme from E12.5 to E14.5, and Ndst2expression was found in the epithelium at E13.5 but expanded into the mesenchyme at a high level at E14.5 (Fig. 6). Ndst4expression was not detected in either incisor or molar until E14.5, with a high level in the epithelium (Fig. 6). Thus, the low levels/absence of syndecan 1 and the possibly reduced extent of heparin sulfation of HSPGs make incisor mesenchyme prone to FGF protection and retention during dissociation and reaggregation.
FGF signaling regulates the subcellular localization of active GSK3βand β-catenin in dental
mesenchymal cells by activating the PI3K/Akt pathway
We next sought to determine the mechanism through which FGF3 prevents nuclear accumulation of β-catenin in dental mesenchymal cells. GSK3β acts a negative modulator of β-catenin signaling by phosphorylating β-catenin for degradation. We investigated the immunocytochemical localization of both inactive and active forms of GSK3β in dissociated E13.5 incisor and molar mesenchymal cells in culture. Whereas the inactive form (p-GSK3βSer9) was similarly localized in the cytoplasm of incisor and molar mesenchymal cells (data not shown), the active form (p-GSK3βY216) showed distinct subcellular localizations. In the majority of molar cells, p-GSK3βY216 was found predominantly in the cytoplasm (Fig. 7A-A⬙), but in the incisor cells p-GSK3βY216was localized exclusively in the nuclei (Fig. 7B-B⬙; 88%, from five independent experiments). Since the degradation of β-catenin by p-GSK3βY216requires the coordination of AXIN2 and APC, which reside only in the cytoplasm (Logan and Nusse, 2004; Ciani and Salinas, 2005), the nuclear localized p-GSK3βY216 in the incisor mesenchymal cells is incapable of degrading β-catenin and modulating β-catenin signaling negatively. Treatment of incisor mesenchymal cells with FGF3 (250 ng/ml) in cell culture changed the subcellular localization of p-GSK3βY216, with 46% (from three independent experiments) of cultured incisor mesenchymal cells exhibiting cytoplasmic localization of p-GSK3βY216 (Fig. 7C-C⬙), indicating that FGF3 promotes the cytoplasmic localization of active GSK3β.
It was reported previously that FGF signaling promotes both the nuclear export and activation of GSK3β through the PI3K/Akt pathway in mouse embryonic stem cells (mESCs) (Bechard and Dalton, 2009; Singh et al., 2012). To determine if similar mechanisms are employed in dental mesenchymal cells, we first performed western blotting to examine the activity levels of the PI3K/Akt
pathway. Similar levels of active Akt (P-AktS473) and total Akt (Pan-Akt) were found in the intact incisor and molar mesenchyme at both E13.5 and E14.5 (Fig. 8A). However, after 4 hours in cell culture, active Akt was completely absent from the incisor mesenchymal cells, but was retained in molar mesenchymal cells (Fig. 8B). Addition of FGF3 (250 ng/ml) to incisor mesenchymal cell culture resumed the expression of active Akt (Fig. 8B).
We further determined whether the active PI3K/Akt pathway regulates the subcellular localization of active GSK3β (p-GSK3βY216) in dental mesenchymal cells by immunocytochemical assay. Dissociated E13.5 molar mesenchymal cells retained the cytoplasmic localization of p-GSK3βY216in ~94.2% (from three experiments) of cells after 12 hours in cell culture (Fig. 8C-C⬙), but exhibited nuclear localization of p-GSK3βY216in the presence of the PI3K/Akt pathway inhibitor BEZ235 (5 μM) in 36% (from three experiments) of cells (Fig. 8D-D⬙; P<0.01). Consistent with the nuclear localization of p-GSK3βY216, 43% (from three experiments) of molar mesenchymal cells displayed the nuclear accumulation of β-catenin in the presence of BEZ235, as compared with 6.2% (from three experiments) nuclear localized control cells (P<0.01; Fig. 8E-F⬙). Thus, FGF signaling appears to promote the cytoplasmic localization of active GSK3β in dental mesenchymal cells by activating the PI3K/Akt pathway to suppress β-catenin signaling.
DISCUSSION
Elevated β-catenin signaling in the dental mesenchyme is detrimental to normal odontogenesis
Multiple Wnt ligands are expressed in the dental epithelium of the developing tooth and have been demonstrated to act in an intra-Fig. 6. Expression of FGFR1, FGFR2 and NDST genes in the
developing tooth.(A-H) Immunohistochemical staining shows expression of FGFR1 (A-D) and FGFR2 (E-H) in E13.5 and E14.5 incisor and molar germs. Note the overlapping patterns of these two receptors in the dental mesenchyme. (I-T) In situhybridization shows expression of Ndst1 (I-L), Ndst2(M-P) and Ndst4(Q-T) in developing incisor and molar. All arrows point to dental mesenchyme. Dashed lines demarcate dental epithelial structures.
[image:7.612.316.561.59.304.2]epithelial manner to regulate early tooth development (Zhu et al., 2013). These epithelially expressed Wnts also act on dental mesenchyme and form a Wnt-BMP feedback circuit with mesenchymally expressed BMP4 to mediate epithelial-mesenchymal interactions during early tooth development (O’Connell et al., 2012). The requirement of β-catenin signaling in the dental mesenchyme for early tooth development was manifested by the arrested molar development at the bud stage and the splitting of the incisor placode in mice carrying tissue-specific inactivation of Catnbin the dental mesenchyme (Chen et al., 2009; Fujimori et al., 2010), although the contribution to the phenotype by impaired cell adhesion in the absence of β-catenin cannot be ruled out. However, whether active β-catenin signaling is operating in the mesenchyme of the early developing tooth remains arguable. Although β-catenin signaling activity was detected in the dental mesenchyme throughout tooth development using an Axin2lacZ knock-in allele (Lohi et al., 2010), other β-catenin signaling reporter lines have failed to show positive activity (Liu et al., 2008). Given the fact that Axin2is expressed in the developing tooth germ and that a lack of Axin2leads to upregulation of β-catenin signaling in a tissue-specific manner (Yu et al., 2005a; Lohi et al., 2010; Qian et al., 2011), the expression of the Axin2lacZ allele in the dental mesenchyme could be a consequence of Axin2haploinsufficiency. Nevertheless, these observations suggest that β-catenin signaling activity in dental mesenchyme is tightly regulated at a low level to execute its physiological function.
In keeping with this notion, our current studies show that β -catenin signaling activity is robustly activated in the mesenchymal cells of incisor reaggregates, leading to a failure of tooth formation and the conversion of odontogenic cells into osteogenic cells, consistent with β-catenin signaling as a potent osteogenic regulator (Hartmann, 2006). Similarly, it has been reported that bone-like tissue formed in the dental pulp is associated with excessive β-catenin activity (Chen et al., 2009; Li et al., 2011a). Thus, elevated β-catenin signaling could alter the odontogenic program in dental mesenchymal cells and convert them into osteogenic cells.
FGF signaling inhibits β-catenin signaling in the dental mesenchyme
[image:8.612.52.298.61.249.2]During development and physiological processes, Wnt signaling is precisely regulated by a number of modulators at intra- and extracellular levels (Clevers and Nusse, 2012). In addition, the intensity of Wnt/β-catenin signaling is also regulated by its crosstalk with other signaling pathways. In the developing tooth, several extracellular Wnt antagonists, including Dkks, Sfrps and SOSTDC1, are expressed (Leimeister et al., 1998; Laurikkala et al., 2003; Fjeld et al., 2005), and loss of Smad4 in the dental mesenchyme results in downregulation of the Wnt inhibitors DKK1 and SFRP1, leading to elevated β-catenin activity and subsequent formation of bone-like structure (Li et al., 2011a). In the present study, we show that exogenously applied DKK1 failed to prevent ectopic activation of β-catenin signaling in the incisor mesenchyme and exogenously applied WNT10B could not induce a canonical signaling response in the molar mesenchyme, indicating the Fig. 7. FGF3 regulates cytoplasmic localization of active GSK3β in
[image:8.612.312.562.62.394.2]dental mesenchymal cells.Immunocytochemical staining reveals (A-A⬙) cytoplasmic localization of active GSK3β in E13.5 molar mesenchymal cells and (B-B⬙) nuclear localization of active GSK3β in E13.5 incisor mesenchymal cells. (C-C⬙) In the presence of FGF3, active GSK3β becomes cytoplasmic (arrows) in incisor mesenchymal cells.
Fig. 8. FGF signaling regulates the subcellular localization of active GSK3β in dental mesenchymal cells by activating the PI3K/Akt pathway. (A) Western blotting assay shows similar levels of total Akt (Pan-Akt) and activated Akt (P-(Pan-Akt) in E13.5 and E14.5 incisor and molar mesenchyme. (B) Western blotting shows unaltered level of P-Akt in E13.5 molar mesenchymal cells after 4 hours in culture, but the complete absence of P-Akt in incisor mesenchymal cells. P-Akt was retained in incisor mesenchymal cells after 4 hours in cell culture in the presence of FGF3. (C-F⬙) Immunocytochemical staining shows cytoplasmic localization of GSK3βY216(C-C⬙) and β-catenin (E-E⬙) in E13.5 molar mesenchymal cells after 12 hours in cell culture, and nuclear localization (arrows) of GSK3βY216(D-D⬙) and β-catenin (F-F⬙) in molar mesenchymal cells in the presence of the PI3K/Akt pathway inhibitor BEZ235.
existence of an intracellular regulatory mechanism of β-catenin activity in the dental mesenchyme. These observations also explain why β-catenin activity is maintained at a very low level, if any, in the dental mesenchyme, despite expression of multiple canonical Wnts in the dental epithelium.
We further show that FGFs, including mesenchymally expressed FGF3 and epithelium-derived FGF4 and FGF8, suppress β-catenin activity in the incisor mesenchyme. Remarkably, application of exogenous FGF3 not only inhibited β-catenin activity and osteogenesis in incisor reaggregates but also resumed odontogenic capability in terms of tooth formation in the reaggregates. Certainly, because mechanochemical control of mesenchymal condensation has been shown to be crucial for tooth development (Mammoto et al., 2011), a contribution of mesenchymal condensation by FGF signaling to tooth formation cannot be ruled out.
These results suggest a novel function for FGF signaling in regulating odontogenic fate by attenuating β-catenin signaling through the prevention of β-catenin nuclear localization. Since epithelium-derived FGF4 and FGF8 could also inhibit β-catenin signaling activity in the dental mesenchyme and as other FGFs, such and Fgf9and Fgf10, are co-expressed in the developing tooth (Kettunen and Thesleff, 1998; Kettunen et al., 2000), the lack of a tooth defect in the Fgf3 null mouse might be attributed to functional redundancy between these FGFs (Mansour et al., 1993). This could also explain why tooth forms in the tissue recombinants of an intact dental epithelium and FGF-free incisor mesenchymal reaggregate (data not shown).
FGF signaling inhibits mesenchymal β-catenin signaling through activating the PI3K/Akt pathway
Since Wnt ligands are expressed predominantly in the dental epithelium of developing tooth germ, the ectopic activation of β -catenin signaling in incisor mesenchymal reaggregates without dental epithelium appears to result from the intracellular relief of β-catenin activity suppression. This point is further supported by the fact that exogenously applied DKK1 failed to prevent activation of β-catenin signaling in isolated incisor mesenchyme and by the failure of exogenous WNT10B to induce β-catenin signaling in molar mesenchyme. It was reported previously that FGF signaling activates canonical Wnt activity by inhibiting GSK3β via the PI3K/Akt pathway in tumorigenesis (Katoh and Katoh, 2006). However, FGF signaling can also suppress β-catenin signaling by activating GSK3β via the PI3K/Akt pathway in mESCs (Singh et al., 2012). In the latter system, the accumulation of active GSK3β (p-GSK3βY216) in the nucleus promotes the differentiation of mESCs, whereas the activated PI3K/Akt pathway relocates the active GSK3β into the cytoplasm and promotes cell proliferation (Bechard and Dalton, 2009).
In this study, we show that in the dental mesenchymal cells FGF signaling suppresses β-catenin signaling by maintaining the active GSK3β in the cytoplasm via activation of the PI3K/Akt pathway. This is evidenced by the increased level of p-AktSer473and the translocation of p-GSK3βY216from the nucleus to the cytoplasm in the dissociated incisor mesenchymal cells in the presence of FGF3. Inhibition of the PI3K/Akt pathway facilitates the importation of both active GSK3β and β-catenin into the nucleus, leading to activation of β-catenin signaling. However, whether other FGF-mediated pathways, such as the Erk/Mek pathway, also contribute to the repression of β-catenin signaling and whether FGF signaling regulates non-canonical Wnt signaling in the dental mesenchymal cells warrant further investigation.
Differential expression of syndecan 1 and NDST genes confers different osteogenic potency on incisor and molar mesenchyme after dissociation and reaggregation
Our studies show that despite Fgf3expression in the mesenchyme of both E14.5 incisor and molar germs, FGF3 was retained on the cell surface of molar mesenchyme but not incisor mesenchyme after dissociation and reaggregation. The retention of FGF3 in molar reaggregates appears to sustain the odontogenic fate and allows odontogenesis, but the lack of FGF3 retention leads to activation of β-catenin signaling and deviates odontogenic fate in incisor reaggregates. This distinct capability for FGF retention could be attributed to the differential expression of syndecan 1 in the incisor and molar mesenchyme. Several syndecans, which are the major cell membrane HSPGs, are expressed in the developing tooth, with syndecan 1 exhibiting the highest expression level (Thesleff et al., 1988; Vainio et al., 1989; Vainio et al., 1991; Vainio and Thesleff, 1992; Bai et al., 1994). The requirement of syndecan 1 for FGF signaling has been reported in mammalian cortical development, epithelial-mesenchymal transition and tumorigenesis (Stepp et al., 2002; McDermott et al., 2007; Wang et al., 2012). However, syndecan 1 null mice do not exhibit a tooth development defect, suggesting functional compensation from other syndecans (Alexander et al., 2000; Stepp et al., 2002). The higher level of syndecan 1 expression in the molar mesenchyme appears to be crucial for FGF3 retention in reaggregates. In addition, NDSTs also regulate FGF signaling during organogenesis, as the heparan sulfate chains provide resistance to enzyme digestion and high FGF binding affinity to the core proteoglycan (Pan et al., 2006; Pan et al., 2008; Hu et al., 2009). The higher level of NDST expression in the molar mesenchyme, as compared with that in the incisor mesenchyme, could further confer higher heparan sulfation of HSPGs, including syndecan 1, in the molar mesenchyme and contribute to FGF3 retention (Lin, 2004; Häcker et al., 2005). Thus, the higher levels of syndecan 1 and NDSTs are responsible for FGF3 retention in molar reaggregates. This notion is further supported by the fact that overdigestion with trypsin or treatment with heparinases resulted in activation of β-catenin signaling, lack of FGF3 retention, and failed tooth formation in molar reaggregates.
In summary, we have shown that elevated β-catenin signaling is associated with the fate change of dental mesenchymal cells, and FGF signaling is able to sustain the odontogenic fate by suppressing intracellular β-catenin signaling. The interplay between FGF and β-catenin signaling appears to regulate the proper fate of craniofacial neural crest cells during tooth and jawbone formation.
Acknowledgements
We thank Dr Xin Zhang of Indiana University School of Medicine for sharing mouse NDST cDNA probes.
Funding
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research grants [R01 DE17792 and DE14044] to Y.C.; Y.Z. was supported by the '973' Project [2010CB944800] from the Ministry of Science and Technology of China and by grants [81100730, 81271102] from the National Natural Science Foundation of China. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
Y.C., C.L. and Y.Z. conceived and designed the experiments. C.L. and Y.Z.
qPCR. C.S. and Z.S. performed histological and in situhybridization assays. W.Y. helped to conduct western blotting and immunocytochemical assays. Y.C., C.L. and S.G. analyzed the data. Y.C. and C.L. wrote the manuscript.
References
Aberg, T., Wang, X. P., Kim, J. H., Yamashiro, T., Bei, M., Rice, R., Ryoo, H. M. and Thesleff, I.(2004). Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev. Biol.270, 76-93.
Alexander, C. M., Reichsman, F., Hinkes, M. T., Lincecum, J., Becker, K. A., Cumberledge, S. and Bernfield, M.(2000). Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Genet.25, 329-332.
Bai, X. M., Van der Schueren, B., Cassiman, J. J., Van den Berghe, H. and David, G.(1994). Differential expression of multiple cell-surface heparan sulfate proteoglycans during embryonic tooth development. J. Histochem. Cytochem.42, 1043-1054.
Bechard, M. and Dalton, S.(2009). Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol. Cell. Biol.
29, 2092-2104.
Bei, M. and Maas, R.(1998). FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development.
Development125, 4325-4333.
Chai, Y., Jiang, X., Ito, Y., Bringas, P., Jr, Han, J., Rowitch, D. H., Soriano, P., McMahon, A. P. and Sucov, H. M.(2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development127, 1671-1679.
Chen, J., Lan, Y., Baek, J. A., Gao, Y. and Jiang, R.(2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev. Biol.334, 174-185.
Chung, I. H., Yamaza, T., Zhao, H., Choung, P. H., Shi, S. and Chai, Y.(2009). Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells27, 866-877.
Ciani, L. and Salinas, P. C.(2005). WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci.6, 351-362.
Clément-Lacroix, P., Ai, M., Morvan, F., Roman-Roman, S., Vayssière, B., Belleville, C., Estrera, K., Warman, M. L., Baron, R. and Rawadi, G.(2005). Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. USA102, 17406-17411.
Clevers, H. and Nusse, R.(2012). Wnt/β-catenin signaling and disease. Cell149, 1192-1205.
D’Souza, R. N., Aberg, T., Gaikwad, J., Cavender, A., Owen, M., Karsenty, G. and Thesleff, I.(1999). Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development126, 2911-2920.
Dassule, H. R. and McMahon, A. P.(1998). Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol.
202, 215-227.
Fjeld, K., Kettunen, P., Furmanek, T., Kvinnsland, I. H. and Luukko, K.(2005). Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev. Dyn.233, 161-166.
Fujimori, S., Novak, H., Weissenböck, M., Jussila, M., Gonçalves, A., Zeller, R., Galloway, J., Thesleff, I. and Hartmann, C.(2010). Wnt/β-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev. Biol.348, 97-106.
Gaur, T., Lengner, C. J., Hovhannisyan, H., Bhat, R. A., Bodine, P. V., Komm, B. S., Javed, A., van Wijnen, A. J., Stein, J. L., Stein, G. S. et al.(2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem.280, 33132-33140.
Häcker, U., Nybakken, K. and Perrimon, N.(2005). Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol.6, 530-541.
Hartmann, C.(2006). A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol.16, 151-158.
Hu, Z., Wang, C., Xiao, Y., Sheng, N., Chen, Y., Xu, Y., Zhang, L., Mo, W., Jing, N. and Hu, G.(2009). NDST1-dependent heparan sulfate regulates BMP signaling and internalization in lung development. J. Cell Sci.122, 1145-1154.
Iwata, J., Ezaki, J., Komatsu, M., Yokota, S., Ueno, T., Tanida, I., Chiba, T., Tanaka, K. and Kominami, E.(2006). Excess peroxisomes are degraded by autophagic machinery in mammals. J. Biol. Chem.281, 4035-4041.
James, M. J., Järvinen, E., Wang, X. P. and Thesleff, I.(2006). Different roles of Runx2 during early neural crest-derived bone and tooth development. J. Bone Miner. Res.21, 1034-1044.
Järvinen, E., Salazar-Ciudad, I., Birchmeier, W., Taketo, M. M., Jernvall, J. and Thesleff, I.(2006). Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA103, 18627-18632.
Jernvall, J. and Thesleff, I.(2000). Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev.92, 19-29.
Jernvall, J., Kettunen, P., Karavanova, I., Martin, L. B. and Thesleff, I.(1994). Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int. J. Dev. Biol.38, 463-469.
Katoh, M. and Katoh, M.(2006). Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol. Ther.5, 1059-1064.
Kettunen, P. and Thesleff, I.(1998). Expression and function of FGFs4, 8, and -9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev. Dyn.211, 256-268.
Kettunen, P., Laurikkala, J., Itäranta, P., Vainio, S., Itoh, N. and Thesleff, I.
(2000). Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev. Dyn.219, 322-332.
Klein, O. D., Minowada, G., Peterkova, R., Kangas, A., Yu, B. D., Lesot, H., Peterka, M., Jernvall, J. and Martin, G. R.(2006). Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell11, 181-190.
Laurikkala, J., Kassai, Y., Pakkasjärvi, L., Thesleff, I. and Itoh, N.(2003). Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev. Biol.264, 91-105.
Leimeister, C., Bach, A. and Gessler, M.(1998). Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech. Dev.75, 29-42.
Li, J., Huang, X., Xu, X., Mayo, J., Bringas, P., Jr, Jiang, R., Wang, S. and Chai, Y.(2011a). SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development138, 1977-1989.
Li, L., Yuan, G., Liu, C., Zhang, L., Zhang, Y., Chen, Y. P. and Chen, Z.(2011b). Exogenous fibroblast growth factor 8 rescues development of mouse diastemal vestigial tooth ex vivo. Dev. Dyn.240, 1344-1353.
Lin, X.(2004). Functions of heparan sulfate proteoglycans in cell signaling during development. Development131, 6009-6021.
Liu, F. and Millar, S. E.(2010). Wnt/beta-catenin signaling in oral tissue development and disease. J. Dent. Res.89, 318-330.
Liu, F., Chu, E. Y., Watt, B., Zhang, Y., Gallant, N. M., Andl, T., Yang, S. H., Lu, M. M., Piccolo, S., Schmidt-Ullrich, R. et al.(2008). Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol.313, 210-224.
Logan, C. Y. and Nusse, R.(2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol.20, 781-810.
Lohi, M., Tucker, A. S. and Sharpe, P. T.(2010). Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev. Dyn.239, 160-167.
Lumsden, A. G.(1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development
103Suppl., 155-169.
Mammoto, T., Mammoto, A., Torisawa, Y. S., Tat, T., Gibbs, A., Derda, R., Mannix, R., de Bruijn, M., Yung, C. W., Huh, D. et al.(2011).
Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev. Cell21, 758-769.
Mandler, M. and Neubüser, A.(2001). FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev. Biol.240, 548-559.
Mansour, S. L., Goddard, J. M. and Capecchi, M. R.(1993). Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development117, 13-28.
Maretto, S., Cordenonsi, M., Dupont, S., Braghetta, P., Broccoli, V., Hassan, A. B., Volpin, D., Bressan, G. M. and Piccolo, S.(2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA100, 3299-3304.
McDermott, S. P., Ranheim, E. A., Leatherberry, V. S., Khwaja, S. S., Klos, K. S. and Alexander, C. M.(2007). Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene26, 1407-1416.
Neubüser, A., Peters, H., Balling, R. and Martin, G. R.(1997). Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell90, 247-255.
Noda, T., Oki, S., Kitajima, K., Harada, T., Komune, S. and Meno, C.(2012). Restriction of Wnt signaling in the dorsal otocyst determines semicircular canal formation in the mouse embryo. Dev. Biol.362, 83-93.
O’Connell, D. J., Ho, J. W. K., Mammoto, T., Turbe-Doan, A., O’Connell, J. T., Haseley, P. S., Koo, S., Kamiya, N., Ingber, D. E., Park, P. J. et al.(2012). A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci. Signal.5, ra4.
Pan, Y., Woodbury, A., Esko, J. D., Grobe, K. and Zhang, X.(2006). Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development133, 4933-4944.
Pan, Y., Carbe, C., Powers, A., Zhang, E. E., Esko, J. D., Grobe, K., Feng, G. S. and Zhang, X.(2008). Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development
135, 301-310.
Powers, C. J., McLeskey, S. W. and Wellstein, A.(2000). Fibroblast growth
Presnell, J. K. and Schreibman, M. P.(1997). Humason’s Animal Tissue Techniques. Baltimore, MD: John Hopkins University Press.
Qian, L., Mahaffey, J. P., Alcorn, H. L. and Anderson, K. V.(2011). Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc. Natl. Acad. Sci. USA108, 8692-8697.
Sarkar, L. and Sharpe, P. T.(1999). Expression of Wnt signalling pathway genes during tooth development. Mech. Dev.85, 197-200.
Singh, A. M., Reynolds, D., Cliff, T., Ohtsuka, S., Mattheyses, A. L., Sun, Y., Menendez, L., Kulik, M. and Dalton, S.(2012). Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell10, 312-326.
Song, Y., Zhang, Z. Y., Yu, X., Yan, M., Zhang, X. Y., Gu, S., Stuart, T., Liu, C., Reiser, J., Zhang, Y. et al.(2006). Application of lentivirus-mediated RNAi in studying gene function in mammalian tooth development. Dev. Dyn.235, 1347-1357.
St Amand, T. R., Zhang, Y., Semina, E. V., Zhao, X., Hu, Y. P., Nguyen, L., Murray, J. C. and Chen, Y. P.(2000). Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage.
Dev. Biol.217, 323-332.
Stepp, M. A., Gibson, H. E., Gala, P. H., Iglesia, D. D., Pajoohesh-Ganji, A., Pal-Ghosh, S., Brown, M., Aquino, C., Schwartz, A. M., Goldberger, O. et al.(2002). Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci.115, 4517-4531.
Thesleff, I. and Mikkola, M.(2002). The role of growth factors in tooth development. Int. Rev. Cytol.217, 93-135.
Thesleff, I., Jalkanen, M., Vainio, S. and Bernfield, M.(1988). Cell surface proteoglycan expression correlates with epithelial-mesenchymal interaction during tooth morphogenesis. Dev. Biol.129, 565-572.
Trumpp, A., Depew, M. J., Rubenstein, J. L. R., Bishop, J. M. and Martin, G. R.
(1999). Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev.13, 3136-3148.
Tummers, M. and Thesleff, I.(2009). The importance of signaling pathways modulation in all aspects of tooth development. J. Exp. Zool.312B, 309-319.
Vainio, S. and Thesleff, I.(1992). Sequential induction of syndecan, tenascin and cell proliferation associated with mesenchymal cell condensation during early tooth development. Differentiation50, 97-105.
Vainio, S., Jalkanen, M. and Thesleff, I.(1989). Syndecan and tenascin expression is induced by epithelial-mesenchymal interactions in embryonic tooth mesenchyme. J. Cell Biol.108, 1945-1953.
Vainio, S., Jalkanen, M., Vaahtokari, A., Sahlberg, C., Mali, M., Bernfield, M. and Thesleff, I.(1991). Expression of syndecan gene is induced early, is transient, and correlates with changes in mesenchymal cell proliferation during tooth organogenesis. Dev. Biol.147, 322-333.
Wang, Q., Yang, L., Alexander, C. and Temple, S.(2012). The niche factor syndecan-1 regulates the maintenance and proliferation of neural progenitor cells during mammalian cortical development. PLoS ONE7, e42883.
Yu, H. M., Jerchow, B., Sheu, T. J., Liu, B., Costantini, F., Puzas, J. E., Birchmeier, W. and Hsu, W.(2005a). The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development132, 1995-2005.
Yu, L., Gu, S., Alappat, S., Song, Y., Yan, M., Zhang, X., Zhang, G., Jiang, Y., Zhang, Z., Zhang, Y. et al.(2005b). Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development132, 4397-4406.
Zhang, Z., Song, Y., Zhang, X., Tang, J., Chen, J. and Chen, Y.(2003). Msx1/Bmp4 genetic pathway regulates mammalian alveolar bone formation via induction of Dlx5 and Cbfa1. Mech. Dev.120, 1469-1479.
Zhang, Y. D., Chen, Z., Song, Y. Q., Liu, C. and Chen, Y. P.(2005). Making a tooth: growth factors, transcription factors, and stem cells. Cell Res.15, 301-316.
Zhu, X., Zhao, P., Liu, Y., Zhang, X., Fu, J., Ivy Yu, H. M., Qiu, M., Chen, Y., Hsu, W. and Zhang, Z.(2013). Intra-epithelial requirement of canonical Wnt signaling for tooth morphogenesis. J. Biol. Chem.288, 12080-12089.