Effect of Allyl pentaerythritol on Mechanical, Swelling and
Thermal Properties of Acrylic Rubber
B. K. Saleh1, S. N. Lawandy1, F. Abdel-Hai2andA. Abdel-Hakim1
1National Institute of Standards,
Tersa Street, El-Haram, El-Giza, 12211, EGYPT. 2Faculty of Science,
Al-Azhar University, Yosief Abbas Street, Cairo, 11754, EGYPT. email: basmasalehnis@yahoo.com
(Received on: August 16, 2016)
ABSTRACT
Different Allyl pentaerythritol (APE) ratios were copolymerized with acrylate monomers via conventional emulsion polymerization. The prepared acrylic rubber (ACM) was compounded with a non-sulfur vulcanizing system using precipitated silica as filler. The effect of APE on the rheometric and mechanical properties of acrylic rubber silica composite was investigated. The torque difference, tensile, elastic modulus and hardness properties were improved by increasing APE percentage while the scorch time and elongation at break were decreased. The crosslink density increased with increase of APE percentage while the relative creep and creep rate were decreased. The swelling technique measurement in different solvents (having different solubility parameters) was used to detect the solubility parameter of different acrylic rubber samples.
Keywords: Acrylic rubber. Allyl pentaerythritol. Mechanical properties.
INTRODUCTION
Acrylic rubber (ACM) was defined in ISO 1629 as a copolymer of ethyl acrylate (EA) and small amount of cure site monomers that facilitate the vulcanization process of ACM1. Cure site monomers containing carboxylic and epoxy cure-sites are relatively safe, whereas the using of chlorinated monomers gives rise to serious problems of toxicity and corrosion2. Acrylic rubbers containing different types of cure-site monomers and their cure behavior with different cross-linking agents were reviewed by Vial3. Brown4 reported the carboxylated ACM and their crosslinkinge with different curing systems.
Matsumoto et al.5 used allylacrylate, allyl methacrylate, 1,4-butanediol diacrylate and dihydrodicyclopentenyl acrylate to improve the compression set of acrylic rubber, where the previous poly functional monomer was copolymerized with acrylic rubber monomers during rubber synthesis. The most researchers used to improve the different properties of rubbers by incorporating the polyfunctional monomers (PFMs) via grafting polymerization (post polymerization) either through electron beam vulcanization or peroxide vulcanization. Trimethylolpropane triacrylate (TMPTA) was used to improve the tensile and morphology of irradiated epoxidized natural rubber (ENR-50), ethylene vinyl acetate copolymer (EVA) and blend of them6. The effect of triallylcyanurate (TAC), triallylisocyanurate (TAIC), trimethylolpropane trimethacrylate (TMPTMA), ethylene glycol dimethacrylate (EDMA) and zinc diacrylate (ZDA) on the mechanical properties of natural rubber (NR) crosslinked by electron beam (EB) processing was investigated by E. Manaila et al.7, where the tensile strength, elongation at break and residual elongation initially increase, reach a maximum at around 100–150 kGy and then decrease with increasing the absorbed dose. Q. Wang et al.8, studied the effect of TMPTA concentration on the crosslink density of electron beam-irradiated styrene–butadiene rubber (SBR), where the crosslink density was increased to a certain level, and then decreased in the irradiation dose range 50–200 kGy. M D Stelescu
et al.9-11 reported that the mechanical properties and crosslink density of chlorinated
polyethylene (CPE), ethylene-propylene–terpolymers (EPDM) and ethylene vinyl acetate copolymer, were increased by introducing PFMs. They compared between EB irradiation and dibenzoyl peroxide vulcanization efficiency and found that EB irradiation gave the best results. Tariq Yasin et al.12 reported that the SBR waste tire blends containing TMPTA exhibited higher tensile strength and thermal stability as compared to the blends containing TMPTMA. The incorporation of PFMs during the preparation of rubber is more effective than radiation crosslinking. Radiation Crosslinking requires additional equipment which increases the production cost. The ACM produced from this work can be vulcanized by the conventional methods without additional equipment or settings.
The aim of our present study is to obtain mouldable acrylic rubber with enhanced properties. The APE was added to acrylate monomers mixture during ACM preparation. We expect that APE monomer (polyfunctional monomer) can react with acrylate monomers via its double bonds to form partially crosslinked acrylic rubber. The effects of incorporation of APE in ACM skeleton on the mechanical and solvent resistance of ACM were investigated.
MATERIALS AND METHODS
Materials
Other chemicals listed in the formulation were obtained as laboratory grade from El-Gomhouria company, Cairo, Egypt.
Preparation of acrylic rubber samples.
Using two liter three necked flask equipped with magnetic stirrer, condenser, N2 inlet thermometer and heater, a mixture of 249g distilled water and 1g of Galaxy les70 were charged. Then the pre-emulsion was prepared by mixing 400 g of monomer mixture showed in table 1, 9 g of Galaxy LES70 and 241 g distilled water. The pre-emulsion was stirred well by magnetic stirrer at 500rpm for 1hr.The initiator solution was prepared by dissolving 2g of sodium persulfate in 98g distilled water. Then the temperature of flask contents was heated at 85˚C and the stirring rate was adjusted at 200 rpm. The pre-emulsion and initiator solution were added continuously over 2hrs, where the temperature remained constant at 85±2. After the addition was finished the reaction temperature was held for 2 h to ensure high percentage of monomer conversion, then the flask charge was cooled, filtered and then precipitated using NaCl solution (1%) and dried at 1050C for 5 hrs. The percentage ratios of the monomers mixtures are shown in table 1.
Table 1 The monomers ratios of the different samples.
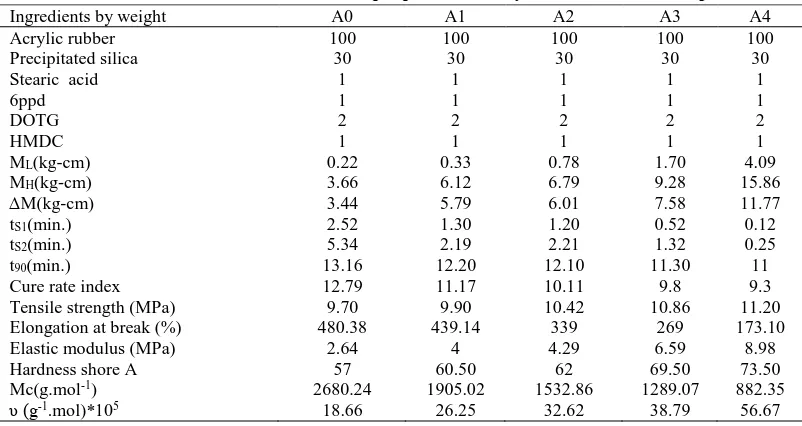
Table 2 Formulation and Rheometeric properties of acrylic rubber/silica composites.
Sample code EA (%) MAA (%) APE (%)
A0 95 5 0
A1 94.75 5 0.25
A2 94.50 5 0.50
A3 94 5 1
A4 93 5 2
Ingredients by weight A0 A1 A2 A3 A4
Acrylic rubber 100 100 100 100 100
Precipitated silica 30 30 30 30 30
Stearic acid 1 1 1 1 1
6ppd 1 1 1 1 1
DOTG 2 2 2 2 2
HMDC 1 1 1 1 1
ML(kg-cm) 0.22 0.33 0.78 1.70 4.09
MH(kg-cm) 3.66 6.12 6.79 9.28 15.86
∆M(kg-cm) 3.44 5.79 6.01 7.58 11.77
tS1(min.) 2.52 1.30 1.20 0.52 0.12
tS2(min.) 5.34 2.19 2.21 1.32 0.25
t90(min.) 13.16 12.20 12.10 11.30 11
Cure rate index 12.79 11.17 10.11 9.8 9.3
Tensile strength (MPa) 9.70 9.90 10.42 10.86 11.20
Elongation at break (%) 480.38 439.14 339 269 173.10
Elastic modulus (MPa) 2.64 4 4.29 6.59 8.98
Hardness shore A 57 60.50 62 69.50 73.50
Mc(g.mol-1) 2680.24 1905.02 1532.86 1289.07 882.35
Mixing procedure and specimen preparation
Mastication and mixing was carried out on two-roll mill (152.4 x330.2 mm) equipped with water cooling. The prepared ACM samples were masticated without fillers for 5 minutes, before adding the other ingredients. The formulations of different rubber mixes are given in table 2. Rheometer MDR 2000, Alpha Technologies was run at 180ºC to determine the cure time (t90), the scorch time(ts2), the minimum torque (ML) and maximum torque (MH) of each composite. Delta torque (ΔH= MH - ML) and cure rate index (CRI= 100/ t90 - ts2) were calculated. Vulcanized sheets (from which standard dumbbell shape samples were cut) were produced by molding in an electrically heated platen press at 180ºC at the time estimated for each sample from the rheometer measurements. The postcuring of test specimens of all mixes was carried out in an oven at 180°C for 4 h.
Crosslink density calculation
The stress–strain relationship was expressed according to the statistical theory of rubber like elasticity 13-15. Where the relation between the force F per unit area A required for stretching elastic network at small extension value λ is given by:
𝐹
𝐴=
ρRT
Mc (λ − λ
−2) = 2C
1(λ − λ−2) (1)
Where ρ is the density of rubber, T is the absolute temperature, R is Boltzman's constant and Mc is the molecular weight between cross-links. The cross link density υ can be calculated from the following equation:
υ = 1
2Mc (2)
Mechanical properties
A dumbbell shape specimens were punched out from the molded sheet of each sample. The mechanical tests were carried out as per ASTM D412-99 using universal testing machine (Zwick-Z010, Germany) at a crosshead speed of 500 mm/min at 25°C, where the tensile strength, elongation and elastic modulus were directly recorded from the apparatus. For the hardness test (Shore A) a Zwick hardness tester was used according to ASTM D2240-05. The average value of five tests for each sample is reported here.
Hysteresis Measurements
Equilibrium Swelling
The swelling in different solvents having solubility parameters in the range (7.3:14.5 cal1/2.cm−3/2) was occurred by immersion of different samples in different solvents for 7 days at ambient temperature. The solvents used and the solubility parameters of them are shown in table 3.
Table 3 Solvents used and their solubility parameters17
The degree of swelling was calculated according to the following equation:
Q =W2−W1
W1
∗ 100 (3)
Where: Q = degree of swelling, %, W1 = initial mass of specimen in air, g, and W2 = mass of specimen in air after immersion, g.
RESULTS and DISCUSSION
Stress-strain results and crosslink density calculation
The stress strain measurements were used to obtain the crosslink density of each composite. Fig. 1 shows the relation between the value (λ-λ-2) and applied stress (F/A), where all composites gave almost straight lines. This indicates that the kinetic theory of elasticity is valid for all composites 18. The values of crosslink density (υ) and molecular weight between crosslinks (Mc) of different acrylic rubber composites containing different concentration of APE are listed in table 2 and the relation between APE percentage and crosslink density is shown in fig. 2. As shown in fig. 2 the crosslink density linearly increased as the APE percentage increased, in other words the incorporation of APE on ACM improved the crosslink density within the ACM composites. The value of crosslink density nearly duplicated more than three times in presence of 2% APE (A4) compared to that of untreated one(A0), while the Mc value decreased.
Solvent Solubility parameter ( cal1/2.cm−3/2)
n-Hexane 7.3
Carbon tetrachloride(CCl4) 8.6
Tetrahydrofuran (THF) 9.1
Acetone 9.9
Ethanol 12.7
Fig. 1 Stress–strain curves of acrylic rubber containing different APE percentage
Fig. 2 Relation between APE % and crosslink density of different composites
Torque rheometry measurements.
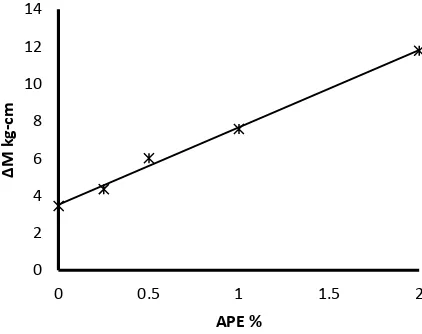
The cure characteristic parameters of ACM silica composites are given in table 2. It is clear that, APE has an effect on acrylic rubber cure properties. The scorch time (ts2), cure time (t90) and the cure rate index decreased with the increase of APE percentage, in other words the incorporation of APE on the ACM accelerate the curing reaction as shown in fig. 3, where the cure rate index gradually decreased with increase of APE percentage. Meanwhile, the torque difference (∆M = MH - ML) linearly increased with increase of APE as shown in fig.4, it is well known that ∆M is a measure of the crosslink density19.So, from the rheometric measurements, it is obvious that the presence of APE, especially at higher concentrations increases the crosslink density between acrylic rubber chains. The produced crosslinks in presence of APE is a summation of the crosslinks formed during the preparation of ACM and the crosslinks formed during curing reaction. The curing reaction involves HMDC decomposition at high processing temperature to hexamethylenediamine (HMD)20, where the amine groups of HMD act as nucleophiles and attacks on electron deficient carbonyl carbon of carboxyl groups in
y = 1.010x - 0.005
y = 1.421x - 0.009
y = 1.766x + 0.004
y = 2.100x - 0.004
y = 3.068x + 0.002
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9
0 0.1 0.2 0.3
F/ A M Pa λ- λ-2 0 0.25 0.5 1 2 6 16 26 36 46 56 66
0 0.5 1 1.5 2
ACM to form amide linkage (during curing reaction). The amide linkage undergoes further rearrangement and forms imide linkage (during post curing)21.
Fig. 3 The relation between APE % and cure rate index
Fig. 4 The relation between APE % and torque difference
The mechanical properties
The values of different mechanical properties of acrylic rubber composites containing different concentration of APE percentage are recorded in table 2. It was observed that the tensile strength, hardness and E-modulus of the composites increased with the increase of the percentage of APE while the elongation at break was sharply decreased. The changes in
8 9 10 11 12 13 14
0 0.5 1 1.5 2
C
u
re
r
ate
in
d
e
x
APE %
0 2 4 6 8 10 12 14
0 0.5 1 1.5 2
∆
M
kg
-cm
mechanical properties may be attributed to the increase of the crosslink density and consequently the rigidity of the composite as the APE ratio increases. The increase of crosslink density also causes restriction of the segmental mobility of the polymer chains and consequently the samples break at lower elongation22, 23. The rigidity and stiffness were increased as the crosslink density increased and thus the modulus and hardness increased24.
Hysteresis Measurements and calculation of relative creep and creep rate
Creep is an important property of polymers, this property represent the deformation in sample with time under constant load25, 26. Dumbbell samples of different mixes undergo a series of loading and unloading cycles in order to produce hysteresis loops. The creep at the end of each cycle represents the difference between the strain at the end of this cycle and the strain at the end of the previous cycle and thus four creep values were recorded for five cycles. The relation between the cycle numbers and the relative creep values of different mixes containing different percentages of APE was shown in fig. 5 where this relation gives straight lines and the slope of these lines represent the creep rate. Fig. 5 shows that the relative creep and creep rate values decrease as the APE percentage increase in the acrylic rubber. The relative creep and creep rate increase with increasing the possibility of segmental motion of polymer. The incorporation of APE increase the crosslink density within the rubber matrix and thus that restricts the segmental motion and by turn reduces the creep rate and relative creep values. Fig. 5 shows that the slope of lines decreased with the increase of APE percentage, this indicates that, the rate of creep of vulcanised ACM composite decreases with increase of APE percentage.
Fig. 5 The relation between the hysteresis cycle number and the relative creep of acrylic rubber silica composite containing acrylic rubber samples with different APE percentages
y= 0.254x + 0.395
y = 0.466x + 0.59
y= 0.535x + 0.725
y = 0.576x + 0.88
y = 0.646x + 0.88
0 0.5 1 1.5 2 2.5 3 3.5 4
0 1 2 3 4
R
e
lati
ve
C
re
e
p
, mm
Cycle Number
2% APE
1% APE
0.5% APE
0.25% APE
The Swelling of the acrylic rubber in different solvents
Fig. 6 shows the relation between the solubility parameter of various solvents having solubility parameter from 7.3 to 14.5 cal1/2.cm−3/2 and the degree of swelling Q of different ACM composites containing different percentages of APE. Fig. 6 clearly shows that all composites have the maximum swelling ratio in THF that have solubility parameter 9.1 cal1/2.cm−3/2, this may be due to the solubility parameter of THF is closest to the solubility parameter value of poly ethyl acrylate (8.76: 9.37 cal1/2.cm−3/2) that represents the main structure of ACM27, 28. The degree of swelling decreased as the solubility parameter difference between the polymer and penetrant increased29. The incorporation of APE did not alternate the solubility parameter of ACM prepared. The degree of swelling Q decreased as the APE percentage increased, where the lowest values of Q for all solvents were observed with the highest value of APE. In other words APE improved the solvent resistance of ACM silica composite to large extent, this is may be attributed to the increasing in crosslink density in the cured samples with increasing APE percentage as previously discussed and hence the free volume available to be included with the solvents molecules were reduced and restrict the polymer chains rearrangement during solvent ingression, thereby causing resistance to the path of penetrants30.
Fig. 6 The relation between solubility parameter of various solvents and the swelling ratio of different ACM composites
CONCLUSION
- The modification of Acrylic rubber by Allyl pentaerythritol (APE) generally improved the mechanical properties.
- Significant increase in the crosslink density was achieved by increasing of the APE percentage. This by turn decreases the degree of swelling in the different solvents. - The solubility parameter of the copolymers was recorded to be about 9.1 cal1/2.cm−3/2. - The relative creep decrease as the APE percentage increase.
0 50 100 150 200 250 300
7 9 11 13 15
Q
%
Solubility parameter
( cal1/2.cm−3/2)
REFERENCES
1. Simpson R. B., Rubber Basics, Smithers Rapra Publishing, Shropshire, 93-95 (2002) 2. Kader M. A., Bhowmick A. K. Acrylic rubber-fluorocarbon rubber miscible blends: Effect
of curatives and fillers on cure, mechanical, aging, and swelling properties, J. Appl .
Polym. Sci., 89:1442-1452 (2003).
3. Vial T.M. Recent Developments in Acrylic Elastomers, Rubber Chem. Technol., 44: 344-362 (1971).
4. Brown H. P. Crosslinking Reactions of Carboxylic Elastomers, Rubber Chem. Technol., 36: 93-962 (1963).
5. Matsumoto K., Minamino E., Mori Y., Noguchi T. Rubber compositions of low compression set, U.S. Patent 5,962,589 (1999).
6. Zurina M., Ismail H., Ratnam C. T. Effect of Trimethylolpropane Triacrylate (TMPTA) on the Properties of Irradiated Epoxidized Natural Rubber (ENR-50), Ethylene-(vinyl acetate) Copolymer (EVA), and an ENR-50/EVA Blend. J. Vinyl. Addit. Techn., doi:10.1002/vnl.20175 (2009).
7. Manaila E., Craciun G., Stelescu M. D., Ighigeanu D., Ficai M. Radiation vulcanization of natural rubber with polyfunctional monomers, Polym. Bull., 71:57–82 (2014).
8. Wang Q., Wang F., Cheng K. Effect of crosslink density on some properties of electron beam-irradiated styrene–butadiene rubber, Radiation Physics and Chemistry, 78:1001– 1005 (2009).
9. Stelescu M. D., Manaila E., Zuga N. The use of polyfunctional monomers in the radical cure of chlorinated polyethylene, Polym. J., 43: 792– 800 (2011).
10. Stelescu M. D., Manaila E., Craciun G., Zuga N. Crosslinking and grafting ethylene vinyl acetate copolymer with accelerated electrons in the presence of polyfunctional monomers,
Polym. Bull., 68:263–285 (2012).
11. Stelescu M. D., Manaila E., Craciun G. Vulcanization of Ethylene-Propylene– Terpolymer-Based Rubber Mixtures by Radiation Processing. J. Appl. Polym. Sci., doi: 10.1002/APP.38231 (2013).
12. Yasin T., Khan S., Shafiq M., Gill R. Radiation crosslinking of styrene–butadiene rubber containing waste tire rubber and polyfunctional monomers, Radiat. Phys. Chem., 106:343-347 (2015).
13. Mooney M. A theory of large elastic deformation, J. Appl. Phys., 11:582-591 (1940). 14. Rivilin R. S. Large Elastic Deformations of Isotropic Materials. IV. Further Developments
of the General Theory, Phil. Trans. R. Soc., 24:379-397 (1948).
15. Mullins L. Determination of degree of crosslinking in natural rubber vulcanizates Part III,
J. Appl. Polym. Sci., 2:1-7 (1959).
16. Lawandy S. N., Abd-ElMageed A. A., Halim S. F. Using Strain Deformation Measurements to Study the Mechanical Behavior of Butyl/Organo-modified Nanoclay Composites International, International Journal of Engineering Science and Innovative
17. Heftmann E.,Chromatography: Fundamentals and applications of chromatography and related differential migration method, Elsevier B.V., Amsterdam, 49(2004).
18. Lawandy S. N., Halim S. F. Effect of vulcanizing system on the crosslink density of nitrile rubber compounds, J. Appl. Polym. Sci., 96:2440-2445 (2005).
19. Micheli C.L., Bluma S.G. Compatibilization Efficiency of carboxylated nitrile rubber and epoxy pre-polymer in nitrile/acrylic rubber blends, Polímeros 23:139-145 (2013).
20. Soares B. G., Santos D. M., Sirqueira A.S. A novel thermoplastic elastomer based on dynamically vulcanized polypropylene/acrylic rubber blends, Express Polym. Lett., 2:602-613 (2008).
21. Holliday J.r., Roger S. Ethylene-Acrylic bonded piston without oven post curing, U.S. Patent 0168015 A1 (2011).
22. Jamal N. A., Anuar H., Shamsul Bahri A. R. Enhancing the Mechanical Properties of Cross-Linked Rubber-Toughened Nanocomposites via Electron Beam Irradiation, Journal
of Nanotechnology, doi:10.1155/2011/769428 (2011).
23. Oprea S., Oprea V. Properties of polyurethane elastomers obtained with various chain extenders, Mat. Plast. J., 45:345-350 (2008).
24. Nabil H., Ismail H., Ratnam C. T. Simultaneous Enhancement of Mechanical and Dynamic Mechanical Properties of Natural Rubber/Recycled Ethylene-Propylene-Diene Rubber Blends by Electron Beam Irradiation, Int. J. Polym. Anal. Ch., 19: 272–285 (2014). 25. Zhang Z., Yang J. L., Friedrich K. Creep resistant polymeric nanocomposites, Polym. J.,
45: 3481- 3485 (2004).
26. Perez C. J., Alvarez V. A., Vazquez A. Creep behaviour of layered silicate/starch– polycaprolactone blends nanocomposites. Mater. Sci. Eng.: A, 480:259-265 (2008). 27. Matsui S., Paul D. R. Pervaporation separation of aromatic/aliphatic hydrocarbons by a
series of ionically crosslinked poly(n-alkyl acrylate) membranes, J. Membr. Sci., 213:67– 83 (2003).
28. Lewin J. L., Maerzke K. A., Nathan Schultz E., Ross R. B., Siepmann I. J. Prediction of Hildebrand solubility parameters of acrylate and methacrylate monomers and their mixtures by molecular simulation, J. Appl. Polym. Sci., 116:1–9 (2010).
29. Mohammadia T., Aroujalianb A., Bakhshi A., Pervaporation of dilute alcoholic mixtures using PDMS membrane, Chem. Eng. Sci., 60:1875 – 1880 (2005).