RESEARCH ARTICLE STEM CELLS AND REGENERATION
Maintenance of
Drosophila
germline stem cell sexual identity in
oogenesis and tumorigenesis
Laura Shapiro-Kulnane, Anne Elizabeth Smolko and Helen Karen Salz*
ABSTRACT
Adult stem cells maintain tissue homeostasis by balancing self-renewal and differentiation. In Drosophila females, germline stem cells (GSCs) requireSex lethal(Sxl) to exit the stem cell state and to enter the differentiation pathway. Without Sxl GSCs do not differentiate and instead form tumors. Previous studies have shown that these tumors are not caused by a failure in the self-renewal/ differentiation switch. Here, we show thatSxlis also necessary for the cell-autonomous maintenance of germ cell female identity and demonstrate that tumors are caused by the acquisition of male characteristics. Germ cells without Sxl protein exhibit a global derepression of testis genes, including Phf7, a male germline sexual identity gene. Phf7 is a key effector of the tumor-forming pathway, as it is both necessary and sufficient for tumor formation. In the absence of Sxl protein, inappropriate Phf7 expression drives tumor formation through a cell-autonomous mechanism that includes sex-inappropriate activation of Jak/Stat signaling. Remarkably, tumor formation requires a novel response to external signals emanating from the GSC niche, highlighting the importance of interactions between mutant cells and the surrounding normal cells that make up the tumor microenvironment. Derepression of testis genes, and inappropriate Phf7 expression, is also observed in germ cell tumors arising from the loss ofbag of marbles(bam), demonstrating that maintenance of female sexual identity requires the concerted actions of Sxlandbam. Our work reveals that GSCs must maintain their sexual identity as they are reprogrammed into a differentiated cell, or risk tumorigenesis.
KEY WORDS:Sxl, Oogenesis, Germline tumors, Jak/Stat, Phf7
INTRODUCTION
Homeostasis of adult tissues depends on a stable population of stem cells that have the capacity to give rise to both self-renewing and differentiating daughter cells. Many cancers exhibit stem cell-like phenotypes, suggesting a direct link between aberrant stem cell behavior and tumor formation (Friedmann-Morvinski and Verma, 2014). The mechanisms involved, however, remain largely unknown. Drosophilaoogenesis is a powerful model system for the study of adult stem cells and their connection to cancer stem cells (Hudson and Cooley, 2014; Spradling et al., 2011; Tipping and Perrimon, 2014). Adult ovaries are composed of individual strands of developing egg chambers called ovarioles. Each ovariole maintains a steady population of two to three germline stem cells (GSCs) located at the tip in a structure called the germarium. The GSCs divide asymmetrically to give rise to one daughter that remains a stem cell and another
daughter, called a cystoblast (CB), that commits to differentiation. The CB then undergoes four mitotic divisions to form an interconnected 16-cell cyst. Only one of these 16 cells differentiates into an oocyte, while the remaining 15 cells develop as polyploid nurse cells. An egg chamber is formed as the somatic follicle cells surround the 16-cell cyst and bud off from the germarium (Fig. 1A). The decision between stem cell maintenance and differentiation is controlled by both intrinsic and extrinsic mechanisms. The GSCs, which are located at the anterior end of the germarium, receive differentiation-inhibiting signals from their somatic neighbors. For example, somatic cells at the tip of the germarium activate BMP signaling in GSCs to directly repress transcription of the differentiation-promoting gene bag of marbles(bam) (Chen and McKearin, 2003a,b, 2005; Song et al., 2004). Cell-intrinsic programs, such as those carried out by the translational repressor Nanos, are also required for stem cell maintenance (Forbes and Lehmann, 1998; Gilboa and Lehmann, 2004; Harris et al., 2011; Wang and Lin, 2004). Following an oriented cell division, the GSC daughter that is specified as a CB displays an increase in Bam protein levels and a decrease in self-renewal factors, including Nanos protein (Chau et al., 2009, 2012; Li et al., 2009).
We recently found that the switch to the CB fate requires the female-specific RNA-binding protein Sex lethal (Sxl) (Chau et al., 2009, 2012). Germ cells without Sxl protein form tumors that comprise a few bona fide GSCs located at the tip of the germarium and cells that coexpress both Bam protein and a set of GSC markers, including Nanos protein. Studies, showing thatnanosis an Sxl target gene and that Nanos downregulation in CB cells is controlled at the level of translation, indicate thatSxlenables the GSC-to-CB switch by directly inhibitingnanostranslation (Chau et al., 2009, 2012; Li et al., 2009). Althoughnanosis clearly necessary for tumor growth, both loss- and gain-of-function studies indicate that the failure to regulate nanosis not a trigger for tumorigenesis (Chau et al., 2012; Harris et al., 2011; Li et al., 2009). Consequently, the mechanism driving tumor formation in the absence of Sxl protein remains unknown.
Previous studies have shown that Sxl also functions in somatic cells, where its activation in early embryogenesis is the central female determining event (Salz, 2011; Salz and Erickson, 2010). Sxl, however, is not essential for establishing sexual identity in the female germline. In the absence ofSxl, germ cells develop normally and exhibit the appropriate female-specific behaviors and expression patterns through the end of the larval period (Casper and van Doren, 2009; Chau et al., 2009; Steinmann-Zwicky, 1994). Germ cells, unlike somatic cells, acquire their female identity by a non-cell-autonomous mechanism, as demonstrated by studies showing that the gene expression program and behavior of embryonic XX germ cells is masculinized if placed in a male somatic environment (Casper and van Doren, 2009; Horabin et al., 1995; Staab et al., 1996; Wawersik et al., 2005). Dictation by somatic signals continues through the larval stages, after which point XX germ cells control their own sexual development in a cell-autonomous manner.
Received 12 August 2014; Accepted 28 January 2015
Department of Genetics and Genome Sciences, Case Western Reserve University School of Medicine, Cleveland, OH 44106-4955, USA.
*Author for correspondence (hks@case.edu)
DEVEL
O
In this study, we establish that, although Sxldoes not play a cell-autonomous role in germ cell sex determination, it is necessary for the maintenance of female identity. We show that, when germ cells lack Sxl protein, tumor formation is accompanied by a global derepression of testis genes, including aberrant expression of the male germline sexual identity genePHD finger protein 7(Phf7) (Yang et al., 2012). We further ascertain thatPhf7 drives tumor formation through a mechanism that includes the sex-inappropriate activation of Janus kinase/Signal transducer and activator of transcription (Jak/Stat) signaling in XX germ cells. Notably, the tumor phenotype depends on paracrine signals from neighboring somatic gonadal cells. Together with previous studies showing that male, but not female, germ cells are able to activate the Jak/Stat pathway in response to signals emanating from the somatic niche (Decotto and Spradling, 2005; Kiger et al., 2001; Leatherman and DiNardo, 2008, 2010; López-Onieva et al., 2008; Tulina and Matunis, 2001; Wang et al., 2008), our work suggests that tumors in this model system form because mutant germ cells respond to their environment as if they were male germ cells. Remarkably, derepression of testis genes, including inappropriate Phf7 expression, is also observed in bam ovarian tumors. This work demonstrates that female GSCs must maintain their female sexual identity, through a mechanism that requires the concerted actions of Sxl and bam, as they differentiate or risk tumor formation.
RESULTS
snf148ovarian tumors inappropriately express a large
number of testis transcripts
Here, as in our earlier studies, we investigateSxlfunction in germ cells by taking advantage of the viable sans fille(snf148) allele,
which interferes withSxlregulation in germ cells without disrupting Sxlregulation and expression in the somatic cells of the ovary (Chau et al., 2009, 2012). In wild-type females Sxl protein is present in all female somatic cells and accumulates to high levels in the cytoplasm of only a few germ cells located at the tip of the germarium (Chau et al., 2009) (Fig. 1B). By contrast, insnf148mutants the ovary is
filled with proliferating undifferentiated germ cells that fail to accumulate Sxl protein even though Sxl protein is clearly detectable in the surrounding somatic cells (Chau et al., 2009) (Fig. 1C). Importantly, all aspects of the snf148 germline tumor phenotype
described to date can be restored by expression ofP{otu::SxlcDNA}, a transgene that expresses Sxl cDNA under the control of a germline-specific promoter (Chau et al., 2009, 2012; Nagengast et al., 2003).
To gain a better understanding of why the loss of Sxl protein in the germline leads to tumors, we used high-throughput sequencing (RNA-seq) to compare the transcriptomes of 3-day-old snf148
[image:2.612.134.477.58.288.2]tumorous ovaries with ovaries from 0- to 24-h-old virgin wild-type females (Fig. 1D,E). Because ovaries from young wild-type females lack late-stage egg chambers, we reasoned that this comparison would minimize the identification of gene expression changes unrelated to the mutant phenotype. Visualization of our RNA-seq data on the UCSC genome browser (UCSCdm3/FlyBase r5) showed that male-specificSxlRNA products are detectable in tumors, but not in wild type (Fig. 1F).Sxltranscripts are sex-specifically spliced to produce mRNAs with different coding potentials (Salz, 2011; Salz and Erickson, 2010). In males, all transcripts include the translation-terminating third exon and encode truncated, inactive proteins. In females, the third exon is always skipped to generate protein-encoding mRNAs. Our finding that tumors contain male-specific transcripts is consistent with earlier studies documenting that loss of Fig. 1.snf148germline tumors.(A) Schematic of a wild-typeDrosophilagermarium. Germline cells are in shades of green. Cells of somatic origin are in shades of blue, red and gray. Spectrosomes and fusomes are yellow. GSC, germline stem cell; CB, cystoblast; sp, spectrosome; TF, terminal filament. (B,C) Germaria from wild-type andsnf148ovaries stained for Sxl (green) and DNA (red). The prominent cytoplasmic Sxl staining observed in wild-type GSCs and their daughter cells is absent insnf148tumors. Arrows mark the anterior tip of the germarium. Scale bar: 50 µm. (D) DAPI-stained ovariole from a 0- to 1-day-old wild-type female. The white bracket indicates the germarium. The yellow bracket indicates the egg chambers. (E) DAPI-stained tumor from a 3-day-oldsnf148mutant female. The arrow (D,E) marks the anterior tip of the germarium. (F) UCSC genome browser view of a portion of theSxllocus. The screen shot is reversed so that the start of transcription is on the left, and tracks are viewed at the same scale. Wild-type reads are in blue andsnf148reads in red. The reads that are unique tosnf148 are highlighted with gray shading. RefSeq annotations (beneath) indicate that the unique read aligns to exon 3. Gray boxes indicate the untranslated exons and black boxes represent the open reading frame. Exon 3, which includes multiple in-frame stop codons, does not produce a functional protein.
DEVEL
O
Sxlactivity in thesnf148germline is caused by a splicing defect that
ultimately prevents Sxl protein production (Nagengast et al., 2003). We identified 804 genes that change expression at least twofold in snf148tumorous ovaries relative to wild type (P<0.05). Of these, 483
are upregulated and 321 are downregulated (supplementary material Tables S1 and S2). Interestingly, Gene Ontology (GO) analysis identified a set of ‘male-meiosis’ (GO:0007140) genes that are significantly over-represented in the upregulated gene set (Fig. 2A). Among these genes are two testis-specific transcription factors,aly (amip130paralog) andrye(Taf12L–FlyBase; aTaf12paralog), that are required for expression of the spermatocyte transcription program (White-Cooper, 2010). A visual survey of the most highly overexpressed genes in tumors revealed several more genes known to be specifically expressed in spermatocytes, such as the testis-specific transcription factor TfIIA-S2 (TFIIA gamma subunit) (Aoyagi and Wassarman, 2000), the bromodomain-containing proteinsmitoshell(mtsh) (Bergner et al., 2010) andtbrd-1(Leser et al., 2012), as well as the eIF4E-3 translation initiation factor (Hernández et al., 2012).
These observations suggested that the expression pattern ofsnf148
tumors includes the anomalous activation of the testis gene expression program. Indeed, of the 483 upregulated genes, 42% (204/483) are expressed in tumors but not in wild type (FPKM<1; Fig. 2B; supplementary material Table S3). Moreover, an examination of the expression data provided by the modENCODE_mRNA-seq_tissues data sets (Brown et al., 2014) reveals that 68% (139/204) of these uniquely expressed tumor genes are expressed in testis (RPKM≥1). Together, these findings indicate that the tumor cells are trending towards a male fate.
The germ cell male sex determining genePhf7is inappropriately expressed in tumors
Since male identity in germ cells is controlled by Phf7 (Yang et al., 2012), we asked whether it is inappropriately expressed in snf148 tumors. Visualization of our RNA-seq data on the
UCSC genome browser (UCSCdm3/FlyBase r5) reveals that the Phf7-RC transcript is expressed in snf148 tumors but not in
wild-type ovaries, whereas Phf7-RA is expressed in both samples (Fig. 3A). Published modENCODE RNA-seq analysis of adult tissues indicates that Phf7-RC is transcribed from an
upstream testis-specific transcription start site (TSS) (Brown et al., 2014).
Using RT-qPCR, we confirmed thatPhf7-RCRNA expression is sexually dimorphic, with higher levels in testis than in ovaries (supplementary material Fig. S1). Moreover, there is a significant increase inPhf7-RClevels in tumors as compared with wild-type ovaries, whereas the level ofPhf7-RC is once more comparable to that of wild-type ovaries in asnf148; P{otu::SxlcDNA}mutant
background (Fig. 3B). Based on these data, we conclude that Phf7-RC misexpression is due to the lack of Sxl protein in the snf148mutant germline.
[image:3.612.61.284.55.232.2]AlthoughPhf7-RChas the same coding potential as Phf7-RA, recent studies have shown that Phf7 protein expression is limited to testis (Yang et al., 2012). There is no available antibody against Fig. 2.snf148tumors ectopically express testis-enriched genes.
(A) Examples of genes overexpressed insnf148mutant ovaries. (B) Of the 483 genes with increased expression, 204 were not expressed in wild-type ovaries (red), and 139 of those transcripts were annotated as testis-enriched by ModENCODE (light blue).
Fig. 3. ThePhf7expression pattern is altered insnf148ovarian tumors. (A) UCSC genome browser view of thePhf7gene locus. The screen shot is reversed so that the start of transcription is on the left, and all tracks are viewed at the same scale. Wild-type RNA-seq reads are in blue andsnf148RNA-seq reads are in red. The reads that are unique tosnf148are highlighted by gray shading. Beneath is the RefSeq gene annotation of the twoPhf7transcripts, Phf7-RCandPhf7-RA.Gray boxes indicate the 5′and 3′UTRs and black boxes represent the open reading frame. Note that both transcripts have the potential to encode identical proteins, but differ in their 5′UTRs. Primers used to detectPhf7-RCby RT-qPCR are indicated by red arrowheads; primers used to determine the total amount ofPhf7transcripts are in blue. (B) RT-qPCR analysis of thePhf7-RCtranscript in gonads isolated from wild-type females (blue),snf148/snf148females (red) andsnf148/snf148; P{otu::SxlcDNA}females (green). Expression, normalized to the total level ofPhf7, is shown as fold change relative tosnf148/snf148. Error bars indicate s.d. of three biological replicates. (C,D) Ovaries from wild-type andsnf148animals carrying an HA-Phf7transgene stained for HA (red),α-Spectrin (green) to visualize spectrosomes (a germ cell-specific organelle), and DNA (white). The prominent HA staining observed in mutant germ cells is absent from wild type. (E,F) Ovaries from wild-type andsnf148animals carrying anHA-Phf7 transgene stained for HA (red), Fas3 (green) to highlight the somatic follicle cell membranes, and DNA (white). Scale bars: 50 µm.
DEVEL
O
Phf7. We therefore used a functional HA-tagged transgene (Yang et al., 2012) to follow Phf7 protein expression. This analysis confirmed that Phf7 protein expression is sex-specifically regulated, with expression detectable in testis but not in ovary (supplementary material Fig. S2). In contrast to wild-type ovaries, HA-Phf7 protein is detectable insnf148ovaries (Fig. 3C-F). In both testis andsnf148
mutant ovaries, expression of the tagged protein is detectable in the cytoplasm and in the nucleus, raising questions about whether the localization of the tagged protein accurately reflects the distribution of the endogenous protein. Nevertheless, our data strongly suggest that Phf7 protein is ectopically expressed insnf148tumors.
Inappropriate expression of Phf7 is responsible for the tumor phenotype
Next, we tested whether snf148tumors are dependent on the
sex-inappropriate expression of Phf7, using RNA interference (RNAi) to knock downPhf7expression. We achieved efficient depletion of Phf7 (>99% efficiency, assayed by RT-qPCR) by driving expression of a short hairpin RNA under the control of the upstream activating sequence (UAS) with the germ cell-specific nanos (nos)-Gal4 driver (van Doren et al., 1998). Remarkably, germline-specific knockdown of Phf7 in the snf148 mutant
background suppressed the tumor phenotype (Fig. 4). Control experiments, in whichPhf7was knocked down in the germline of wild-type females (nos>Phf7RNAi) with comparable efficiency, had
no effect on oogenesis (data not shown), confirming previously published data (Yang et al., 2012).
In contrast to snf148 females, which are sterile with germline
tumors, the majority ofsnf148; nos>Phf7RNAifemales lay eggs and
many, but not all, of their ovaries contain late stage egg chambers, indicating that inhibition ofPhf7expression suppresses the tumor phenotype. To quantitate the efficiency of suppression, we stained ovaries with an antibody against Orb, a marker for oocyte determination. In the wild-type ovary, Orb protein concentrates in
the cytoplasm of the oocyte (Fig. 4A). In the majority ofsnf148
mutant egg chambers, Orb staining remains diffuse, consistent with earlier findings that the tumor is filled with germ cells that are blocked prior to oocyte determination (Chau et al., 2009). Similarly, Orb staining is diffuse in the majority of control ovaries fromsnf148females carrying the driver alone (Fig. 4B). On
occasion, however, we observe Orb protein accumulation in the cytoplasm of a mutant germ cell (2.5%, n=277), even though differentiation is clearly blocked and the ovariole remains tumorous (supplementary material Fig. S3A). In sharp contrast, 42% (n=196) of ovarioles obtained from snf148; nos>Phf7RNAimutant females
contained at least one egg chamber with localized Orb staining (Fig. 4C). Based on these data, we conclude that sex-inappropriate expression of Phf7 is a key cause of the snf148 ovarian tumor
phenotype.
Finding thatsnf148tumor formation is dependent on anomalous
Phf7expression suggests that forcingPhf7expression in wild-type female germ cells might be sufficient for tumorigenesis. Previous studies have reported that forcedPhf7expression can be achieved by driving the expression ofPhf7EY03023, an EP insertion line that
contains aUASsequence inserted within the first intron ofPhf7, with the germline-specific nos-Gal4driver (Yang et al., 2012). However, it was reported that forced expression leads to an empty gonad phenotype due to germ cell death during the larval period (Yang et al., 2012). To bypass this early germ cell lethality, we induced ectopic expression in the adult using a temperature shift protocol in whichtub-gal80ts; nos>Phf7EY03023flies were reared at
[image:4.612.51.415.56.344.2]the restrictive temperature (18°C) and then shifted as adults to the permissive temperature (27°C) for 10 days to allow for significant expression of Phf7. We found that 18% (n=156) of the Phf7-expressing ovarioles showed evidence of tumor formation, based on the number of round spectrosome-like structures present in the germarium (Fig. 4F,G). The spectrosome is a spherical α-Spectrin-containing structure that is normally found only in
Fig. 4. EctopicPhf7expression is responsible for the tumor phenotype. (A-C) Knockdown ofPhf7suppresses the snf148tumor phenotype. Ovarioles from wild-type,snf148andsnf148; nos>Phf7RNAi females stained for Orb (green) and DNA (red). In wild type the Orb protein accumulates around the presumptive oocyte. Insnf148tumors Orb protein does not accumulate in any one location. In snf148; nos>Phf7RNAiat least one site of localized Orb accumulation was observed in 42% (n=196) of all ovarioles scored. Arrows mark the anterior end of the germarium. (D-G) ForcedPhf7expression in the female germline elicits tumor formation. Germaria from wild-type, snf148/snf148andnos>Phf7EYfemales stained forα-Spectrin (green) to visualize spectrosomes (sp) and fusomes (fu), and DNA (red). Note thatα-Spectrin also stains somatic cell membranes. (G) Quantification of the number of round spectrosome-like structures per germarium in wild type (WT),nos>Phf7EY andsnf148/snf148(snf). Scale bars: 50 µm.
DEVEL
O
GSCs and CB cells (∼5 per ovariole). During the subsequent divisions, the round spectrosome elongates and branches out to form a fusome that connects the synchronously dividing germ cells (Fig. 4D). Tumors innos>Phf7EY03023 ovaries are composed of
cells that contain round spectrosomes or abnormal fusome-like structures, resembling snf148 tumors (Fig. 4E,F). The low
penetrance of the tumor phenotype could be due to the inefficient production of Phf7 protein or might indicate that other key downstream targets in addition toPhf7 are needed to foster tumorigenesis.
Phf7 drives tumor formation through aberrant Jak/Stat activation
Interestingly, our RNA-seq data reveal that the levels of two Jak/ Stat pathway activating cytokines, unpaired 2 (upd2) and unpaired 3 (upd3) (Agaisse et al., 2003; Gilbert et al., 2005; Hombría et al., 2005), and of a downstream effector of the Jak/Stat signaling pathway,chronologically inappropriate morphogenesis (chinmo) (Flaherty et al., 2010), are significantly increased in snf148ovarian tumors (supplementary material Table S2). Jak/Stat
activity in adult germ cells is sexually dimorphic, being required in male but not female cells (Decotto and Spradling, 2005; Kiger et al., 2001; Leatherman and DiNardo, 2008, 2010; Tulina and Matunis, 2001). Consistent with these earlier studies, we find that germline-specific knockdown ofStat92Eorhopscotch(hop), the sole Jak kinase in the Drosophila genome, does not disrupt oogenesis (supplementary material Fig. S3B,C). Together, these
data suggest the possibility that Jak/Stat is aberrantly activated in thesnf148mutant germline.
Using RT-qPCR, we confirmed that there is a significant increase inupd2,upd3andchinmolevels in tumors as compared with wild-type ovaries (Fig. 5A). The levels of these three transcripts are once more comparable to those of wild type in asnf148; P{otu::SxlcDNA}
mutant background, confirming that the effects that we are observing are due to the absence of Sxl protein in the mutant germline. Notably, we also observed a genetic dependence on inappropriatePhf7expression, as the levels of these three transcripts were restored to near wild-type levels in a snf148; nos>Phf7RNAi
mutant background. These data suggest a genetic pathway in which the lack of Sxl protein leads to anomalousPhf7expression, which in turn results in increasedupd2and upd3production, inappropriate Jak/Stat signaling, andchinmoexpression (Fig. 5B).
In agreement with these findings, Jak/Stat activity, as assayed by staining with an antibody against Stat92E, is detectable insnf148
tumors (Fig. 5C,D). Stat92E encodes the only Stat transcription factor in theDrosophilagenome, and activation of the pathway is accompanied by increased levels or stability of Stat92E protein (Chen et al., 2003). In wild-type ovaries, Stat92E protein is highly expressed in the somatic cells of the germarium, but is not detectable in the germline of wild-type ovaries (Fig. 5C). By contrast, Stat92E is detectable throughout the snf148 tumor
(Fig. 5D).
These observations, together with studies that have connected both aberrant Jak/Stat signaling and ectopicchinmoexpression to tumor formation in other contexts (Flaherty et al., 2010), suggest that anomalous activation of this pathway might play a role in the formation of snf148 germline tumors. Consistent with this
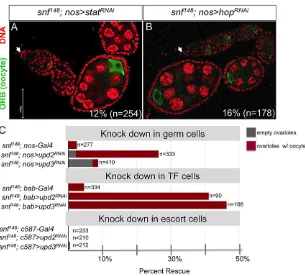
hypothesis, we find suppression of the tumor phenotype upon inactivation of key components of the Jak/Stat pathway (Fig. 6). When Stat92Ewas knocked down in a tumor background, 12% (n=254) of thesnf148; nos>Stat92ERNAimutant ovarioles contained
at least one egg chamber with localized Orb staining (Fig. 6A). Similarly, whenhopwas knocked down, 16% (n=178) of thesnf148;
nos>hopRNAimutant ovarioles contained at least one egg chamber
(Fig. 6B).
Rescue of sterility phenotypes reveals a role for both autocrine and paracrine signaling
In wild-type ovaries, the Upd2 and Upd3 cytokines are thought to be produced by the somatic terminal filament (TF) cells located at the tip of the germarium (López-Onieva et al., 2008). It is possible, therefore, that thesnf148mutant germ cells have acquired a novel
ability to receive signals from the surrounding somatic cells that normally produce these signals. On the other hand, the increased level ofupd2andupd3observed in tumors is dependent on aberrant Phf7expression in germ cells (Fig. 5A), raising the possibility that snf148germ cells produce these cytokines and activate Jak/Stat in an
autocrine manner. These two possibilities are not mutually exclusive, as the tumor phenotype might reflect a novel response to paracrine signals as well as an additional production and response to autocrine signals.
These scenarios may be distinguished by determining the cellular source of the cytokines important forsnf148tumor formation. We
therefore performed tissue-specific knockdown ofupd2 and upd3 in thesnf148mutant germline (with thenos-Gal4driver), TF cells [with
[image:5.612.57.283.380.638.2]thebab1-Gal4driver (Cabrera et al., 2002)] and escort cells [with the c587-Gal4driver (Song and Xie, 2003)]. Interestingly, we observed that knockdown in the germline and in TF cells, but not in escort cells, led to suppression of the tumor phenotype (Fig. 6C). In germ cells, Fig. 5. EctopicPhf7expression leads to aberrant Jak/Stat activity.(A)
RT-qPCR analysis ofchinmo,upd2andupd3transcripts in gonads isolated from wild-type (blue),snf148/snf148(red),snf148/snf148; P{otu::SxlcDNA}(green) and snf148/snf148; nos>Phf7RNAi( purple) females. Expression, normalized torp49, is shown as fold change relative to wild type. Error bars indicate s.d. of three biological replicates. (B) The genetic pathway leading to tumorigenesis. (C,D) Ovarioles from wild type andsnf148tumors stained for Stat92E (green) and DNA (red). Whereas Stat staining is limited to somatic cells in wild-type ovaries, staining is observed throughout thesnf148/snf148mutant ovariole.
Arrows mark the anterior end of the germarium. Scale bars: 50 µm.
DEVEL
O
knockdown ofupd2effectively suppressed the tumor phenotype, with 24% (n=333) of ovarioles obtained from snf148; nos>upd2RNAi
females containing at least one egg chamber with localized Orb staining. Knockdown of upd3 in the germline was significantly weaker, with only 2% (n=410) of snf148; nos>upd3RNAi mutant
ovarioles showing evidence of Orb protein localization. In TF cells, bothupd2-RNAi andupd3-RNAi effectively suppressed the tumor phenotype; 41% (n=90) ofsnf148; bab1>upd2RNAiovarioles and 46%
(n=166) ofsnf148; bab1>upd3RNAiovarioles showed evidence of Orb
localization. By contrast, knockdown in escort cells had no effect on the tumor phenotype; no Orb localization was observed in snf148;
c587>upd2RNAi ovarioles (n=210) or in snf148; c587>upd3RNAi
ovarioles (n=212). Overall, these studies demonstrate the importance of both autocrine and paracrine signaling in establishing and/or maintaining the tumor phenotype.
snfandbamovarian tumors express a common subset of testis transcripts
bam mutant ovaries, like snf148 ovaries, are highly enriched for
proliferating germ cells that fail to differentiate (McKearin and Ohlstein, 1995; McKearin and Spradling, 1990). We, and others, have previously noted the anomalous expression of several testis transcripts inbam ovarian tumors (Chau et al., 2009; Kai et al., 2005; Wei et al., 1994). To determine whether there is a global depression of testis genes inbamovarian tumors, we examined the previously published RNA-seq analysis in which the transcriptomes ofbamtumorous and wild-type ovaries from 0- to 24-h-old females were compared (Gan et al., 2010). From this analysis we identified 2661 genes that are upregulated at least twofold in bam tumors. Of these, 48% (1278/2661) are expressed in tumors but not in wild type (RPKM<1; Fig. 7A). Furthermore, 54% (692/1278) of these uniquely expressed tumor genes are expressed in testis (RPKM≥1). Our finding that the bam mutant gene expression program is masculinized is further supported by RT-qPCR analysis showing aberrant expression of the male-specific transcript Phf7-RC in bamΔ86ovarian tumors (Fig. 7B).
Together, these expression studies suggest that the failure to maintain sexual identity, as indicated by the global depression of testis genes, is a common feature of both snf and bam tumors. Interestingly, although there is a strong overlap between the uniquely expressed genes in snf and bam tumors, the overlap is not complete (Fig. 7C; supplementary material Table S4). We do not yet know whether these differences are important, but our finding that the bamΔ86 tumor phenotype is not suppressed by
germline-specific knockdown of Phf7 (supplementary material Fig. S4) suggests that there are fundamental biological differences betweenbamandsnftumors.
DISCUSSION
When housed in a normal ovary, female germ cells lackingSxlare unable to differentiate and instead form tumors. AlthoughSxlhas a pivotal role in the cell fate switch from a self-renewing GSC to a differentiation-competent CB, tumor formation is not simply the result of the failure of this switch (Chau et al., 2009, 2012). By taking advantage of asnfmutant allele that specifically eliminates Sxl protein expression in the germline, we have shown that it is the loss of female sexual identity and the acquisition of male characteristics that leads to tumor formation. Our finding thatSxl jointly controls exit from the stem cell state and the maintenance of sexual identity provides the basis for a model (Fig. 8A) in which Sxl safeguards the previously made sexual decision as the stem cell reprograms itself towards a differentiated fate.
Taking a genome-wide approach, we establish thatSxlis a potent repressor of testis genes. This global analysis supports and extends earlier studies that identified several inappropriately expressed testis genes in ovarian tumors (Chau et al., 2009; Wei et al., 1994). Although these mutant germ cells are not sex reversed, as they continue to express ovary-specific genes, the degree to which they are in fact masculinized is illustrated by our key observation that Phf7, a gene known to be required for male germline sexual identity (Yang et al., 2012), is expressed in the absence of Sxl. This suggests thatSxlmaintains female identity by silencingPhf7. Sex- and tissue-Fig. 6. Knockdown of Jak/Stat pathway components suppresses thesnf148tumor phenotype.An ovariole was scored as rescued when at least one site of localized Orb accumulation was observed. (A)snf148/snf148;
nos>Stat92ERNAiand (B)snf148/snf148;
nos>hopRNAiovarioles stained for Orb (green) and DNA (red). Arrows mark the anterior end of the germarium. Scale bar: 50 µm. (C) Frequency of rescuedsnf148/snf148 ovarioles upon knocking downupd2orupd3in the germline, somatic TF cells or somatic escort cells.
DEVEL
O
[image:6.612.52.357.55.331.2]specific regulation ofPhf7appears to be achieved by a mechanism that involves alternative TSSs and translational regulation. Although Sxl is known to inhibit translation in other contexts (Salz and Erickson, 2010), the lack of putative Sxl binding sites (multiple polyuridine runs of seven or more nucleotides) inPhf7 transcripts argues that Sxl is likely to regulate Phf7 expression indirectly.
Interestingly,Phf7encodes a methyl-histone-binding protein that preferentially recognizes lysine 4-dimethylated histone H3 (H3K4me2) (Yang et al., 2012). We therefore propose that Sxl maintains germline sexual identity by impacting epigenetic regulation. In this model, the global depression of testis genes observed in the absence of Sxl would be due to unscheduled epigenetic changes. In this regard, we find it intriguing that disrupting the H3K4 methylation pathway in female germ cells by knocking downSet1, the H3K4 di- and trimethyltransferase, also leads to germ cell tumors (Yan et al., 2014). A future challenge will be to understand the molecular connection between Sxl, which is an RNA-binding protein, and sexually dimorphic epigenetic programming.
Through our pathway analysis (Fig. 8B) we establish that tumor formation is caused by the activation of a sex-inappropriate gene expression network that is unleashed by the loss of Sxl protein. Phf7 is a key effector of this pathway, as we have shown that it is both necessary and sufficient for tumor formation. Our findings indicate that Phf7 drives tumor formation through inappropriate activation of the Jak/Stat pathway. It is intriguing that activation of Jak/Stat signaling leads to tumor formation because in male GSCs activity is only needed for adhesion to the somatic hub cells (Leatherman and DiNardo, 2010). It is possible, therefore, that the tumors express a Stat-regulated pathway that is unrelated to the pathway expressed in the male germline. Our data also suggest that Phf7 drives tumor formation through a mechanism that leads to increased levels of two of the Upd cytokine family members known to activate the Jak/Stat pathway. How inappropriate Phf7 expression leads to upregulation of Upd cytokines remains an open question. One possibility,
suggested by our transcriptional profiling experiments, is that Phf7 expression leads to the derepression of genes that include, but is not limited to, testis-specific genes. Irrespective of the mechanism, the finding that depletion of Upd2, and to a lesser extent Upd3, in germ cells reverts the tumor phenotype indicates a requirement for autocrine signaling. Furthermore, aberrant autocrine signaling is consistent with data emerging from numerous studies connecting hyperactive Jak/Stat signaling to otherDrosophila tumor models and human cancers (Amoyel et al., 2014).
A remarkable and unexpected aspect of our analysis is that tumor formation also depends on Upd2 and Upd3 produced by somatic cells in the neighboring microenvironment. The somatic microenvironment at the tip of the germarium consists of three cooperating cell types: TF cells, cap cells and escort cells. In wild type, secretion of the Upd family of cytokines from TF cells activates Jak/Stat signaling in cap and escort cells, but not in the adjacent germ cells (Decotto and Spradling, 2005; López-Onieva et al., 2008; Wang et al., 2008). The situation is different in males, where somatic cytokine production activates Jak/Stat in neighboring somatic and germ cells (Kiger et al., 2001; Leatherman and DiNardo, 2008, 2010; Tulina and Matunis, 2001). Because germ cells lacking Sxl protein are masculinized, we hypothesize that mutant germ cells interpret the information provided by the microenvironment as if they were male, leading to sex-inappropriate Jak/Stat activation. Although we do not understand how these mutant cells acquired the ability to receive activating signals from the surrounding somatic cells, these results highlight the importance of interactions between mutant cells and the surrounding normal cells that make up the tumor microenvironment in driving tumor formation.
[image:7.612.316.563.57.229.2]Masculinization of the gene expression program is also observed in bam ovarian tumors, suggesting that maintenance of sexual identity requires both Sxl and bam. A Sxl-bam partnership is Fig. 7.bamandsnf148tumors ectopically express an overlapping set of
[image:7.612.51.301.58.231.2]genes.(A) Analysis of previously published RNA-seq data (Gan et al., 2010) reveals that, of the 2661 genes with increased expression inbamtumors, 1278 are not expressed in wild-type ovaries (red), and 692 of these uniquely expressed genes are expressed in testis (blue). (B)Phf7-RCexpression as assayed by RT-qPCR in gonads isolated from wild-type,snf148andbamΔ86 mutant females.Phf7-RCexpression, normalized to the total level ofPhf7, is shown as fold change relative tosnf148animals. Error bars indicate s.d. of three biological replicates. (C) The overlap in genes uniquely expressed bybamand snftumors.
Fig. 8. Model for the integration of maintenance of female sexual identity with the self-renewal/differentiation switch in adult GSCs.(A) The genetic pathways employed by aSxl-bampartnership to control the GSC/CB switch (left) while maintaining female sexual identity (right). (B) The genetic pathway leading to tumor formation in germ cells lacking Sxl protein. In the absence of Sxl protein, the presumptive GSCs located at the tip of the ovariole respond appropriately to signals emanating from the somatic niche (black arrow). Upon division they fail to exit the stem cell stage, continue to proliferate and form a tumor. The tumor cells co-express GSC markers (including Nanos protein), the differentiation marker Bam and the male germline sexual identity gene Phf7. Ectopic Phf7 expression causes the tumor cells to interpret the signals emanating from the somatic niche (red arrow) as if they were male germ cells, leading to the sex-inappropriate Jak/Stat activation.
DEVEL
O
strongly supported by genetic epistasis experiments, which show that the two proteins jointly repress expression of the stem cell maintenance factornos(Chau et al., 2009, 2012; Li et al., 2013). Based on these observations we propose that Sxl and bam co-regulate at least two independent pathways, one of which leads to germ cell differentiation whereas the other maintains female identity (Fig. 8A).
Altogether, our studies establish that sexual identity must be maintained in an adult stem cell lineage, or risk tumor formation. Although the gene regulatory networks that specify sex vary between species, the need for a germ cell to remember its sexual identity is likely to extend beyondDrosophila. In humans, germ cell tumors occur most frequently in individuals with intersex disorders (Hersmus et al., 2012; Kraggerud et al., 2013; Pleskacova et al., 2010). There is also increasing evidence of a connection between testicular germ cell tumors and the disruption of sex-specific processes (Kanetsky et al., 2011; Koster et al., 2014; Matson et al., 2011; Turnbull et al., 2010). Non-reproductive cells and tissues also exhibit sexually dimorphic gene expression programs, suggesting that the failure to maintain this dimorphism could have a role in cancer and other diseases (Bellott et al., 2014; Nakada et al., 2014; Ronen and Benvenisty, 2014). It will be important, therefore, to determine whether there is a mechanism to maintain sexual identity in tissues other than gonads and whether the failure in this process leads to disease.
MATERIALS AND METHODS
Fly stocks and culture conditions
Flies were reared on standard media at 25°C unless otherwise indicated. Mutant alleles, transgenic, enhancer trap and Gal4 drivers used in this study include snf148 [Bloomington Drosophila Stock Center (BDSC) #7398 (Nagengast et al., 2003)], P{HA-Phf7}(Yang et al., 2012),Phf7EY03023 (BDSC #15894), nanos-Gal4 [BDSC #4937 (van Doren et al., 1998)],
c587-Gal4(Song and Xie, 2003),bab1-Gal4[BDSC #6802 (Cabrera et al., 2002)], tub-Gal80ts[BDSC #7019 (McGuire et al., 2003)] and bamΔ86 [BDSC #5427 (McKearin and Ohlstein, 1995)]. Additional information about theDrosophilastrains used in this study is available from FlyBase (http://flybase.org).
Knockdown studies with UAS-RNAi were carried out at 27°C with the following lines from theDrosophilaTransgenic RNAi Project (TRiP; Ni et al., 2011): Phf7-P{TRiP.GL00455} (BDSC #35807), stat-P{TRiP. GL00437} (BDSC #35600), hop-P{TRiP.GL00305} (BDSC #35386),
upd2-P{TRiP.HMS00948}(BDSC #33988) andupd3-P{TRiP.HMS00646}
(BDSC #32859). The HMS RNAi lines are constructed in the VALIUM20 vector (upd2,upd3) and are expressed in both somatic and germline cells. The GL RNAi lines are constructed in the VALIUM22 vector [Phf7, stat
(Stat92E),hop] and show strong expression in the female germline and weak expression in the soma. The Gal4/Gal80tssystem was used to forcePhf7 expression in adult germ cells. Animals were raised at 18°C to maintain the Gal80-dependent repression of Gal4 until adulthood. To induce Gal4 activity, adult females were transferred to 27°C for 10 days before immunostaining.
Gene expression analysis by RNA-seq
Total RNA was isolated using the RNeasy Kit (Qiagen), including the on-column DNase I digestion. RNA purity was assessed with the Nano 6000 RNA chip on an Agilent 2100 bioanalyzer, and quantification was performed using a RiboGreen fluorescence assay (Invitrogen). RNA-seq libraries were constructed using the Illumina Truseq Total Stranded RNA Kit, with 800 ng of input total RNA and 14 cycles of PCR amplification. 100 bp paired-end mRNA sequencing was performed on biological duplicates from each genotype on an Illumina HiSeq 2500 by the CWRU Genomics Sequencing Core. After quality assessment, the sequenced reads were aligned to the
D. melanogastergenome (UCSCdm3/FlyBase r5.23) using TopHat (2.0.9) (Trapnell et al., 2009) with the RefSeq annotated transcripts as a guide. The differential expression analysis was performed using CuffDiff (2.0.2) (Trapnell et al., 2010). Genes with P≤0.05 after adjusting to a false
discovery rate (FDR) with the Benjamini and Hochberg method and a twofold or higher change were considered significant. GO term enrichment analysis was performed separately on the upregulated and downregulated gene sets using High-Throughput GoMiner (Zeeberg et al., 2005).
Gene expression analysis by RT-qPCR
Total RNA was isolated using TRIzol (Invitrogen) and treated with DNase (Promega). RNA yield and quality were assessed with a NanoDrop spectrophotometer (NanoDrop Technology), followed by reverse transcription using random hexamers with the SuperScript First-Strand Synthesis System Kit (Invitrogen). Transcript levels were measured with Quanta PerfeCTa SYBR Fastmix (VWR) in an MJ Research PTC-200 gradient cycler. Initial activation was performed at 95°C for 30 s, followed by 39 cycles of: 95°C for 5 s, 60°C for 15 s, 70°C for 10 s. The melting curve was generated ranging from 50°C to 95°C with an increment of 0.5°C each 5 s. Primers used for RT-qPCR are listed in supplementary material Table S5. Measurements were performed on biological triplicates, with technical duplicates of each biological sample.chinmo,
upd2andupd3RNA levels were normalized torp49(RpL32–FlyBase).
Phf7-RClevels were normalized to the total level ofPhf7using a primer set that detects bothPhf7-RAandPhf7-RC. The relative transcript levels were calculated using the 2−ΔΔCTmethod (Livak and Schmittgen, 2001).
Immunofluorescence and image analysis
Ovaries and testes were fixed and stained by standard methods. The following primary antibodies were used: mouse Sxl-M18 [1:100, Developmental Studies Hybridoma Bank (DSHB)], mouse Orb-4H8 (1:50, DSHB), mouse Orb-6H4 (1:50, DSHB),α-Spectrin (1:100, DSHB), rat HA high affinity (1:500, Roche, 11867423001), rat Vasa (1:100, DSHB), rabbit Stat92E [1:1000 (Chen et al., 2002)] and mouse Fas3-7G10 (1:50, DSHB). Secondary antibodies conjugated to Alexa Fluor 555 (Life Technologies) or FITC (Jackson ImmunoResearch Labs) were used at 1:200. Images were acquired on a Leica TCS SP8 confocal microscope and assembled using Photoshop (Adobe) and PowerPoint (Microsoft). A full description of these data can be found at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE65932
Acknowledgements
We thank M. Van Doren, J. McDonald, the TriP at Harvard Medical School, the BloomingtonDrosophilaStock Center and the Iowa Developmental Studies Hybridoma Bank for fly stocks and antibodies; the CWRU GTSC sequencing core for RNA-seq library generation and sequencing; A. Miron and S. Bai for help with the bioinformatic analysis; and R. Conlon, I. Greenwald, J. McDonald, M. Van Doren and M. Wawersik for helpful discussions.
Competing interests
The authors declare no competing or financial interests.
Author contributions
L.S.-K., A.E.S. and H.K.S. conceived, designed and performed the experiments. H.K.S. wrote the paper.
Funding
This work was supported by the National Institutes of Health (NIH) [R01GM102141] and a CTSC Core Utilization Grant [funded under NIH UL1TR000439]. Imaging was performed using equipment purchased through NIH S10OD016164. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at
http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.116590/-/DC1
References
Agaisse, H., Petersen, U.-M., Boutros, M., Mathey-Prevot, B. and Perrimon, N.
(2003). Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury.Dev. Cell5, 441-450.
Amoyel, M., Anderson, A. M. and Bach, E. A. (2014). JAK/STAT pathway dysregulation in tumors: a Drosophila perspective.Semin. Cell Dev. Biol.28, 96-103.
Aoyagi, N. and Wassarman, D. A. (2000). Genes encoding Drosophila melanogaster RNA polymerase II general transcription factors: diversity in TFIIA and TFIID components contributes to gene-specific transcriptional regulation.
J. Cell Biol.150, F45-F50.
DEVEL
O
Bellott, D. W., Hughes, J. F., Skaletsky, H., Brown, L. G., Pyntikova, T., Cho, T.-J., Koutseva, N., Zaghlul, S., Graves, T., Rock, S. et al.(2014). Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators.Nature508, 494-499.
Bergner, L. M., Hickman, F. E., Wood, K. H., Wakeman, C. M., Stone, H. H., Campbell, T. J., Lightcap, S. B., Favors, S. M., Aldridge, A. C. and Hales, K. G.
(2010). A novel predicted bromodomain-related protein affects coordination between meiosis and spermiogenesis in Drosophila and is required for male meiotic cytokinesis.DNA Cell Biol.29, 487-498.
Brown, J. B., Boley, N., Eisman, R., May, G. E., Stoiber, M. H., Duff, M. O., Booth, B. W., Wen, J., Park, S., Suzuki, A. M. et al.(2014). Diversity and dynamics of the Drosophila transcriptome.Nature512, 393-399.
Cabrera, G. R., Godt, D., Fang, P.-Y., Couderc, J.-L. and Laski, F. A.(2002). Expression pattern of Gal4 enhancer trap insertions into the bric àbrac locus generated by P element replacement.Genesis34, 62-65.
Casper, A. L. and van Doren, M.(2009). The establishment of sexual identity in the Drosophila germline.Development136, 3821-3830.
Chau, J., Kulnane, L. S. and Salz, H. K.(2009). Sex-lethal facilitates the transition from germline stem cell to committed daughter cell in the Drosophila ovary.
Genetics182, 121-132.
Chau, J., Kulnane, L. S. and Salz, H. K.(2012). Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis.Proc. Natl. Acad. Sci. USA109, 9465-9470.
Chen, D. and McKearin, D.(2003a). Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells.Curr. Biol.13, 1786-1791.
Chen, D. and McKearin, D. M.(2003b). A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell.
Development130, 1159-1170.
Chen, D. and McKearin, D.(2005). Gene circuitry controlling a stem cell niche.
Curr. Biol.15, 179-184.
Chen, H.-W., Chen, X., Oh, S.-W., Marinissen, M. J., Gutkind, J. S. and Hou, S. X.
(2002). mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family.Genes Dev.16, 388-398.
Chen, X., Oh, S.-W., Zheng, Z., Chen, H.-W., Shin, H.-H. and Hou, S. X.(2003). Cyclin D-Cdk4 and cyclin E-Cdk2 regulate the Jak/STAT signal transduction pathway in Drosophila.Dev. Cell4, 179-190.
Decotto, E. and Spradling, A. C.(2005). The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals.Dev. Cell9, 501-510.
Flaherty, M. S., Salis, P., Evans, C. J., Ekas, L. A., Marouf, A., Zavadil, J., Banerjee, U. and Bach, E. A.(2010). chinmo is a functional effector of the JAK/ STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila.Dev. Cell18, 556-568.
Forbes, A. A. and Lehmann, R. R.(1998). Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells.Development 125, 679-690.
Friedmann-Morvinski, D. and Verma, I. M. (2014). Dedifferentiation and reprogramming: origins of cancer stem cells.EMBO Rep.15, 244-253.
Gan, Q., Chepelev, I., Wei, G., Tarayrah, L., Cui, K., Zhao, K. and Chen, X.(2010). Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq.Cell Res.20, 763–783.
Gilbert, M. M., Weaver, B. K., Gergen, J. P. and Reich, N. C.(2005). A novel functional activator of the Drosophila JAK/STAT pathway, unpaired2, is revealed by an in vivo reporter of pathway activation.Mech. Dev.122, 939-948.
Gilboa, L. and Lehmann, R. (2004). Repression of primordial germ cell differentiation parallels germ line stem cell maintenance.Curr. Biol.14, 981-986.
Harris, R. E., Pargett, M., Sutcliffe, C., Umulis, D. and Ashe, H. L.(2011). Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling.Dev. Cell20, 72-83.
Hernández, G., Han, H., Gandin, V., Fabian, L., Ferreira, T., Zuberek, J., Sonenberg, N., Brill, J. A. and Lasko, P.(2012). Eukaryotic initiation factor 4E-3 is essential for meiotic chromosome segregation, cytokinesis and male fertility in Drosophila.Development139, 3211-3220.
Hersmus, R., Stoop, H., White, S. J., Drop, S. L. S., Oosterhuis, J. W., Incrocci, L., Wolffenbuttel, K. P. and Looijenga, L. H. J.(2012). Delayed recognition of disorders of sex development (DSD): a missed opportunity for early diagnosis of malignant germ cell tumors.Int. J. Endocrinol.2012, 671209.
Hombrı́a, J. C.-G., Brown, S., Häder, S. and Zeidler, M. P. (2005). Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288, 420-433.
Horabin, J. I., Bopp, D., Waterbury, J. and Schedl, P.(1995). Selection and maintenance of sexual identity in the Drosophila germline. Genetics 141, 1521-1535.
Hudson, A. M. and Cooley, L.(2014). Methods for studying oogenesis.Methods 68, 207-217.
Kai, T., Williams, D. and Spradling, A. C.(2005). The expression profile of purified Drosophila germline stem cells.Dev. Biol.283, 486-502.
Kanetsky, P. A., Mitra, N., Vardhanabhuti, S., Vaughn, D. J., Li, M., Ciosek, S. L., Letrero, R., D’Andrea, K., Vaddi, M., Doody, D. R. et al.(2011). A second
independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility.Hum. Mol. Genet.20, 3109-3117.
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. and Fuller, M. T.(2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue.Science294, 2542-2545.
Koster, R., Mitra, N., D’Andrea, K., Vardhanabhuti, S., Chung, C. C., Wang, Z., Erickson, R. L., Vaughn, D. J., Litchfield, K., Rahman, N. et al.(2014). Pathway-based analysis of GWAs data identifies association of sex determination genes with susceptibility to testicular germ cell tumors.Hum. Mol. Genet.23, 6061-6068.
Kraggerud, S. M., Hoei-Hansen, C. E., Alagaratnam, S., Skotheim, R. I., Abeler, V. M., Rajpert-De Meyts, E. and Lothe, R. A.(2013). Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr. Rev. 34, 339-376.
Leatherman, J. L. and DiNardo, S.(2008). Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal.Cell Stem Cell3, 44-54.
Leatherman, J. L. and DiNardo, S.(2010). Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes.Nat. Cell Biol. 12, 806-811.
Leser, K., Awe, S., Barckmann, B., Renkawitz-Pohl, R. and Rathke, C.(2012). The bromodomain-containing protein tBRD-1 is specifically expressed in spermatocytes and is essential for male fertility.Biol. Open1, 597-606.
Li, Y., Minor, N. T., Park, J. K., Mckearin, D. M. and Maines, J. Z.(2009). Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance.Proc. Natl. Acad. Sci. USA106, 9304-9309.
Li, Y., Zhang, Q., Carreira-Rosario, A., Maines, J. Z., McKearin, D. M. and Buszczak, M.(2013). Mei-p26 cooperates with bam, bgcn and sxl to promote early germline development in the Drosophila ovary.PLoS ONE8, e58301.
Livak, K. J. and Schmittgen, T. D.(2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.Methods 25, 402-408.
López-Onieva, L., Fernández-Miñán, A. and González-Reyes, A.(2008). Jak/ Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary.Development135, 533-540.
Matson, C. K., Murphy, M. W., Sarver, A. L., Griswold, M. D., Bardwell, V. J. and Zarkower, D.(2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis.Nature476, 101-104.
McGuire, S. E., Le, P. T., Osborn, A. J., Matsumoto, K. and Davis, R. L.(2003). Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765-1768.
McKearin, D. and Ohlstein, B.(1995). A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells.Development 121, 2937-2947.
McKearin, D. M. and Spradling, A. C.(1990). bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 4, 2242-2251.
Nagengast, A. A., Stitzinger, S. M., Tseng, C.-H., Mount, S. M. and Salz, H. K.
(2003). Sex-lethal splicing autoregulation in vivo: interactions between SEX-LETHAL, the U1 snRNP and U2AF underlie male exon skipping.Development 130, 463-471.
Nakada, D., Oguro, H., Levi, B. P., Ryan, N., Kitano, A., Saitoh, Y., Takeichi, M., Wendt, G. R. and Morrison, S. J.(2014). Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy.Nature505, 555-558.
Ni, J.-Q., Zhou, R., Czech, B., Liu, L.-P., Holderbaum, L., Yang-Zhou, D., Shim, H.-S., Tao, R., Handler, D., Karpowicz, P. et al.(2011). A genome-scale shRNA resource for transgenic RNAi in Drosophila.Nat. Methods8, 405-407.
Pleskacova, J., Hersmus, R., Oosterhuis, J. W., Setyawati, B. A., Faradz, S. M., Cools, M., Wolffenbuttel, K. P., Lebl, J., Drop, S. L. and Looijenga, L. H.
(2010). Tumor risk in disorders of sex development.Sex. Dev.4, 259-269.
Ronen, D. and Benvenisty, N.(2014). Sex-dependent gene expression in human pluripotent stem cells.Cell Rep.8, 923-932.
Salz, H. K. (2011). Sex determination in insects: a binary decision based on alternative splicing.Curr. Opin. Genet. Dev.21, 395-400.
Salz, H. K. and Erickson, J. W.(2010). Sex determination in Drosophila: the view from the top.Fly (Austin)4, 60-70.
Song, X. and Xie, T.(2003). Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila.Development130, 3259-3268.
Song, X., Wong, M. D., Kawase, E., Xi, R., Ding, B. C., McCarthy, J. J. and Xie, T.
(2004). Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary.Development131, 1353-1364.
Spradling, A., Fuller, M. T., Braun, R. E. and Yoshida, S.(2011). Germline stem cells.Cold Spring Harb. Perspect. Biol.3, a002642–a002642.
Staab, S., Heller, A. and Steinmann-Zwicky, M.(1996). Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development 122,
4065-4071.
DEVEL
O
Steinmann-Zwicky, M.(1994). Sex determination of the Drosophila germ line: tra and dsx control somatic inductive signals.Development120, 707-716.
Tipping, M. and Perrimon, N.(2014). Drosophila as a model for context-dependent tumorigenesis.J. Cell. Physiol.229, 27-33.
Trapnell, C., Pachter, L. and Salzberg, S. L.(2009). TopHat: discovering splice junctions with RNA-Seq.J. Gerontol.25, 1105-1111.
Trapnell, C., Williams, B. A., Pertea, G., Mortazavi, A., Kwan, G., van Baren, M. J., Salzberg, S. L., Wold, B. J. and Pachter, L.(2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation.Nat. Biotechnol.28, 511-515.
Tulina, N. and Matunis, E.(2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling.Science294, 2546-2549.
Turnbull, C., Rapley, E. A., Seal, S., Pernet, D., Renwick, A., Hughes, D., Ricketts, M., Linger, R., Nsengimana, J., Deloukas, P. et al.(2010). Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer.
Nat. Genet.42, 604-607.
van Doren, M., Williamson, A. L. and Lehmann, R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells.Curr. Biol. 8, 243-246.
Wang, Z. and Lin, H.(2004). Nanos maintains germline stem cell self-renewal by preventing differentiation.Science303, 2016-2019.
Wang, L., Li, Z. and Cai, Y.(2008). The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche.J. Cell Biol.180, 721-728.
Wawersik, M., Milutinovich, A., Casper, A. L., Matunis, E., Williams, B. and van Doren, M.(2005). Somatic control of germline sexual development is mediated by the JAK/STAT pathway.Nature436, 563-567.
Wei, G., Oliver, B., Pauli, D. and Mahowald, A. P.(1994). Evidence for sex transformation of germline cells in ovarian tumor mutants of drosophila.Dev. Biol. 161, 318-320.
White-Cooper, H. (2010). Molecular mechanisms of gene regulation during Drosophila spermatogenesis.Reproduction139, 11-21.
Yan, D., Neumüller, R. A., Buckner, M., Ayers, K., Li, H., Hu, Y., Yang-Zhou, D., Pan, L., Wang, X., Kelley, C. et al.(2014). A regulatory network of Drosophila germline stem cell self-renewal.Dev. Cell28, 459-473.
Yang, S. Y., Baxter, E. M. and van Doren, M.(2012). Phf7 controls male sex determination in the Drosophila germline.Dev. Cell22, 1041-1051.
Zeeberg, B. R., Qin, H., Narasimhan, S., Sunshine, M., Cao, H., Kane, D. W., Reimers, M., Stephens, R. M., Bryant, D., Burt, S. K. et al. (2005). High-throughput GoMiner, an“industrial-strength” integrative gene ontology tool for interpretation of multiple-microarray experiments, with application to studies of common variable immune deficiency (CVID).BMC Bioinformatics 6, 168.