Application of mesoporous metal oxides in catalytic oxidation reactions
Full text
(2) Application of mesoporous metal oxides in catalytic oxidation reactions. By: Ndzondelelo Sigqibo Bingwa. Thesis Submitted in fulfillment of the requirements for the degree PHILOSOPHIAE DOCTOR. in Chemistry. at the University of Johannesburg. Supervisor(s): Prof R. Meijboom Dr M. Haumann. November 2017.
(3) Dedications. I dedicate this thesis to my parents, Nomagugu Elina Bingwa and Mzimasi Wilmot Nywabeni. I wholeheartedly thank you for everything you have done for me and thank you for soldiering on and taking care of me during my childhood health scares.. ii.
(4) Acknowledgements. I would like to express my gratitude to both my supervisors, Prof Reinout Meijboom and Dr Marco Haumann. I thank you for making this journey an interesting and a fruitful one.. I would also like to extend my gratitude to Dr Nathan Charles Antonels, Dr Matumuene Joe Ndolomingo, and Dr Ji-Hyang Noh for their invaluable contributions and advice. I thank you wholeheartedly, and I hope you will continue inspiring other candidates.. I would like to send a special thank you to my other parents, Sithembiso Bingwa and Feza Bingwa. You guys have shown me that you don’t have to be a biological parent in order to raise and nurture a young person. In my entire life I have witnessed very few people who sacrificed their family needs to financially support the education and help mold a future of a black child.. I would like to also extend my gratitude to the Metacatalysis research group, I thank you all for the time we had in the lab and for the continuous constructive scientific engagements we had. This journey has been a special one because of all of you. I would like to send a special thank you to Semakaleng Bewana, Sifelani Dube, and Tafadzwa Mabate for providing extra hands when needed. I hope and wish that you guys will one day grow and become the best in your scientific careers.. Furthermore, I would like to thank the entire Chemistry department for providing a conducive environment for research.. I also thank the Lehrstuhl für Chemische Reaktionstechnik (CRT) of Friedrich-AlexanderUniversität Erlangen-Nürnberg (FAU) for allowing me to use their facilities during my shortterm visit in Germany.. The financial support from the National Research Foundation of South Africa (NRF) and the University of Johannesburg is greatly acknowledged.. Last but not least, I would like to thank the Lord for the strength He has given me.. iii.
(5) Declaration. I hereby declare that all parts of the experimental work reported herein have been performed by myself and that all the scientific publications that result from this thesis have been written by myself. Both my supervisors have been involved in the planning of the projects and they were responsible for proof-reading the manuscripts prior to publication.. iv.
(6) Keywords. Mesoporous metal oxides; nanocasting; soft-template; oxidation kinetics; LangmuirHinshelwood model; Mars-van Krevelen model; doped-metal oxides; morin oxidation; hybrid oxides; aerobic oxidation; ethanol oxidation; dendrimer-encapsulated nanoparticles; Pt nanoparticles; AuPd nanoalloys; ionic liquids; solid catalysts with ionic liquid layer (SCILL).. v.
(7) Abstract. This work reports on the synthesis, characterization, and the catalytic activity of mesoporous metal oxides (MMOs) of manganese (Mn), cobalt (Co), and cerium (Ce). The hard-template, often referred to as nanocasting, and the soft-template approaches were used to successfully synthesize these mesoporous metal oxides. In the hard-template synthesis, KIT-6 (a typical silica material) was used as a template, while for the soft-template synthesis P-123 was used as the surfactant to template the synthesis of mesoporous metal oxides. Also reported herein is the synthesis of dendrimer-encapsulated monometallic and bimetallic nanoparticles (DENs). Bimetallic gold (Au) and palladium (Pd) nanoalloys were synthesized inside the cavities of poly(amidoamine) (PAMAM) dendrimer (AuPd-DENs). Furthermore, monometallic platinum (Pt) dendrimer-encapsulated nanoparticles were successfully synthesized using PAMAM dendrimer (Pt-DENs). In order to achieve stable heterogeneous catalytic systems containing nanoparticles, the synthesized DENs were immobilized on the as-synthesized MMOs.. Prior to catalytic evaluation, all the catalysts were fully characterized using various techniques. Mesoporous metal oxides were characterized using nitrogen sorption measurements (BET) for physicochemical properties, powder X-ray diffraction (p-XRD) for phase identification, temperature-programmed reduction/oxidation (TPR/TPO) and desorption (TPD) for surface redox properties and nature of active sites, respectively, transmission electron spectroscopy (TEM) for porosity identification, and X-ray photoelectron spectroscopy (XPS) for elemental composition and oxidation states. While the synthesis of dendrimer-encapsulated nanoparticles was monitored using Ultraviolet-Visible spectroscopy (UV-Vis) and transmission electron spectroscopy (TEM) was used to check the morphology of the nanoparticles.. The hard-templated mesoporous MnO2 was used as a catalyst to study kinetics of morin oxidation as a model reaction. Two different kinetic models were used to elucidate the surface reaction, namely, Langmuir-Hinshelwood and Mars-van Krevelen models. The LangmuirHinshelwood model which assumes adsorption of both the substrate and the oxidant onto the catalyst surface appeared to describe the kinetics of morin oxidation better than the Mars-van Krevelen kinetic model which assumes participation of the catalyst’s lattice oxygen in the oxidation of the substrate.. vi.
(8) Furthermore, the effect of alkali and alkaline earth metal dopants on mesoporous cobalt oxide was investigated using morin oxidation as a model reaction. Prior to the catalytic evaluation of the doped cobalt oxide, full characterization was performed and results suggested that the amount of dopants used altered the electronic structure of the cobalt oxide as revealed by temperature-programmed reduction (TPR) and X-ray photoelectron spectroscopy (XPS). The dopants had a negative effect on the catalytic activity of cobalt oxide.. Also, synthesis of bimetallic oxides of cobalt and tin was undertaken and the resulting materials were highly active as catalysts in morin oxidation. Preliminary characterization results showed that the CoSn hybrid catalysts have high surface area. The nature of the active sites present in CoSn oxide catalysts was investigated using temperature-programmed techniques. The activity of the catalyst showed good correlation with the nature of the active sites.. The aerobic oxidation of ethanol is also reported. A synergistic effect between nanoparticles and the mesoporous metal oxides was observed. For the bimetallic AuPd nanoalloys it was observed that the amount of each component of the nanoalloys plays a crucial role in the selectivity profile of aerobic oxidation of ethanol. Complete oxidation of ethanol to carbon dioxide was observed when Pt nanoparticles were used. Furthermore, the use of ionic liquid in the solid catalyst with ionic liquid layer (SCILL) significantly reduced the formation of carbon dioxide and improved selectivity towards acetaldehyde. The amount of ionic liquid coating impacted the activity of the catalysts.. vii.
(9) Publications. 1. N. Bingwa, S. Bewana, M. Haumann, and R. Meijboom; Revisiting kinetics of morin oxidation: Surface kinetics analysis, Appl. Surf. Sci. (2017) 426, 497-503.. 2. N. Bingwa, S. Bewana, M.J Ndolomingo, N. Mawila, B. Mogudi, P. Ncube, E. Carleschi, B.P. Doyle, M. Haumann, and R. Meijboom; Effect of alkali and alkaline earth metal dopants on catalytic activity of mesoporous cobalt oxide evaluated using a model reaction, Appl. Catal. A: Gen., Accepted.. 3. N. Bingwa, M.J. Ndolomingo, S. Dube, T. Mabate, M. Haumann, and R. Meijboom; Surface property-activity relations of Co/SnO2 oxide nanocatalysts evaluated using a model reaction: Surface characterization study, (Manuscript to be submitted). 4. N. Bingwa, N.C. Antonels, M.B. Williams, M. Haumann, and R. Meijboom; Application of mesoporous metal oxide immobilized gold-palladium nanoalloys as catalysts for ethanol oxidation, (Manuscript to be submitted) 5. N. Bingwa, M.J. Ndolomingo, J-H. Noh, N.C. Antonels, E. Carleschi, B.P. Doyle, M. Haumann, and R. Meijboom; Synergistic effect of mesoporous metal oxides and PtOx nanoparticles in aerobic oxidation of ethanol and ionic liquid induced acetaldehyde selectivity. (Manuscript to be submitted). Other papers 6. N. Bingwa, R. Patala, J-H. Noh, M.J. Ndolomingo, S. Tetyana, S. Bewana, and R. Meijboom; Synergistic effects of Gold-Palladium nanoalloys and reducible supports on the catalytic reduction of 4-nitrophenol, Langmuir (2017) 33, 7086-7095. (Not part of the thesis).. 7. B. Mogudi, P. Ncube, N. Bingwa, N. Mawila, S. Mathebula, and R. Meijboom; Promotion effects of alkali- and alkaline earth metals on catalytic activity of mesoporous Co3O4 for 4-nitrophenol reduction, Appl. Catal. B: Environm. (2017), 218, 240-248. (Not part of the thesis).. viii.
(10) Conference presentations. N. Bingwa, M. Haumann, and R. Meijboom, Metal oxides as catalysts and catalytic supports for oxidation reactions, Oral presentation, CATSA 2016, Drakensburg, KwaZulu Natal, South Africa.. ix.
(11) List of abbreviations. µM. micromolar. µL. microliters. β. Beta. °C. degrees Celcius. ΔH. enthalpy change. ΔS. entropy change. ΔG. Gibb’s free energy change. a. interface area. AA. acetaldehyde. A0. Initial absorbance / absorbance at time 0 min. Af. Final absorbance. At. Absorbance at any given time t. AuPd. gold-palladium. BET. Brunauer Emmett Teller. cm2. square centimeter. cm3/g. cubic centimeter per gram. CTAB. cetyl trimethylammonium bromide. DaII. Damköhler constant. DENs. dendrimer-encapsulated nanoparticles. Ea. activation energy. EMIM NTf2. 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide. EtOH. ethanol. eV. electron volts. FT-IR. fourier transform infrared spectroscopy. FID. flame ionization detector. g. grams. GC. gas chromatography. G4. generation 4. G5. generation 5. hrs. hours. x.
(12) HRTEM. High resolution transmission electron microscopy. ICP-OES. inductively coupled plasma-omission emission spectroscopy. ICP-MS. inductively coupled plasma-mass spectroscopy. I-L. ionic liquid. J. Joules. Jmol-1. Joules per mole. k. surface rate constant. kapp. apparent rate constant. kobs. observed rate constant. Ka. adsorption constant. K. Kelvins. kV. kilovolt. L-H. Langmuir-Hinshelwood. M. molar. MvK. Mars-van Krevelen. mol%. mole percent. MΩ·cm. mega Ohm’s centimeter. MMO. mesoporous metal oxide. mmol. millimole. mM. millimolar. NPs. nanoparticles. nm. nanometer. PAMAM. poly(amidoamine). PAMAM-NH2. amine-terminated poly(amidoamine). PAMAM-OH. hydroxyl-terminated poly(amidoamine). PXRD. powder X-ray diffractometer. P-123. poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide). SCILL. solid catalyst with ionic liquid layer. SEM. scanning electron microscope. s-1. per second. TEOS. tetraethyl orthosilicate. TBHP. tert-butyl hydroperoxide. xi.
(13) TPR. temperature-programmed reduction. TPO. temperature-programmed oxidation. UV-vis. Ultraviolet-visible. XPS. X-ray photoelectron spectroscopy. xii.
(14) Table of Contents Dedications......................................................................................................................................... ii Acknowledgements .......................................................................................................................... iii Declaration........................................................................................................................................ iv Keywords ........................................................................................................................................... v Publications .................................................................................................................................... viii Conference presentations ................................................................................................................ ix List of abbreviations ......................................................................................................................... x Chapter 1 ................................................................................................................................................. 1 Introduction and Literature Review ........................................................................................................ 1 Synopsis of the project ........................................................................................................................ 2 1.1.. Introduction to metal oxides and their application as heterogeneous catalysts for oxidation. reactions .............................................................................................................................................. 3 1.1.1.. Metal oxides and related materials.................................................................................. 3. 1.1.2. Synthesis of metal oxides ...................................................................................................... 4 1.2.. Application of metal oxides in catalysis ................................................................................. 7. 1.2.1. Oxidation reactions ............................................................................................................... 7 1.2.2. Established mechanisms in metal oxide catalyzed reactions ................................................ 8 1.3. Introduction to transition metal nanoparticles and their application as heterogeneous catalysts in oxidation reactions ............................................................................................................................ 10 1.3.1 Synopsis into nanoparticles and catalysis ............................................................................ 10 1.3.2. Preparation and stabilization of nanoparticles .................................................................... 11 1.3.3. Origin of dendrimers ........................................................................................................... 13 1.3.4. Application of metal nanoparticles as catalysts .................................................................. 15 1.4. Introduction to ionic liquids and their role in catalysis .............................................................. 22 1.4.1. The Solid Catalyst in Ionic Liquid Layer (SCILL) ............................................................. 23 1.5. Aims and objectives of the dissertation ..................................................................................... 24 1.6. References .................................................................................................................................. 26 xiii.
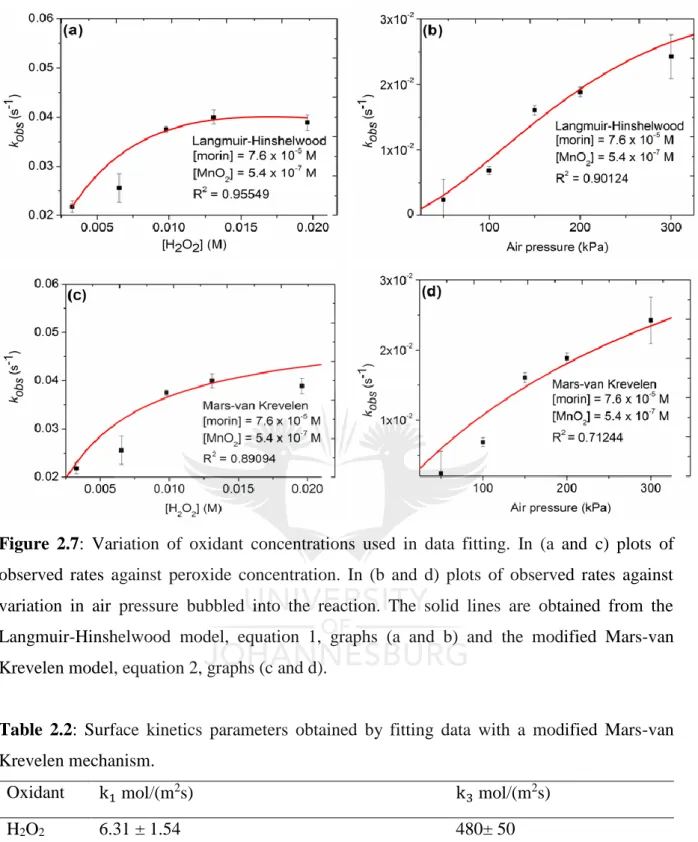
(15) Chapter 2 ............................................................................................................................................... 35 Revisiting kinetics of morin oxidation: Surface kinetics analysis ........................................................ 35 Abstract ............................................................................................................................................. 36 2.1. Introduction ................................................................................................................................ 37 2.2. Experimental section .................................................................................................................. 39 2.2.1. Chemicals and instruments ................................................................................................. 39 2.2.2. Hard-template synthesis (KIT-6) ........................................................................................ 39 2.2.3. Mesoporous MnO2 synthesis............................................................................................... 39 2.2.4. Catalyst characterization ..................................................................................................... 40 2.3. Results ........................................................................................................................................ 41 2.4. Discussions ................................................................................................................................ 49 2.5. Conclusions ................................................................................................................................ 50 2.6. References .................................................................................................................................. 52 Chapter 3 ............................................................................................................................................... 54 Effect of alkali and alkaline earth metal dopants on catalytic activity of mesoporous cobalt oxide evaluated using a model reaction .......................................................................................................... 54 Abstract ............................................................................................................................................. 55 3.1. Introduction ................................................................................................................................ 56 3.2. Experimental .............................................................................................................................. 57 3.2.1. Materials and chemicals ...................................................................................................... 57 3.2.2. Catalyst synthesis ................................................................................................................ 57 3.2.3. Catalyst characterization ..................................................................................................... 58 3.2.4. Catalytic evaluation............................................................................................................. 59 3.3. Results ........................................................................................................................................ 60 3.4. Discussions ................................................................................................................................ 67 3.5. Conclusion ................................................................................................................................. 69 3.6. References .................................................................................................................................. 70 Chapter 4 ............................................................................................................................................... 74 xiv.
(16) Surface property-activity relations of Co/Sn oxide nanocatalysts evaluated using a model reaction: Surface characterization study .............................................................................................................. 74 Abstract ............................................................................................................................................. 75 4.1. Introduction ................................................................................................................................ 76 4.2. Experimental .............................................................................................................................. 77 4.2.1. Materials and instruments ................................................................................................... 77 4.2.2. Synthesis of Co/Sn oxide materials .................................................................................... 78 4.2.3. Material characterization..................................................................................................... 78 4.2.4. Catalytic studies .................................................................................................................. 79 4.3. Results ........................................................................................................................................ 79 4.4. Discussions ................................................................................................................................ 87 4.5. Conclusions ................................................................................................................................ 88 4.6. References .................................................................................................................................. 90 Chapter 5 ............................................................................................................................................... 92 Application of mesoporous metal oxide immobilized gold-palladium nanoalloys as catalysts for ethanol oxidation ............................................................................................................................................... 92 Abstract ............................................................................................................................................. 93 5.1. Introduction ................................................................................................................................ 94 5.2. Experimental .............................................................................................................................. 96 5.2.1. Preparation of AuPd nanoalloys ......................................................................................... 97 5.2.2 Preparation of mesoporous metal oxides ............................................................................. 97 5.2.3. Preparation of AuPd/MMO catalysts .................................................................................. 98 5.2.4. Catalytic evaluation............................................................................................................. 98 5.3. Results and discussion ............................................................................................................... 99 5.3.1. Catalyst characterization ..................................................................................................... 99 5.3.2. Catalytic ethanol oxidation ............................................................................................... 102 5.4. Conclusion ............................................................................................................................... 108 5.5. References ................................................................................................................................ 109. xv.
(17) Chapter 6 ............................................................................................................................................. 112 Synergistic effect of mesoporous metal oxides and PtO2 nanoparticles in aerobic oxidation of ethanol and ionic liquid induced acetaldehyde selectivity............................................................................... 112 6.1. Introduction .............................................................................................................................. 114 6.2. Experimental section ................................................................................................................ 116 6.2.1. Materials and instruments ................................................................................................. 116 6.2.2. Synthesis of Pt dendrimer-encapsulated nanoparticles (Pt-DENs) ................................... 116 6.2.3. Synthesis of the nanocasting template (KIT-6) ................................................................. 117 6.2.4. Synthesis of mesoporous metal oxides (MMOs) .............................................................. 117 6.2.5. Immobilization of Pt nanoparticles on mesoporous metal oxides..................................... 118 6.2.6.. Preparation. of. solid. catalysts. with. ionic. liquid. layer. (SCILL. catalysts:. [EMIm][NTf2]/Pt/MMOs) .......................................................................................................... 118 6.2.7. Catalytic studies ................................................................................................................ 118 6.2.8. Catalyst characterization ................................................................................................... 119 6.3. Results ...................................................................................................................................... 121 6.3.1. Catalyst characterization ................................................................................................... 121 6.3.2. Catalytic evaluation........................................................................................................... 128 6.4. Discussion ................................................................................................................................ 130 6.5. Conclusions .............................................................................................................................. 133 6.6. References ................................................................................................................................ 134 Chapter 7 ............................................................................................................................................. 137 Conclusions and recommendations ..................................................................................................... 137 Conclusions ..................................................................................................................................... 138 7.1. Synthesis of mesoporous metal oxides .................................................................................... 138 7.2. Synthesis of alkali- and alkaline earth metal-doped mesoporous Co3O4 ................................. 138 7.3. Synthesis of mesoporous Co/Sn oxide catalysts ...................................................................... 138 7.4. Synthesis dendrimer-encapsulated nanoparticles ..................................................................... 138 7.5. Immobilization of Pt, AuPd nanostructures on as-synthesized mesoporous metal oxides ...... 139. xvi.
(18) 7.6. Catalytic activity of mesoporous metal oxides in the oxidation of morin ............................... 139 7.7. Catalytic activity of mesoporous metal oxides and immobilized Pt, and AuPd nanoparticles in aerobic oxidation of ethanol............................................................................................................ 140 Recommendations ........................................................................................................................... 140 Chapter 8 ............................................................................................................................................. 142 Supplementary information ................................................................................................................ 142 Chapter 2 ......................................................................................................................................... 143 Derivation of the Mars-van Krevelen and Langmuir-Hinshelwood rate equations .................... 143 References ................................................................................................................................... 148 Chapter 3 ......................................................................................................................................... 149 Crystallite size calculations using Scherer equation: .................................................................. 149 Chapter 5 ......................................................................................................................................... 156 Chapter 6 ......................................................................................................................................... 160. xvii.
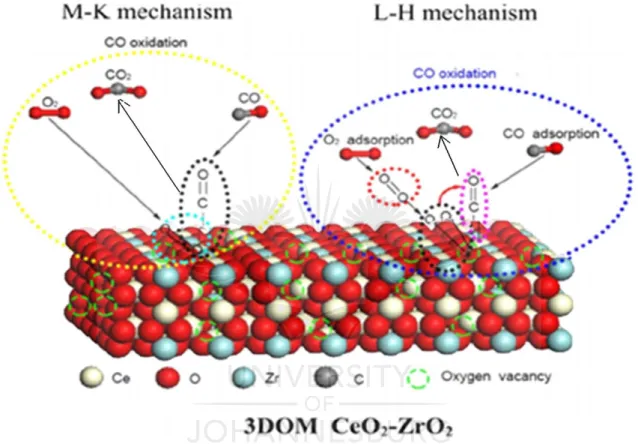
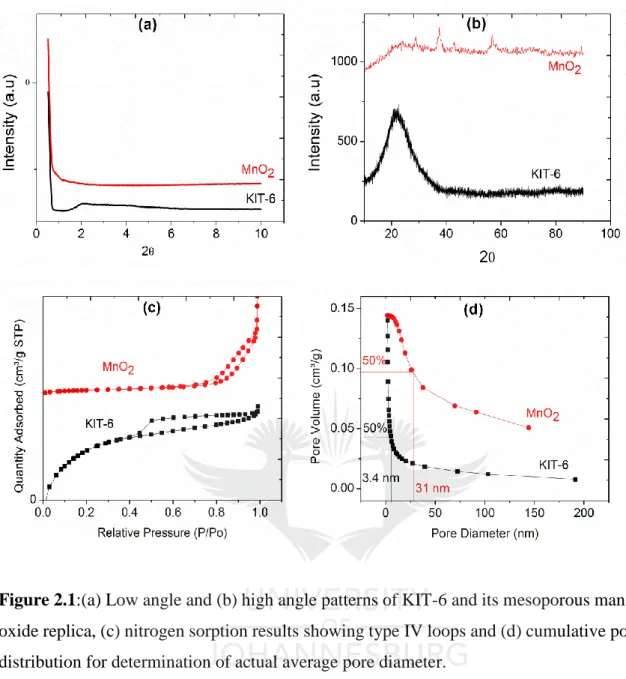
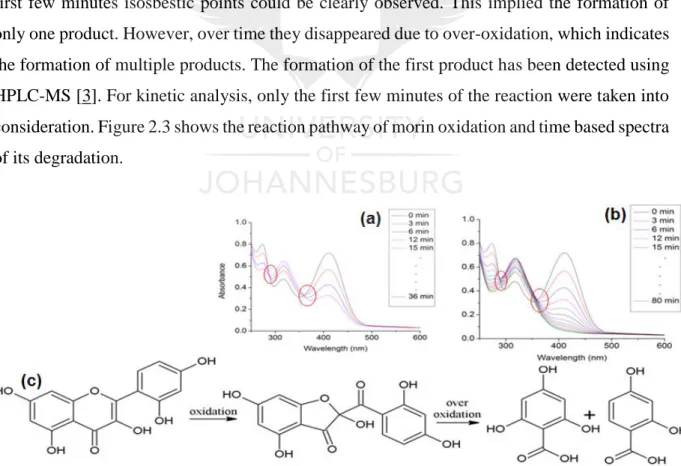
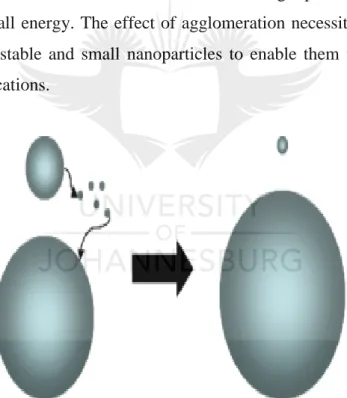
(19) List of Figures. Chapter 1 Figure 1.1. Mechanism of formation of mesoporous materials via the soft-template approach .................................................................................................................................................... 5 Figure 1.2. Inverse micelle sol-gel preparation of mesoporous metal oxides .......................... 6 Figure 1.3. Illustration of hard-template synthesis of mesoporous metal oxides. .................... 6 Figure 1.4. Oxidative dehydrogenation mechanism illustrating oxygen insertion to hydrocarbons. ............................................................................................................................. 9 Figure 1.5. Illustration of the dehydrogenation mechanism for methanol conversion to formaldehyde ........................................................................................................................... 10 Figure 1.6. Ostwald ripening phenomenon showing aggregation of nanoparticles into inactive larger nanoparticles. ................................................................................................................. 11 Figure 1.7. Illustration of the divergent approach for the synthesis of dendrimer. ................. 13 Figure 1.8. Synthesis of dendrimer-encapsulated and dendrimer-stabilized nanoparticles. ... 14 Figure 1.9. Illustration of Mars-van Krevelen and Langmuir-Hinshelwood mechanisms on mixed-metal oxide catalysts in oxidation of carbon monoxide. .............................................. 16 Figure 1.10. The commonly used cations and anions that make up the ionic liquid. ............. 22 Figure 1.11. Schematic representation of SCILL catalyst. ..................................................... 23. Chapter 2 Figure 2.1:(a) Low angle and (b) high angle patterns of KIT-6 and its mesoporous manganese oxide replica, (c) nitrogen sorption results showing type IV loops and (d) cumulative pore size distribution for determination of actual average pore diameter. .............................................. 42 Figure 2.2: HRTEM images of (a) templating KIT-6 and (b) the corresponding mesoporous manganese oxide. ..................................................................................................................... 43 Figure 2.3: (a) Spectra of oxidation of morin as model reaction signified by formation of isosbestic points, circled in red and (b) its over-oxidation to more than one product, shown by disappearance of isosbestic points, circled in red, (c) reaction scheme for the oxidation of morin to the first product and over-oxidation to multiple products. .................................................. 43 Figure 2.4: Kinetic plots showing oxidation of morin over time and (b) verification of pseudo first order kinetics shown by linear plots. ................................................................................ 44 Figure 2.5: Variation of catalyst concentration at constant morin and peroxide concentrations shown by plot of observed rates versus concentration of the catalyst. .................................... 44 xviii.
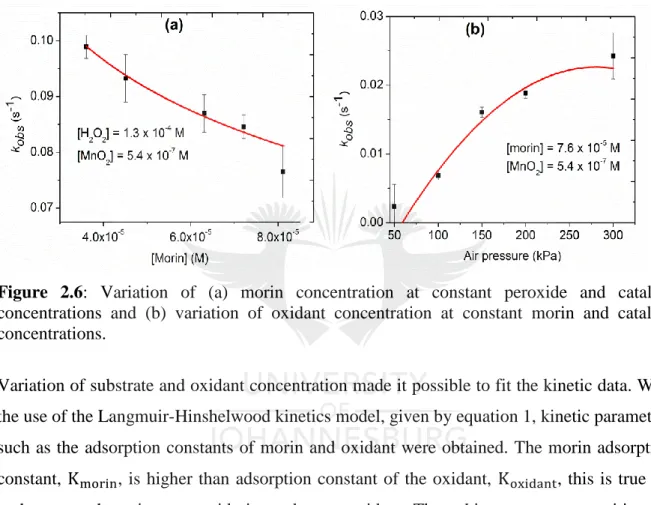
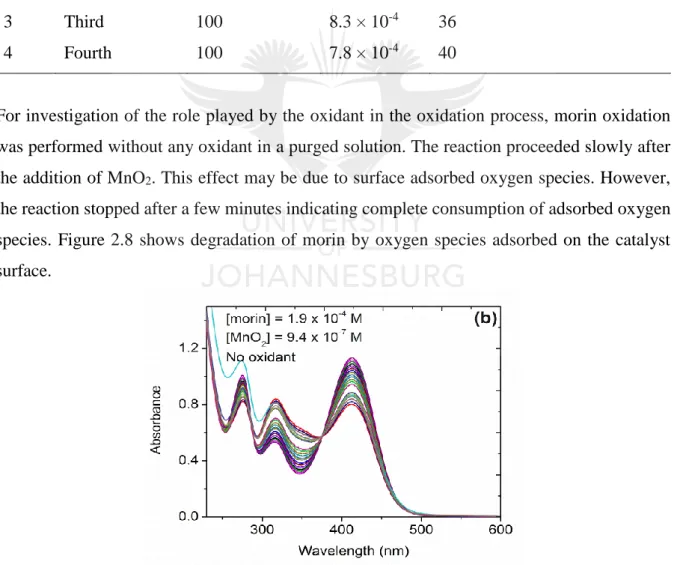
(20) Figure 2.6: Variation of (a) morin concentration at constant peroxide and catalyst concentrations and (b) variation of oxidant concentration at constant morin and catalyst concentrations. ......................................................................................................................... 45 Figure 2.7: Variation of oxidant concentrations used in data fitting. In (a and c) plots of observed rates against peroxide concentration. In (b and d) plots of observed rates against variation in air pressure bubbled into the reaction. The solid lines are obtained from the Langmuir-Hinshelwood model, equation 1, graphs (a and b) and the modified Mars-van Krevelen model, equation 2, graphs (c and d). ........................................................................ 47 Figure 2.8: Proof of morin oxidation by surface adsorbed oxygen indicating a LangmuirHinshelwood mechanism. ........................................................................................................ 48. Chapter 3 Figure 3.1: Diffraction pattern of pure and doped cobalt oxides. Graphs in (a) show low angle diffraction between 0.5° and 2° 2θ range and in (b) is the wide angle diffraction patterns measured between 20° and 65° 2θ. The N2 sorption results showing (c) type IV hysterisis loops and (d) the pore size distribution of pure and doped cobalt oxides. ........................................ 60 Figure 3.2: TEM images of (a) pure cobalt oxide and (b) lithium doped cobalt oxide showing mesoporous nature. SEM images for (c) pure cobalt oxide and (d) lithium-doped cobalt oxide showing the morphology of the catalysts................................................................................. 62 Figure 3.3: H2-TPR for pure and doped-Co3O4 catalysts and (b) the O 1s core level electrons XPS spectrum for undoped and doped Co3O4 catalysts........................................................... 63 Figure 3.4: Changes in binding energy for pure and doped metal oxides determined by XPS analysis. (a) Pure Co3O4 and (b) Cs/Co3O4.............................................................................. 64 Figure 3.5: (a) Time-based spectral evolution of morin oxidation measured at time intervals of three minutes. (b) Kinetic traces for catalyzed oxidation of morin monitored at λ = 410 nm. (c) Natural logarithmic plots for the determination of reaction order. (d) Dependence of apparent rate constants on the Co3O4 concentration monitored at λ = 410 nm. ..................................... 66 Figure 3.6: Arrhenius plots used in calculating apparent activation energies for pure and doped cobalt oxides catalysts.............................................................. Error! Bookmark not defined. Figure 3.7: Catalyst reusability study using the Li/Co3O4 catalysts showing slight changes in apparent rate constants with each catalytic cycle. ................... Error! Bookmark not defined.. xix.
(21) Chapter 4 Figure 4.1: (a) Powder XRD patterns for the synthesized Co/Sn oxide nanoparticles and (b) the electron dispersion spectrum (EDS) of Co/Sn oxide. ........................................................ 80 Figure 4.2: Nitrogen sorption isotherms for the catalysts with different ratios of Co/Sn oxide (a, c, e) and the corresponding pore size distributions (b, d, f). ............................................... 81 Figure 4.3: SEM images for the different ratios of Co:Sn (a-c) and (d-f) the corresponding elemental mapping of the catalytic materials with different ratios. ......................................... 83 Figure 4.4: TEM images of different ration of Co/Sn oxide catalysts. ................................... 83 Figure 4.5: Temperature-programmed reduction profiles of the as-synthesized catalytic materials. .................................................................................................................................. 84 Figure 4.6: Temperature-programmed desorption for the determination of (a) acidic sites using NH3, and (b) basic sites using CO2. ......................................................................................... 85 Figure 4.7: (a) Blank run of the oxidation of morin with hydrogen peroxide and (b) Co/Sn oxide catalyzed oxidation of morin by hydrogen peroxide. ..................................................... 86 Figure 4.8: Plots of (a) observed rate constants against catalyst amount at constant morin and peroxide concentrations and constant temperature and (b) change in absorbance against time with different oxidant concentration at constant morin and catalyst concentration and constant temperature. ............................................................................................................................. 87. Chapter 5 Figure 5.1: (a) Nitrogen sorption studies of hard-template KIT-6 and resulting metal oxides and (b) wide angle powder XRD measurements of template and metal oxides. ..................... 99 Figure 5.2: Transmission electron microscopy of the hard-template KIT-6; b) TEM of the prepared Co3O4 metal oxide using KIT-6. ............................................................................. 100 Figure 5.3: TEM analysis of a) Au28Pd28, b) Au45Pd9 and c) Au50Pd5. Respective size distribution histograms of d) Au28Pd28, e) Au45Pd9 and f) Au50Pd5....................................... 101 Figure 5.4: Hydrogen temperature-programmed reduction profiles of MnO2 and (b) AuPd/MnO2............................................................................................................................ 102 Figure 5.5: Conversion (top) and selectivity (bottom) versus temperature for ethanol oxidation using Au28Pd28/Co3O4 catalysts. ............................................................................................ 105. Chapter 6 Figure 6.1. Powder-XRD patterns for templating KIT-6 and the corresponding metal oxide replicas in (a) low angle pattern and (b) high angle pattern. (c) Type IV hysteresis loops xx.
(22) indicating mesoporous nature of KIT-6 and the corresponding mesoporous metal oxide replicas. (d) Cumulative pore volume distribution showing the average pore size of KIT-6 and the corresponding mesoporous metal oxides ......................................................................... 122 Figure 6.2. TEM image of (a) Pt40-DENs and (b) particle size distribution. TEM images of (c) KIT-6, (d) cobalt oxide and (e) Pt nanoparticles immobilized on the coresponding cobalt oxides. .................................................................................................................................... 124 Figure 6.3. XPS plots a) showing the Co 2p electrons and b) showing the Pt 4f electrons. . 125 Figure 6.4. (a) Isotherm for H2 chemisorption on PtO2/Co3O4, and (b) Adsorption isotherm of 2-MBI on PtO2/Co3O4 and Langmuir isotherm of the adsorption of 2-MBI on PtO2/Co3O4. ................................................................................................................................................ 126 Figure 6.5. Reduction temperatures of pure CeO2 (black line) and PtO2/CeO2 (red line) with corresponding shifts in temperatures upon immobilization of PtO2 nanoparticles................ 127 Figure 6.6. Catalytic activity of (a) the synthesized mesoporous metal oxides at different temperatures and constant pressure, 3 bar and (b) Activity of PtO2 nanoparticles immobilized on SiO2 (black bars) at constant temperature and pressure and the contribution of MMOs and the synergy between MMO and PtO2 nanoparticles (red bars).............................................. 128 Figure 6.7. Effect of temperature on conversion of ethanol and product selectivity at constant pressure catalyzed by (a) PtO2/CeO2 and (b) PtO2/MnO2 as catalysts. ................................. 129 Figure 6.8. Influence of the [EMIm][NTf2] ionic liquid on (a) Pt/MnO2 and (b) Pt/Co3O4 activity and acetaldehyde selectivity at 3 bar and 120 °C. Pressure effect on the activity of (c) Pt/MnO2 and (d) Pt/Co3O4 at constant temperature and [EMIm][NTf2] loading. ................. 130. xxi.
(23) List of Tables. Chapter 2 Table 2.1: Surface kinetic parameters obtained by fitting data with Langmuir-Hinshelwood mechanism and comparison with literature data. ..................................................................... 46 Table 2.2: Surface kinetics parameters obtained by fitting data with a modified Mars-van Krevelen mechanism. ............................................................................................................... 47 Table 2.3: Catalytic reusability study showing changes in apparent rate constants. .............. 48. Chapter 3 Table 3.1: Surface properties of pure and doped cobalt oxide obtained from N2 sorption experiments. ............................................................................................................................. 61 Table 3.2: Determination of Damköhler constants using kinetic parameters obtained at constant morin concentration and with the use of catalysts surface properties. ...............Error! Bookmark not defined. Table 3.3: Thermodynamic data obtained from pure and doped-mesoporous cobalt oxide and comparison with literature values. ........................................... Error! Bookmark not defined.. Chapter 4 Table 4.2: Physicochemical properties of the as-synthesized catalysts determined using nitrogen sorption measurements. ............................................................................................. 82 Table 4.3: Summary of the H2-TPR reduction profiles for the as-synthesized catalytic material and their comparison with literature systems........................................................................... 85. Chapter 5 Table 5.1: Surface properties of mesoporous metal oxides obtained from nitrogen sorption analysis. .................................................................................................................................. 100 Table 5.2: Overview of the reduction temperatures of metal oxides determined by TPR. ... 102 Table 5.3: Ethanol oxidation using mesoporous metal oxides (MMO) as support for Pd28Au28 nanoalloys at 120 °C. ............................................................................................................. 104 Table 5.4: Apparent activation energies for native mesoporous metal oxides and Au28Pd28/MMO catalysts. ...................................................................................................... 106 Table 5.5: Ethanol oxidation using different AuPd nanoalloys supported on MnO2............ 107. xxii.
(24) Chapter 6 Table 6.1: Surface properties of the templating KIT-6 and corresponding mesoporous metal oxides. .................................................................................................................................... 123 Table 6.2: Reduction temperatures of empty mesoporous metal oxides and Pt-loaded mesoporous metal oxides. ...................................................................................................... 127. xxiii.
(25) List of Scheme. Scheme 5.1: Reaction network of ethanol oxidation using Au catalysts. ............................... 94 Scheme 5.2: General methodology for preparation of dendrimer-encapsulated gold-palladium nanoparticles [17]..................................................................................................................... 95. Scheme 6.1: Illustration of the preparation of mesoporous metal oxides, immobilized nanoparticles and SCILL-type catalysts. ............................................................................... 118. xxiv.
(26) Chapter 1: Introduction and Literature Review. Chapter 1 Introduction and Literature Review. 1.
(27) Chapter 1: Introduction and Literature Review. Synopsis of the project A tremendous amount of research has focused its attention on the synthesis of environmentally friendly catalysts. For industrial applications, these environmentally friendly catalysts must be highly active at ambient reaction conditions, highly selective towards the desired products, should be easily separated from the reaction mixture after completion of the reaction, and be reusable for as many catalytic cycles as possible. Also, the preparation of such catalysts must be cost effective. However, synthesis of such catalysts has proved to be a difficult task because of the non-uniform surfaces and difficulty in studying their surface properties.. Heterogeneous catalysts are the most preferred catalysts in industry because of the ease of separation after completion of the catalytic reaction. Elucidation of heterogeneously catalyzed reaction mechanisms led to the realization of surface reactions. This thesis describes heterogeneous catalysis using reducible metal oxides. More emphasis will be placed on oxidation reactions. Enhancement of catalytic activity on the surface of metal oxide catalysts can be achieved by immobilization of catalytically active nano-structures. This realizes the possibility of synergistic effects. The synergistic effect can be brought about by the change in electronic structures of both components of the catalyst. There are many kinetic models developed to explain the reasons of enhanced catalytic activity. One model that is widely accepted is the Mars-van Krevelen model which assumes participation of the lattice oxygen of the oxide.. Herein, an attempt to define preparation of metal oxides and get more insights into their catalytic capabilities by applying them in their pure form, by doping them with alkali and alkaline earth metal ions, combining with another less precious metal, and by immobilization of nano-structures on their surface is made. Also an attempt to study kinetic mechanisms of oxidation of a model reaction, and extend the mechanism to industrially related reactions such as oxidation of ethanol using air as an oxidant is made. Lastly, the effect of tuning the product selectivity by use of ionic liquids in solid catalyst with ionic liquid layer (SCILL) concept is herein investigated in gas-phase oxidation reaction.. 2.
(28) Chapter 1: Introduction and Literature Review. 1.1.. Introduction to metal oxides and their application as heterogeneous catalysts for. oxidation reactions. 1.1.1. Metal oxides and related materials Metal oxides are defined as chemical species with repeating units of metal-oxygen bonds. They are classified according to the position of the metal in the periodic table. Thus, there are metal oxides from the main groups and transition metal oxides. Transition metal oxides are an interesting area of study due to the partial filling of d-orbitals of the metal component(s) which bring about varied chemical and physical properties [1]. They are further classified based on the number of different metals they contain.. 1.1.1.1.. Binary oxides. Binary oxides consist of one cation type such as a transition metal and an oxygen. Although by definition the binary oxide is by far the simplest oxide, there is a complicated phenomenon of non-stoichiometry associated with many oxides of this type. This usually happens when the elemental composition of one or both constituents of the oxide is not an integer number. The fractional composition of elements lead to a very complicated task when assigning oxidation states of the metal component of the oxide. However, these fractional values indicate the presence of two or more atomic states of the metal component of the oxide [1]. A clear example would be cobalt oxide in the form of Co3O4. Assignment of atomic states would yield Co2.6+ if the oxygen is given its usual O2- atomic state. However, the most appropriate way of interpretation is not to think of 2.6+ value as purely fractional but as ratio of the most electropositive to the less electropositive cations. In this case the value would mean the ratio of Co3+ to Co2+ ions. Such metal oxides are termed spinel oxides because of the existence of different oxidation states of the cation. The existence of spinel oxides leads to different geometries. For instance, with Co3O4 the species would have the Co3+ ions in the octahedral positions and the Co2+ in tetrahedral positions. This unusual arrangement in a crystal lattice can be utilized in different applications such as in supercapacitors, optics, sensors, and catalysis [2-4]. The non-stoichiometric characteristic of metal oxides is advantageous in catalysis as they at times lead to formation of elemental vacancies or occupation of sites that are usually not occupied and thus for some reasons enhance or suppress catalytic activity [1, 5].. 3.
(29) Chapter 1: Introduction and Literature Review. 1.1.1.2.. Ternary oxides. Ternary metal oxides consist of two or more cations and an oxygen. Ternary oxides also provide alternatives for better materials in various applications such as catalysis, energy storage and supercapacitors [6-8]. The presence of an extra metallic element tunes the electronic properties of the oxide. Due to partially filled d-orbitals of the oxides of the first row transition metals the electron transitions of the parent metal oxide are in most cases significantly affected by the addition of another metal. This is brought about by the metal-metal bonding(s) present in the resulting oxide that splits the d-orbitals in a different way [9]. The change in splitting energy is mainly accompanied by change in magnetic, optical, and lattice interactions in the oxide which directly affect performance in catalysis.. 1.1.2. Synthesis of metal oxides The activity of these metal oxide species in different applications can be altered significantly by applying synthetic methods that lead to oxide particles of relatively small size. Particle sizes in the nanometer range have been proven to enhance catalytic activity for a range of materials. Herein, emphasis will be placed on the synthesis of mesoporous metal oxides in their nm range instead of bulk oxide preparation. The most important reason for this choice is that porous structures have been reported to have a higher surface area and abundant transport channels that help to enhance activity in different applications [10-14]. Various metal oxides are prepared by two traditional methods, namely the soft-template and the hard-template method.. 1.1.2.1. The soft-template method The soft-template approach makes use of surfactants to control the synthesis of mesoporous metal oxides. The surfactants are reported to self-assemble and form micelles under certain reaction conditions [15]. Deposition of metal precursor on the micelles and the removal of the micelles by solvent extraction results in the formation of mesoporous metal oxides. This synthesis has been reported by many research groups [16, 17], and has its origin in the Mobil Oil Corporation where one of the first synthesis of mesoporous metal oxides was reported [18]. One of the interesting factors about this synthetic route is the tunability of the surface properties such as surface area, pore size, and pore volume. Vunain et al. reported that the variation of aging time and recrystallization time alters the pore size of mesoporous materials synthesized using the soft-template approach [15]. Figure 1.1 shows the synthesis route for the soft-template method using cetyl trimethylammonium bromide (CTAB) and tetraethyl orthosilicate (TEOS). 4.
(30) Chapter 1: Introduction and Literature Review. Figure 1.1. Mechanism of formation of mesoporous materials via the soft-template approach [15].. In this type of synthesis, water plays a crucial role and the pH is an important factor. Depending on the pH, the ionic and cationic species either create strong Coulombic interactions or strong ligand-metal interactions via the hydrogen bonding and systems with such interactions have low thermal stability. Poyraz et al. reported the sol-gel chemistry for the synthesis of mesoporous transition metal oxides [14]. They reported the role of nitrate ions in the synthesis where they used HNO3 to prevent condensation and the metal oxo-clusters were stabilized and confinement of the clusters occurred in hydrated inverse micelles. The stabilized oxo-clusters then interact with the surfactant via hydrogen bonding. The formation of micelles from the surfactant helps form a physical barrier between the oxo-clusters and prevents uncontrolled aggregation. Figure 1.2 illustrates the formation of mesoporous metal oxides via the inverse micelle sol-gel chemistry route.. 5.
(31) Chapter 1: Introduction and Literature Review. Figure 1.2. Inverse micelle sol-gel preparation of mesoporous metal oxides [14].. 1.1.2.2. The hard-template method This synthesis approach, often referred to as nanocasting, makes use of a porous hard-template (typically silica) to cast the resulting mesoporous metal oxide [19, 20]. The advantage of hard-template is the tunability of the pores of the template itself, thus synthesis of desired pore sizes of the resulting metal oxide is possible [21]. The synthesis of metal oxides using the hard-template method has been reported by many research groups and the hard-template used was mainly the silicon-based KIT-6 [20]. Metal ions are then incorporated into the pores of the template with solvent assisted evaporation, then calcination in air at high temperature results in the formation of the metal oxide [19]. Figure 1.3 is a schematic illustration of the hard-template synthesis of mesoporous metal oxides.. Figure 1.3. Illustration of hard-template synthesis of mesoporous metal oxides.. 6.
(32) Chapter 1: Introduction and Literature Review. 1.2.. Application of metal oxides in catalysis. Based on the interconnected network of repeating units of metal-oxygen bonds that make up an oxide, the catalytic properties differ depending on the redox properties of the active metal ion. The change in catalytic properties is brought about by changes in bond length, strength, and subsequently bond energy in metal-oxygen bond. Also, the nature of the metal ion plays a crucial role in tuning catalytic properties. The oxygen mobility on the surface of the oxide is another factor that determines the activity of the oxide catalysts [22, 23]. The availability of oxygen vacancies on the oxide surfaces is also believed to trigger different catalytic behavior in many oxides [24, 25]. This phenomenon has been well documented for ceria [26, 27].. Amongst the many applications of both binary and ternary metal oxides, catalysis has been an interesting one [17]. Metal oxides are capable of catalyzing a wide range of reactions such as oxidation [20, 28-31], reduction [32], and ammoxidation [33, 34]. However, based on the limited scope of this work, emphasis will be placed on oxidation reactions. The choice of oxidation reactions is solely based on the expected synergistic effect that the oxygen-rich metal oxides and the active nanostructures could possess.. 1.2.1. Oxidation reactions Oxidation reactions are an important class of reactions both in academia and industrially [35]. Academically their importance derives from the complex scientific challenge they pose to scientists trying to study their properties. Industrially, oxidations continue to take center stage in the production of large scale fine chemicals and pharmaceuticals. The production of these chemicals via the oxidation processes is an old process which dates back decades. The value of oxidation products back in the 1980’s was estimated to be between US$ 20-40 billion per annum and the value kept on increasing with time [36]. Though there is a tremendous profit generated in the production of the above chemicals, the task of producing them is a difficult one. There are many drawbacks such as the use of environmentally unfriendly reactants and solvents, and most importantly, the over-oxidation of the desired partial oxidation products. Thus, ways to design catalysts that are efficient in environmentally friendly reaction conditions have been the subject of research for a long time. These environmentally friendly conditions include the use of peroxide-free oxidants and chlorine free catalysts. Thus, the use of molecular oxygen from air has been utilized as a cost effective and a greener oxidant. The catalysts themselves should be synthesized with cost effective methods and be fully characterized to study the surface properties. 7.
(33) Chapter 1: Introduction and Literature Review. 1.2.1.1. Types of oxidation catalysts Industrially, the production of oxygenates using the oxidation route employs a variety of catalysts such as coordination compounds and metal oxides, to mention a few. Both sets of catalytic systems mentioned had been extensively used in many oxidation reactions and have had their successes and drawbacks.. 1.2.1.1.1. Coordination complexes The palladium-based coordination compounds have been successfully used in the Wacker-Hoeschst process for the production of acetaldehyde [37, 38]. In the Wacker-Hoeschst process, the chloride is the ligand of choice and the process is precisely homogeneously catalyzed.. The catalytic coordination compounds are not limited to palladium complexes, vanadium metal can actively catalyze the oxidation of olefins. Oxovanadium(IV) complexes with different ligand systems successfully oxidized styrene to benzaldehyde [39]. These type of catalysts have drawbacks. The presence of chlorine is considered as a catalyst poison and affects the catalytic activity. Also the stability of these catalytic systems is questionable.. 1.2.1.1.2. Metal oxides Metal oxides have been utilized as catalysts for oxidation reactions [28, 40]. However, studies to uncover their surface properties have proved to be a complex task. This leads to poorly drawn kinetic trends between catalyst structures and reaction mechanisms [35]. The complexity in understanding the oxide catalyst structures also leads to the difficult task of tuning product selectivity in many oxidation reactions. However, attempts to study oxidation mechanisms using oxides have been made and progress to date is promising. One example is the use of bismuth molybdate-based catalysts in the oxidation of alkanes [23].. 1.2.2. Established mechanisms in metal oxide catalyzed reactions. 1.2.2.1. Oxidative dehydrogenation This oxidation process is initiated by dissociation of adsorbed oxygen on the surface of the oxide [5]. Direct participation of dissociated reactive oxygen species in the oxidation of the substrate is assumed [41]. This type of mechanism is often referred to as oxygen assisted dehydrogenation. Furthermore, for oxidation to take place on the surface of the catalytic metal 8.
(34) Chapter 1: Introduction and Literature Review. oxide, both reactants must be activated on the surface. The oxidative dehydrogenation is often associated with the Langmuir-Hinshelwood mechanism. However, in some instances, the involvement of the lattice oxygen in the oxidation process, which is latter replenished by surface-dissociated oxygen from the oxidant of choice, makes this mechanism to be viewed in terms of the Mars-van Krevelen model. Figure 1.4 shows the oxidative dehydrogenation mechanism for the formation of propanal.. Figure 1.4. Oxidative dehydrogenation mechanism illustrating oxygen insertion to hydrocarbons.. 1.2.2.2. Dehydrogenation Unlike the oxidative dehydrogenation, dehydrogenation assumes hydrogen transfer by the substrate onto the catalyst surface [5]. The substitution of oxygen as an oxidant by hydrogen acceptor molecules confirmed the hydrogen transfer mechanism [42]. The role of the oxygen is the oxidation of the byproduct, hydrogen, to form water. Similar to the oxidative dehydrogenation, dehydrogenation also requires adsorption of the substrate on the oxide surface. Figure 1.5 shows dehydrogenation of methanol on oxide catalyst.. 9.
(35) Chapter 1: Introduction and Literature Review. Figure 1.5. Illustration of the dehydrogenation mechanism for methanol conversion to formaldehyde Due to the requirement of surface adsorption of the substrate in both mechanisms, reactivity on the oxide surface has been the subject of extensive research. There are kinetic models that have been developed to study the surface reactions. There are three models that have been studied extensively, both in liquid- and gas-phase reactions. They are (1) The Eley-Rideal, (2) Langmuir-Hinshelwood, and (3) the Mars-van Krevelen models. They all differ in kinetic interpretation and they are all mathematical approximations. However, when supported by precise experimental and theoretical evidence, they become useful in the design and application of novel metal oxide catalysts.. 1.3. Introduction to transition metal nanoparticles and their application as heterogeneous catalysts in oxidation reactions. 1.3.1 Synopsis into nanoparticles and catalysis Defined as a cluster of atoms with dimensions of less than 100 nm in diameter, nanoparticles have drawn tremendous attention among researchers in different fields due to their novel properties. Compared to their bulk counter-parts, nanoparticles posses enhanced optical, electronic, magnetic, and catalytic properties. All these properties are brought about by oscillation of electrons in the conduction band and they can be manipulated depending on the intended application [43]. In fields like catalysis, researchers take advantage of the high surface area to volume ratio of the nanoparticles [44]. Thus, a variety of reactions have been catalyzed. 10.
(36) Chapter 1: Introduction and Literature Review. by nanoparticles including carbon-carbon coupling [45, 46], reduction [47-50], and oxidation reactions [51, 52].. The stability of nanoparticles is one aspect that needed careful consideration in the past. The tendency of these small particles to agglomerate and form larger inactive particles has been a drawback in many applications. The aggregation of these particles into larger inactive particles is called Ostwald ripening (see Figure 1.6). Ostwald ripening, also called particle coarsening, was first observed by Wilhelm Ostwald in 1896. This spontaneous process is a thermodynamically driven process where large particles are energetically more stable than the smaller particles. The molecules on the surface of the particles are energetically less stable than the ones in the interior. This then suggests that large particles have a lower surface area to volume ratio and lower surface energy. The smaller, energetically unfavorable, and higher surface energy particles tend to attach to the surface of the larger particles and in that way the system lowers its overall energy. The effect of agglomeration necessitates carefully planned preparation routes for stable and small nanoparticles to enable them to retain their activity across a range of applications.. Figure 1.6. Ostwald ripening phenomenon showing aggregation of nanoparticles into inactive larger nanoparticles.. 1.3.2. Preparation and stabilization of nanoparticles There are two routes that have been reported extensively on for the preparation of nanoparticles. The first synthetic route is the bottom-up approach. The bottom-up approach makes use of decomposition of an organometallic precursor [53]. The main chemical reduction taking place is the reduction of the metal ion by a suitable reducing agent to form zerovalent 11.
(37) Chapter 1: Introduction and Literature Review. metal nanoparticles. The second route is the top-down approach which involves decomposition of larger metal structures through thermal, mechanical, or chemical decomposition [54]. However, in both synthetic routes, the use of stabilizers is necessary to avoid agglomeration. Different types of stabilizing agents have been employed to reduce or to completely eliminate the possibility of agglomeration of the metallic nanostructures. These stabilization techniques are classified into different types, namely, (i) electrostatic, (ii) steric, and (iii) electrosteric stabilization.. 1.3.2.1. Electrostatic stabilization Electrostatic stabilization, often referred to as electronic stabilization, is a theoretical concept developed by Derjaugin et al. in the 1940s [53]. It assumes binding of anionic species to the coordinated unsaturated, electrophilic nanocluster surface and in that way providing a stabilizing layer that provides Coulombic repulsion between the two particles [55, 56].. 1.3.2.2. Steric stabilization Steric stabilization results from ligation of the surface of the nanoparticles. Nanoparticles stabilized in this manner in most cases loose activity when used as catalysts. This is due to passivation of the catalytically active surface atoms by stabilizers. Thus, in the field of catalysis, steric stabilized nanoparticles are considered to be ligand-poisoned nanoparticles. However, with small ligands that result in a large fraction of the surface atoms remaining coordinatively unsaturated, the nanoparticles remain catalytically active. This type of stabilization makes use of a range of polymers that act as ligands to the nanoparticles’ surface atoms and also by providing steric hindrance in the space around the nanoparticles [57-61]. Amongst many other stabilizers, poly(vinyl pyrolidone), PVP, is the most used stabilizer because of its non-toxicity and high solubility in many polar solvents [62]. Polymeric micromolecular dendritic structures have recently drawn attention as nanoparticle stabilizers. They have been used to a great effect in the synthesis of mono-dispersed nanoparticles with a narrow size distribution [63, 64]. The scope of this work will put more emphasis on the use of dendrimers as nanoparticle stabilizers.. The combination of the two stabilization methods, electrostatic and steric stabilization is known as electrosteric stabilization. 12.
(38) Chapter 1: Introduction and Literature Review. 1.3.3. Origin of dendrimers Dendrimers are defined as three dimensional polymeric macromolecules with high density of pheripheral groups [64, 65]. They are characterized by the core, the branching groups, and the terminal or pheripheral groups. The synthesis of dendrimers dates back to 1941 when the first theoretical work was reported by Paul Flory [66]. Flory’s work triggered many researchers to further the research into the synthesis of these dendritic structures. The next set of reports into dendritic materials came in 1978 and in 1985 by Vögtle [67], and Newkome and Tomalia [68, 69], respectively. Tomalia’s poly(amido amine) dendrimers and Newkome’s arborol systems were extensively studied.. 1.3.3.1. Synthetic routes towards higher generation dendrimers. There are two well-known synthetic routes for the synthesis of dendrimers. The two synthetic routes differ in the direction of structural growth. The first route, the divergent approach, shown in Figure 1.7, starts from the core and grows to the peripheral groups [68, 70]. While on the other hand the convergent approach is the opposite of the divergent approach. The convergent approach was developed by Frèchet [71].. Figure 1.7. Illustration of the divergent approach for the synthesis of dendrimer [80].. 1.3.3.2. Dendrimers as templating and stabilizing agents for nanoparticles The structure of the dendrimer contains internal voids within the three dimensional framework. The availability of such voids is advantageous as they are utilized to host foreign nanoparticles with less passivation of surface atoms [72, 73]. The poly(amidoamide), PAMAM, dendrimer was utilized by Tomalia et al. [74] and Crooks et al. to host a range of transition metal nanoparticles [75, 76]. The PAMAM dendrimer was also utilized to template the synthesis of. 13.
(39) Chapter 1: Introduction and Literature Review. these nanoparticles. They achieved the templated synthesis by making use of the tertiary nitrogen branching groups within the dendrimer. Coordination of most metal ions to these nitrogen bearing groups inside the dendrimer is aided by intermediate donor nature of the nitrogens which makes them capable of binding to either soft or hard Lewis acids. Reduction with a suitable reducing agent of the coordinated metal ions results in nanoparticles encapsulated within the dendrimer and are referred to as dendrimer-encapsulated nanoparticles (DENs).. Although the dendrimer-templated synthesis yields nanoparticles with narrow size distribution and well-dispersed nanoparticles, there is a possibility of forming nanoparticles that are not encapsulated within the dendrimer but stabilized by two or more dendrimers, known as dendrimer-stabilized nanoparticles (DSNs). The formation of these two phenomena is illustrated in Figure 1.8. The difference between the dendrimer-encapsulated and dendrimerstabilized nanoparticles is the manner in which they are stabilized. The dendrimer-encapsulated nanoparticles’ surface atoms are less passivated because the ligating effect of the nitrogens is minimal as the nanoparticles are held by steric hindrance in three dimensions from the branching groups. While the dendrimer-stabilized nanoparticles’ surface atoms are passivated by the peripheral groups which have a high density of atoms. To avoid coordination to the peripheral groups, which subsequently results in the formation of dendrimer-stabilized nanoparticles, protection of peripheral groups is necessary.. Figure 1.8. Synthesis of dendrimer-encapsulated and dendrimer-stabilized nanoparticles.. 14.
(40) Chapter 1: Introduction and Literature Review. 1.3.4. Application of metal nanoparticles as catalysts Colloidal nanoparticles have been applied as catalysts in many reactions. The versatility of many metal nanoparticles in a range of reactions is mainly because of the ease at which they transfer electrons [77, 78]. More emphasis was put on the use of dendrimer-encapsulated nanoparticles as catalysts because the minimal passivation of surface atoms allows for a comprehensive kinetic studies at the catalyst surface. The use of dendrimer-encapsulated copper nanoparticles was reported by Feng et al. for the first time in the reduction of 4nitrophenol to 4-aminophenol [79]. Soon after, many reactions using dendrimer-encapsulated nanoparticles were reported [80-82]. These include oxidation, carbon-carbon coupling [83], and other reduction reactions [84]. Furthermore, all these reactions are governed by certain type of kinetics. Most of these reactions require adsorption of one or more reactants onto the surface of the heterogeneous catalyst.. 1.3.4.1. Kinetic models There are various types of kinetic models that govern adsorption of reactant(s) onto the surface of the catalyst and the reaction that takes place on the surface. The Eley-Rideal model takes into account a catalytic bimolecular reaction in gas-phase. This type of kinetic model assumes adsorption of only one reactant onto the surface of the catalyst [85, 86]. The adsorbed species then reacts with the second reactant which is in the gas-phase to form the product [87]. The Langmuir-Hinshelwood model is by far the most reported kinetic model. It assumes the adsorption of both the substrate and the reactant onto the surface of the catalyst [87, 88]. The interpretation of the Langmuir-Hinshelwood model is a complicated process because one of the assumptions is that all catalyst sites are identical, which is very rare for many catalysts. The assumption that most reaction steps are in thermodynamic equilibrium also proves to be ambiguous. With the Langmuir-Hinshelwood model the rate determining step is the surface reaction of the adsorbed species. However, careful analysis of the experimental data can lead to a possibility of different rate determining steps.. While on the other hand, the Mars-van Krevelen model is purely based on the redox properties of the catalyst. This model was developed by Mars and van Krevelen for the oxidation of naphthalene in 1954 [89]. It assumes participation of the loosely bound lattice oxygen of the catalyst during the oxidation process. It then assumes that the catalyst undergoes a redox cycle where it is reduced by loss of lattice oxygen to the substrate and it is reoxidized by an oxygen 15.
(41) Chapter 1: Introduction and Literature Review. species from the reaction oxidant [90]. However, Vannice identified shortcomings of the original derivation of the Mars-van Krevelen rate equation [90]. Thus, throughout this work a modified rate equation is used. The model remains relevant in the kinetic interpretation of many oxidation reactions catalyzed by oxygen-rich catalysts such as metal oxides [20, 91-93]. Figure 1.9 shows the illustration of both the Mars-van Krevelen and Langmuir-Hinshelwood models.. Figure 1.9. Illustration of Mars-van Krevelen and Langmuir-Hinshelwood mechanisms on mixed-metal oxide catalysts in oxidation of carbon monoxide. The derivations of both the Mars-van Krevelen and the Langmuir-Hinshelwood rate equations have been reported on previously. Mars-van Krevelen model which assumes the participation of the catalyst’s lattice oxygen as an oxidant was best suited for this study [94]. However, the original derivation by Mars and van Krevelen has been reported to be erroneous, thus, it required modification [90]. The derivation yielded equation 1.. 16.
(42) Chapter 1: Introduction and Literature Review. 𝑟=. (𝑘1 𝑘2 [𝐴][𝑜𝑥𝑖𝑑𝑎𝑛𝑡]𝑛 ) 𝑘1 [𝐴] + 𝑘2 [𝑜𝑥𝑖𝑑𝑎𝑛𝑡]𝑛. (1). where 𝑟, 𝑘1 , and 𝑘2 are rates constants, rate constant for the formation of the product at the catalyst surface and rate constant for catalyst reoxidation, respectively.. The participation of lattice oxygen is assumed to result in the reduced catalyst and the catalyst is reoxidized by oxygen from the oxidant or absorbed oxygen. The Mars-van Krevelen equation was derived from this analogue, and the following formulations were made: 𝐴 + 𝑐𝑎𝑡𝑎𝑙𝑦𝑠𝑡 → 𝐵 + 𝑟𝑒𝑑𝑢𝑐𝑒𝑑 𝑐𝑎𝑡𝑎𝑙𝑦𝑠𝑡. (i). 𝑅𝑒𝑑𝑢𝑐𝑒𝑑 𝑐𝑎𝑡𝑎𝑙𝑦𝑠𝑡 + 𝑂2 → 𝐶𝑎𝑡𝑎𝑙𝑦𝑠𝑡. (ii). where A is the substrate to be oxidized and B is the oxidation product. From this, the following assumptions were made, (1) formation of the product, B, in step (i), is first order with respect to substrate A concentration, (2) lattice oxygen ions are participating in the oxidation of substrate A, (3) the rate of reoxidation of the catalyst is proportional to [𝑜𝑥𝑖𝑑𝑎𝑛𝑡]𝑛 and the number of unoccupied sites, (1-θ). Albert Vannice’s analysis of the original Mars-van Krevelen rate equation based on the above assumptions revealed errors in its derivation [90]. One of the errors in the rate equation is the nondissociative adsorption of oxygen on the surface, whereas most of molecular oxidation reactions require oxygen ions. The use of non-elementary steps in the final rate equation, and the absence of other species in site balance are some of the errors reported by Vannice.. If we consider an oxidation reaction that produces no byproducts and consider the participation of lattice oxygen in the oxidation process, then the following kinetic steps can be postulated: 𝑘1. 𝐴 + 2𝑂 ∗ → 𝐵 + 𝑂 ∗ + ∗. (2). 𝑘2. (3). 𝑘3. (4). 𝐴+𝑂∗+∗→𝐵+ 2∗ 𝑂2 + 2 ∗ → 2𝑂 ∗. 17.
Figure
![Figure 1.1. Mechanism of formation of mesoporous materials via the soft-template approach [15]](https://thumb-us.123doks.com/thumbv2/123dok_us/9046136.2802482/30.892.288.614.138.788/figure-mechanism-formation-mesoporous-materials-soft-template-approach.webp)
![Figure 1.2. Inverse micelle sol-gel preparation of mesoporous metal oxides [14].](https://thumb-us.123doks.com/thumbv2/123dok_us/9046136.2802482/31.892.232.659.128.1024/figure-inverse-micelle-sol-preparation-mesoporous-metal-oxides.webp)
![Figure 1.7. Illustration of the divergent approach for the synthesis of dendrimer [80]](https://thumb-us.123doks.com/thumbv2/123dok_us/9046136.2802482/38.892.168.735.483.875/figure-illustration-divergent-approach-synthesis-dendrimer.webp)
Outline
Related documents
Robertson NL, Moore CM, Ambler G et al (2013) MAPPED study design: A 6 month randomised controlled study to evaluate the effect of dutasteride on prostate cancer volume using
Example of AWS Protocol utilizing CIWA-Ar Symptom Triggered Pharmacologic
Researcher not only present discussion on the various determinants factors for the soil fertility, but also propose a methodology with different complex analysis
The results (Fig. 1A) show that fluctuations in Nanog:VNP expression can be detected in mESCs grown in both conditions, with the major difference being the number of cells that do
If the three above conditions are satisfied at the same time, then the generalized multivariable Gimel-function reduces in the generalized multivariable H-function (exten s ion
( G ) Representative tissue sec- tions showing that SAN-targeted eGFP and CaMKIIN expression protects against Ang II–induced TUNEL staining compared with SAN-targeted eGFP
To elucidate the characteristics of this CDK/cyclin pair implied by its association in a tight complex, its different regulation due to no abundance fluctuation by the
[r]