Copyright 8 1995 by the Genetics Society of America
Comparison of Germline
Mosaics of
Genes
in
the
Polycomb
Group of
Drosophila melanogaster
Martha C.
Soto,*
Tze-Bin
Chou
and
Welcome Bender
*
*
Haruard Medical School, Department of Biological Chemistry and Molecular Pharmacology, Boston, Massachusetts 021 15 andtHaruard Medical School, Department of Genetics, Boston, Massachusetts 02115
Manuscript received November 19, 1994 Accepted for publication February 14, 1995
ABSTRACT
The genes of the Polycomb group ( PcG) repress the genes of the bithorax and Antennapedia com- plexes, among others. To observe a null phenotype for a PcG gene, one must remove its maternal as well as zygotic contribution to the embryo. Five members of the PcG group are compared here: Enhancer
of Polycomb [ E ( P c ) ] , Additional sex combs (Asx), Posterior sex combs (Psc), Supp-essor of m t e 2 [ Su ( 2 ) 21 and
Polycomblike (Pcl). The yeast recombinase (FLF’) system was used to induce mitotic recombination in
the maternal germline. Mutant embryos were analyzed by staining with antibodies against six target genes of the PcG. The loss of the maternal component leads to enhanced homeotic phenotypes and to unique patterns of misexpression. E(Pc) and S u ( 2 ) 2 mutations had only subtle effects on the target genes, even when the maternal contributions were removed. Asx and Pcl mutants show derepression of
the targets only in specific cell types. Psc shows unusual effects on two of the targets, ultrabithorax and
abdominal-A. These results show that the PcG genes do not act only in a common complex or pathway;
they must have some independent functions.
T
HE Polycomb (PC) mutation causes transformations in flies reminiscent of gain of function mutations in the segment identity homeotic genes of the Anten- napedia and bithorax complexes. We will refer to these genes as the homeotics. Pcwas proposed to be a genetic repressor of the homeotics based on the observations that PC does not map to either of the complexes, and that the defects in segmental identity were caused by the loss of PC function (LEWIS 1978; DENELL and FREDERICK 1983). PC has since been shown to cause ectopic expres- sion of the homeotic proteins. For example, Ultrabi- thorax (Ubx) protein is normally expressed with an anterior boundary at parasegment 5 (PS5) (WHITE and WILCOX 1985a). In a PC background, Ubx protein is initially expressed with the appropriate PS5 anterior boundary. However, in later developmental stages Ubx is ectopically expressed in the segments anterior to PS5 in the central nervous system (CNS) (BEACHY et al. 1985; WHITE and WILCOX 1985b) and in the epidermis( MCKEON and BROCK 1991 )
.
Other mutations similar to PC have been found ( JOR- GENS 1985)
.
They are referred to as the Polycomb group ( PcG) after the first mutation that was described( JORGENS 1985 )
.
Genes have been included in the Poly- comb group for a variety of reasons. Some are included because their loss of function phenotypes are similar toCorresponding author: Welcome Bender, Harvard Medical School,
BCMP Department, 240 Longwood Ave., Boston, MA 02115. E-mail: bender@bcmp.med.harrd.edu
’
h e n t address: Department of Hematology/Oncology, Chang Gung Memorial Hospital, 5 Fushing St., Kweishan, Taoyuan, Taiwan 333.Genetics 140: 231-243 (May, 1995)
PC. Others are included because they enhance PC or because they enhance a gene thought to be like PC.
Like PC, they do not map to the homeotic complexes. Not only are the phenotypes similar, but the combina- tion of two of these genes typically gives more than additive effects. This “synergy” suggests that they func- tion in the same process ( J ~ R G E N S 1985)
.
Five of the PcG genes have been cloned: PC, Enhancer of zeste [E(%)], polyhomeotic (ph), Posterior sex combs (Psc) and Polycomblike (Pcl) (DURA et al. 1987; ZINK et al. 1989; BRUNK et al. 1991a; VAN LOHUIZEN et al. 1991; JONESand GELBART 1993; LONIE et al. 1994). The sequences of these genes do not appear to be related to each other, nor do they provide clues to their biochemical functions. PC and ph proteins can be immunoprecipi- tated with each other ( FRANKE et al. 1992). Also, four of the proteins have been localized on polytene chro- mosomes; PC, ph, and Pcl colocalize to the same 100 specific sites and Psc overlaps approximately 45 sites. This supports the idea that they are part of a protein complex ( DECAMILLIS et al. 1992; MARTIN and ADLER
1993; LONIE et al. 1994).
There are striking differences among the PcG pheno- types due to zygotic loss. MCKEON and BROCK (1991 )
232 M. C. Soto, T.-B. Chou and W. Bender
ences may be misleading, because there may be differ- ential perdurance of the maternal products.
All of the PcG genes that have been tested are s u p plied by the mother to the embryo, However, the amount of the maternal product varies greatly from one locus to the next. PC shows little maternal contribution. Zygotic homozygous mutant embryos die at the first instar stage with all of their segments transformed to- ward abdominal segment 8 (AS) ( LEWIS 1978; DENELL and FREDERICK 1983). Embryos lacking both maternal and zygotic PC look only slightly more transformed
( HAYNIE 1983; LAWRENCE et al. 1983). On the other end of the spectrum is extra sex combs (esc) in which homozygous zygotic mutant embryos have no cuticular phenotype and can develop into fertile adults. Embryos lacking both maternal and zygotic esc (derived from homozygous esc parents) resemble PC zygotic mutants
( STRUHL 1981 ) .
With the exception of esc, the PcG mutations are embryonic lethals. Mutants lacking maternal product must be made using mosaic female germlines. Mutant germline clones were made by pole cell transplantation for mutations in Pcl, Asx , Sex combs extra (Sce), Sex combs
on midleg (Scm), 1(4)29 (also known as pleiohomeotic)
(BREEN and DUNCXN 1986), and super sex combs (sxc)
( INGHAM 1984). polyhomotic ( p h ) and Psc germline clones were produced by X-ray-induced mitotic recom- bination. Dominant female sterile mutations were used to eliminate nonrecombinant germline cells (DURA et
al. 1988; SMOUSE et al. 1988; MARTIN and ADLER 1993). The maternal product of E ( z ) has been removed using a temperature-sensitive allele (JONES and GELBART
1990). These studies showed that all nine PcG genes have at least some maternal contribution. The germline mutant phenotypes can be grouped into three classes based on the cuticles: those weaker than maternal esc mutants (Pcl, Asx, sxc), those similar to esc [ P C , Sce, Scm, E ( % ) ] and those with defects much more pleiotropic than esc [ 114) 29, ph, Psc]
.
Most of these analyses fo- cused on the cuticle of the embryos, which forms at the end of embryogenesis and is secreted by the epidermis. We used the dominant female sterile (DFS) recombi- nase (FLP) system ( GOLIC and LINDQUIST 1989; CHoU and PERRIMON 1992; CHOU et al. 1993) to generate germline clones of five of the PcG genes on chromo- some arm 2R. We wished to investigate whether this group of genetically related genes would have similar phenotypes once the maternal contributions were re- moved. We studied E ( P c ) , Asx, Pcl, Psc, and S u ( z ) 2 ,using antibodies to follow the patterns of target gene products at different times in development. When we study the true nulls, there is still significant variation in the patterns of the homeotic proteins. Most of the PcG mutations cause misexpression of all the homeotics to a similar extent. Psc is distinctive in that two of the homeotics, abdominal-A (abd-A) and Ubx, are re- pressed everywhere. We also find that the ectopic ex-
pression of the homeotics is tissue specific in two PcG mutants. Asx and Pcl.
MATERIALS AND METHODS
Mutant alleles: esc2 is an apparent null; esc" is a deficiency for the esc locus (STRUHL 1981, 1983). There is only one allele of E ( P c ) ( SATO et al. 1983). Df(2R) e7tX3'removes E ( P c )
and en ( SATO et al. 1983, 1984). Pc3, previously considered an antimorph, is a protein null (R. PARO, personal communi- cation). Asx x7'29 is a 1.2-kb deletion in the middle of the Asx
coding region, but it is not known if this mutation is a true null
( D. SINCLAIR, personal communication)
.
Df(2R) trix removesAsx (BREEN and DUNCAN 1986). Pcl' (DUNCAN 1982) and
PclD5 are apparent nulls (BREEN and DUNCAN 1986).
Df(2R) 7B removes the Pcl locus (DUNCAN 1982)
.
Psc"~ andPsc"' are representative loss of function mutations ( HOWE
and WU 1995) . Df(2R) u$' removes most of the Psc transcrip- tion unit ( U s K O and PARDUE 1988; BRUNK et al. 1991b). Su(z)2' b7 is a 2-kb deletion at the 5' end of the S u ( z ) 2 pro-
moter that behaves like a null (Wu 1984; ADLER et al. 1989)
.
Su(z)2' 'I8 is a deficiency that removes Psc, Su(z)2, and possi- bly more distal DNA ( ADLER et at. 1989; HOWE and WU 1995).Maintenance of stocks for crosses: Stocks containing the FLP recombinase under the control of the heat shock pro- moter (hs-FLP) were maintained at 18" due to reports of possible constitutive recombination at higher temperatures. The cross to generate males carrying hs-FLP and ova"' was done at 18". All other crosses and the collection of embryos for antibody staining were done at 25".
mS experiments We recombined the above null or poten- tial null alleles of the PcG genes on chromosome arm 2R
onto a chromosome bearing an FRT marked with white+ at the base of 2R (FRT 42B, T-B. CHOU and N. PERRIMON, un- published results). We collected males that carried hs-FLP on their X chromosomes. One of the second chromosomes of these males had the same 2R FRT and the dominant female sterile mutation ouo"'. These males were mated to virgin fe- males bearing the 2R FRT and one of the various PcG alleles to be tested. The progeny of these crosses were heat shocked for 1 hr at 37" during their larval phases, on days 4, 5 and 6 after egg deposition. Experiments done to optimize the pro- cess suggested heat shocks done on these days yield the high- est percentage of females with mosaic germlines, and germ- lines that produce the most eggs (CHOU and PERRIMON 1992; M. C. SOTO and W. BENDER, unpublished observations). The heat shocked female progeny that bore the PcG mutation and the ova'" chromosome were then mated to males heterozy- gous for a deficiency for the locus or for a mutation in the locus.
Number of embryos examined Antibody results cited in this paper were based on the examination of 2 100 embryos. The only exceptions were: Df(2R) enx3' homozygous germline mothers crossed to E ( P c ) fathers, stained with Abd-B; the lac2
experiment cited in Figure 5, right panel; the Df(2R) S u ( z ) 2'
'"
zygotic- ; Abd-B- embryos stained with abd-A antibody. In these cases we examined between 30 and 100 embryos.Germline Mosaics of Polycomb Group 233
Description of the mosaic females from the FLP tech- nique: The females that produced the embryos described be- low as maternal- zygotic- are expected to be mosaic for the PcG gene being tested. We examined these females for Pc-type phenotypes. The females mosaic for the PcG genes E(Pc),
Asx, Pcl, and Psc had alterations in the pigmentation of their abdomens. Often, the sixth tergite had less pigmentation than wild type while the anterior tergites had ectopic anterior pig- mentation. These females often had small abdomens. They sometimes had wing defects similar to those described for heterozygous Pcflies (DUNCAN and LEWIS 1982). Surprisingly, the male siblings did not show these defects. The S u ( z ) 2
mosaic females also looked quite normal in pigmentation, size, and wing appearance.
Construction of recombinant chromosomes: Alleles of Asx, Pcl, Psc, Su (2) 2, and the double mutant for Psc and Su ( z ) 2
were recombined individually onto an FRT chromosome marked with white' (on the FRT) , scabrous (sca) and brown
( bw). Recombinants were recovered between w+ and bw [ PscSz4, PschZ7, Su ( z ) 21.b7, Su ( z ) 2'.@] , between white+ and sca (Pcl'), or between sca and bw (Asx*IZ9, PclD5). The E(Pc)
allele was marked with cn and bw. It was recombined to an FRT chromosome only marked with the w+ at the FRT, choos
ing white+, bw recombinants. These recombination events may
have removed second-site lethals to the left of the mutations. In some cases the recombinant stocks were visibly healthier than the parental stocks.
LacZ lines: bxdl4 is a Pelement construct carrying 14.5 kb
of DNA from the bxd regulatory region of the bithorax com- plex placed next to a Ubx promoter driving lacZ (SIMON et al.
1990). It expresses lacZ from PS6 to PS12 in embryos aged 6-15 hr. A line carrying this Pelement construct on the third chromosome was used for the experiment illustrated in Figure
5 . A Cy0 chromosome bearing the bxdl4 Pelement was used
to balance all of the fathers crossed to germline clone moth- ers. This allowed us to identify embryos that receive a wild- type copy of a given locus by the presence of lacZ expression. Antibody staining: Embryos were fixed and stained as de- scribed ( SIMON et al. 1990) using a mouse monoclonal anti- body against Abd-B ( CELNIJER et al. 1989), a mouse mono- clonal and a rabbit polyclonal antibody against abd-A ( KARCH
et al. 1990; KELLERMAN et al. 1990), the mouse monoclonal,
FP3.38, against Ubx (WHITE and WILCOX 1985a), a rat poly- clonal against Antp (M. HORNER and M. SCOTT, unpublished data), a rat polyclonal against Scr (A. MCCORMICK and M. SCOTT, unpublished data) and the 4D9 mouse monoclonal against engrailed (en) ( PATEL et al. 1989). Stained embryos were dissected and mounted as described (SIMON et al. 1990) .
RESULTS
Generation of maternal- zygotic- embryos: We gen- erated embryos maternally mutant for PcG alleles using the dominant female sterile (DFS) recombinase (FLP) technique (see MATERIALS AND METHODS) This method takes advantage of the introduction of the yeast 2 pm plasmid recombination enzyme into flies (GOLIC and LINDQUIST 1989) to promote recombination between chromosome arms carrying FLP recombination targets
( FRTs)
.
One copy of the chromosome carries the DFS mutation ovoD' ( o n a Pelement) and the other carries the mutation of interest ( CHOU and PERRIMON 1992;CHOU et al. 1993)
.
Only females whose germlines un- dergo the appropriate recombination event can lay eggs, because they recombine away the female sterile mutation.Mosaic mothers were crossed to fathers carrying a deficiency for the same gene over a lac2 marked bal- ancer chromosome; the resulting embryos without the lac2 marker were designated maternal - zygotic -
.
For comparison, zygoti- embryos were generated by cross- ing heterozygous parents; these embryos typically car- ried a null allele over a deficiency. For most of these PcG genes, the cuticular transformations of germline mosaics have been described. We examined the cuticles of our germline mosaics to verify that we were obtaining the expected genotypes.The major advantage of the DFS/FLP/FRT tech- nique for making germline clones is that one usually obtains many embryos from each experiment (see MA- TERIALS AND METHODS). This permits one to study mu- tant effects with a variety of antibodies. Antibodies stain internal as well as external tissues, providing a more complete view of the consequences of a mutation.
We examined PcG loci on chromosome arm 2R in- cluding E(Pc), Asx, Pcl, Psc, and Su ( z ) 2. We could not study sxc because it is located proximal to the most proximal FRT site available ( INCHAM 1984). We stained the mutant embryos with antibodies against Abd-B, abd- A, Ubx, Antp, Scr and en proteins. The Abd-B, abd-A and Ubx expression patterns in zygotic- mutants of some of these genes have been reported ( Asx, Pcl, and Psc) (MCKEON and BROCK 1991; SIMON et al. 1992).
Survey of the genes: We describe the more notable results of these antibody stainings on a gene by gene basis. When relevant, we describe the cuticles as well. We begin with esc, our standard of comparison, which is known to strongly affect the homeotics. The genes are described in order of increasing severity of overall effects, from E(Pc) to Psc
.
We group S u ( z ) 2 with Psc because they are part of a gene complex. We concen- trate mainly on Abd-B in the first part of the results because it is not known to be regulated by the other homeotics. The Abd-B expression pattern has been de- scribed. In wild-type embryos at stage 14 ( l l hr at 25") it is expressed in PSlO to 15 in the epidermis, in PS 10 to 14 of the CNS, and in the visceral mesoderm and in amnioserosa cells in the corresponding region (CELNIKER et al. 1989). The developmental times are esti- mated according to the stages of CAMPOSORTEGA and
HARTENSTEIN (1985). The zygotic- and maternal- zy- gotic- Abd-B results are shown in Figure 1. Table 1 summarizes three types of information about the Abd- B pattern of each mutant: the earliest ectopic expres- sion in zygotic - and maternal- zygotic - mutants, the affected tissues and the segmental limits of ectopic ex- pression.
234 M. C. Soto, T.-B. Chou and W. Bender
zygotic-
maternal
-,
zygotic-
Ann
.,
L. . .c
M
I
RGURE 1.-Abd-B antibody expression comparing embryos lacking zygotic product (zygotic-) or lacking both maternal and zygotic froduct (maternal- zygotic-) for: esc2/esc'*,
E(Pc), AmXT' ', Pcl', Psch2', SU(Z)~'.~', and the deficiency for Pscand Su(z)2, D f ( 2 R ) S u ( ~ ) 2 ' . ~ . The arrows in C , D, G and H indicate the presumptive anterior spiracles. The embryos are dissected and shown with anterior to the left.
2)
,
the CNS from PS3 to PS9 has a few spots of expres- sion. This late zygotic esc misexpression has not been reported. We examined zygotic mutant esc embryos for ectopic abd-A. A few embryos, less than the expected onequarter zygotically mutant, also show late misex- pression in the CNS (data not shown). esc maternal- zygotic- embryos show dramatic ectopic expression of Abd-B (SIMON et al. 1992) (Figure 1B; Table 1 ).
At germ band extension, stage 9, in maternal- zygotic-esc embryos, Abd-B expression extends to PS3. By stage 14, when head involution is underway and dorsal clo- sure begins, all of the segments in the epidermis, vis- ceral mesoderm and CNS show strong expression, in what appears to be all of the cells. This is shown in Figure 1B as a standard for comparison.
The abd-A misexpression in esc maternal - zygotic- mutant embryos has been described (SIMON et al.
1992). Curiously, the abd-A monoclonal antibody ( KEL LERMAN et al. 1990) but not the polyclonal antibody
(KARCH el al. 1990) shows decreased expression in esc
maternal- zygotic- embryos. This difference was not seen in embryos missing other PcG products. The re- duced staining by the monoclonal may reflect a distinc- tive posttranslational modification of abd-A in esc mu- tant embryos.
The Ubx, Antp, and Scr expression patterns in esc
maternal- zygotic- embryos have been described (STRUHL and AKAM 1985; GLICKSMAN and BROWER 1990; MCKEON and BROCK 1991). We see ectopic Scr expression in more cells than others have reported. There is anterior epidermal expression in the mandibu- lar lobe, the maxillary lobe, and the clypeolabrum, as
well as the previously reported posterior spread to PS13 in the lateral epidermis. The wild-type Scr expression is missing in the CNS.
Although it is considered to be mostly maternal, there is partial paternal rescue of esc ( STRUHL 1981 )
.
Embryos from homozygous mutant mothers that re- ceive one wild-type copy from the fathers show Abd-B misexpression, but it does not extend beyond PS4 into the head, in either the epidermis or CNS, and the brain lobes do not stain (SIMON et al. 1992).
Enhancer of Polycomb [ E(Pc)] : E(Pc) has been shown to enhance the mutant phenotype of other PcG muta- tions when tested as transheterozygotes. However, the loss of zygotic E(Pc) activity alone does not produce an embryonic phenotype, although zygotic- embryos die at the first instar stage ( SATO et al. 1983,1984).
We find that E(Pc) mutants are not much affected by removal of the maternal contribution. The cuticles of E(Pc) maternal- zygotic- mutants still look wild type, with no evidence of segmental transformations. E(Pc)
Germline Mosaics of Polycomb Group 235
TABLE 1
Abd-B ectopic expression in zygotic- and maternal- zygotic- PcG embryos
Gene
Maximum extent of ectopic expression First ectopic expression
Visceral
Status Stage Tissues Epidermis mesoderm CNS
esc
PC E m )
Asx
PC1
Psc
su (x) 2
Df Psc & Su(z)2
15 9 10 12 12 12 10 12 10 15 10
-
-
12 10CNS Epi,
VM
Epi Epi Epi Epi,
VM
Epi,
VM
Epi,
VM
Epi, VM
CNS Epi,
VM
-
Epi, V M , CNS Epi, V M , CNS
-
All All
As As PS6/7-9 PS3-9
PS6/7-9 &
As
PS3-9
All
-
-
-
All
A l l
-
For comparison, wild-type Abd-B protein expression begins at stage 8 in the epidermis and visceral mesoderm, is in PS10-15 of the epidermis and PS10-14 of the visceral mesoderm and CNS. Epi, epidermis; V M , visceral mesoderm; CNS, central nervous system; A s , anterior spiracles; All, cells in all segments are staining.
nor spiracles toward posterior spiracles. The embryos which receive a wild-type copy of E(Pc) from the father,
as confirmed by lac2 staining, do not show the ectopic spots. This half of the embryos is paternally rescued to adulthood. The expression patterns of abd-A, Ubx, and en antibodies are also unchanged. The single available
E(Pc) mutation could have partial function. Therefore, we examined embryos maternally and zygotically mu- tant for the deficiency e n x 3 ’ , which removes E(Pc) and
a ( SATO et al. 1983). Because the segment polarity gene engrailed (a) has no maternal contribution (LAW- RENCE et al. 1983), removing a in the germline should
?e*
RGURE 2.- esc zygotic- embryos show late misexpression when stained with Abd-B. The embryo is of the genotype esc2/ esc” and is shown at stage 17. The ectopic expression is evident in the CNS. The wild-type Abd-B pattern is shown in Figure 1A. Anterior is to the left.
not complicate the results. Mothers with mosaic germ- lines were mated to E(Pc) fathers; the mutant embryos show no additional misexpression of Abd-B.
Additional sex combs (Asx): The loss of Asx has tissue specific effects. The homeotic proteins are misex- pressed in the epidermis and visceral mesoderm, but not in the CNS, even when the maternal contribution is removed. In Asx zygotic- mutant embryos (Figure
1E; Table 1 ) the Abd-B misexpression affects only epi- dermal and visceral mesoderm cells. Removal of the maternal and zygotic contribution leads to earlier mi+ expression (stage 10 instead of stage 12)
.
By germ band retraction, or stage 14 (Figure 1F; Table 1 ) the ectopic expression in the epidermis extends further anteriorly, to posterior PS3, and it is in more cells. Abd-B is now also misexpressed throughout the visceral mesoderm, and in the pericardial cells to PS6. It is not misexpressed in the CNS.The abd-A expression pattern in Asx mutant embryos also shows tissue specificity. Embryos lacking both ma- ternal and zygotic Asx again show earlier misexpression and more anterior spread than zygotic mutants (SIMON et al. 1992). By stage 14 there is ectopic expression up to PS3 in the epidermis, in a few cells in the clypeola- brum, and in the visceral mesoderm to its anterior end. There is no ectopic expression in the CNS in the
zy-
gotic- or maternal- zygotic- embryos. Ubx staining gives similar results.
Scr staining of Asx maternal- zygotic- embryos reveals only late misexpression. At stage 15 there is faint dorsal epidermal expression in two segments behind the PS3
236 M. C. Soto. T . B . Chou and W. Bender
BREEN and DUNCAN ( 1986) have carefully described the cuticles of Asx mutants. Zygotic mutant embryos show only subtle segmental transformations, while ma- ternal - and zygotic - mutants have increased anterior to posterior transformations. The intermediate level of homeotic misexpression in A s x maternal- zygotic- em- bryos that we see is in agreement with these cuticular results. We find that one half of the embryos from het- erozygous fathers and mosaic mothers show wild-type homeotic staining, in agreement with the paternal res- cue to adulthood seen by BREEN and DUNCAN (1986). Double labeling with lacZ antibody confirms that the apparently wild-type embryos receive a wild-type copy of Asx from the father.
Polycomb like (Pcl): The Abd-B expression of Pcl ma- ternal and zygotic mutants shows some tissue specificity. In early embryos there is moderate misexpression in the epidermis and full misexpression in the visceral mesoderm, and in late embryos there is strong misex- pression in the CNS. The maternal- zygotic- embryos show earlier and more extensive ectopic expression than the zygotic- embryos, but the tissue specificity still holds (Figure 1, G and H; Table 1 )
.
This is in contrast to esc in which ectopic expression is similarly strong in all tissues. The abd-A and Ubx patterns of misexpres- sion of zygotic- and maternal- zygotic- embryos are similar to the Abd-B pattern.For the Scr protein, as for Abd-B, the misexpression largely switches from epidermis and visceral mesoderm to the CNS. The later Scr visceral mesoderm staining, after stage 14, is not suppressed, in spite of the earlier misexpression of abd-A and Ubx in the anterior visceral mesoderm. In other PcG mutant backgrounds, in which Ubx and abd-A are misexpressed in the visceral meso- derm throughout development, the late Scr visceral mesoderm staining is suppressed (data not shown)
.
The intermediate extent of homeotic misexpression of Pcl maternal- zygotic - embryos is in agreement with the known cuticular transformations. Zygotic mutant embryos show partial transformations of A6 and A7 den- ticle bands towards A8. Embryos obtained from pole cell transplants lacking both maternal and zygotic Pcl show more severe segmental transformations (BREEN and DUNCAN 1986). We obtained wild-type homeotic staining in one half of our maternal- zygotic- embryos. This agrees with the paternal rescue to adulthood seen by BREEN and DUNCAN (1986).
Posterior sex combs (Psc) : Psc is known to have a s u b stantial maternal contribution (MARTIN and ADLER
1993). The gene is part of a complex locus which in- cludes S u ( z ) 2 , and probably a third gene (WU 1984). The PscA'@ allele, a probable null, is the best character- ized, but we could not use it because it is in a Pcytotype stock. We find that the two alleles of Psc that we exam- ined, e24 and h27, have the same cuticular transforma- tions as those seen with PscAq2 embryos, zygotically mu- tant (ADLER 1991 ) or maternally and zygotically mutant (MARTIN and ADLER 1993). Zygotic- Psc embryos show
minimal homeotic misexpression, only obvious at late times (SIMON et al. 1992). At stage 15 (dorsal closure) these embryos show Abd-B staining in a few cells in each segment of the CNS, and a few cells in the brain (Figure 11)
.
Maternal - zygotic - Psc mutants show Abd- B misexpression like that of esc, strong in all tissues and at all times (Figure 1J; Table 1 ) (MARTIN and ADLER1993). Late embryos are morphologically quite abnor- mal. The abd-A and Ubx results are significantly differ- ent so they are described below with the Antp and Scr results.
The strong Abd-B misexpression of Psc maternal- zygotic- embryos could not be easily predicted from the known cuticular phenotype. The cuticles of PscAqp.l zygotic mutants show head defects, and minor segmen- tal transformations (ADLER et al. 1991 )
.
MARTIN andADLER (1993) obtained PscAqZ germline clones by mi- totic recombination. Maternal- zygotic- mutants have more severe defects, which include head involution problems, missing denticle bands, segmental transfor- mations, and twisting of the embryos. Remarkably, all these defects are rescued by one paternal wild-type chromosome and embryos develop to adulthood. In agreement with this, we find that one half of the Psc
maternal- zygotic- embryos show wild-type homeotic staining.
Suppressor of zeste 2 [ S u ( z ) 21 : Although it is not al- ways included with the PcG, S u ( z ) 2 does show syner- gistic interaction with Sex combs on midleg (Scm), another PcG gene (ADLER et al. 1989). The cuticles of S u ( z ) 2
zygotic mutants show no obvious segmental transforma- tions (ADLER et al. 1989). We find that the cuticles of S u (z) 2 maternal- zygotic - embryos also show no segmental transformations. The only defects in the one half of the cuticles assumed to be the mutants were slight kinks on the dorsal side at about PS13. Occasion- ally, but in less than half the animals, there were head abnormalities. The other half of the embryos look com- pletelywild type, indicating full paternal rescue of these defects. Because we did not examine other null alleles and because the background chromosome is not avail- able for comparison, these defects may not be related to S u ( z ) 2 . Neither zygotic- (Figure 1K) nor maternal- zygotic - (Figure 1L) S u ( z ) 2 embryos show Abd-B mis- expression. abd-A, Ubx, Antp and Scr staining of mater- nal- zygotic- embryos also show no obvious misexpres- sion, as might be expected from the cuticular phenotype.
DeJiciency for Psc and S u (z) 2: Psc and S u (z) 2 are simi- lar enough in their protein sequences that their func- tions may be redundant. We therefore tested a defi- ciency that removes both genes. The deficiency
S u (z) 21.b8 removes Psc, S u (z) 2, and possibly more distal DNA. We find that zygotic mutants of the l.b8 defi- ciency show strong misexpression of Abd-B, as strong as that of the Psc maternal- zygotic- mutants or esc
Germline Mosaics of Polycomb Group 237
earlier misexpression. Surprisingly, these embryos show fewer morphological abnormalities than Psc maternal- zygotic- embryos (compare Figure. 1, J and N )
.
This seems paradoxical, given the apparent redundancy of Psc and Su ( z ) 2 function, as illustrated by the compari- son of zygotic phenotypes (compare Figure 1, I, K, and M ).
On the other hand, there are suggestions that the relative levels of Psc and Su ( z ) 2 are important. Psc defi- ciencies cause elevated Su ( z ) 2 transcription ( BRUNK etal. 1991b), and a duplication for the Su(z)2 complex suppresses the gain-of-function chaetae loss that some Psc deficiencies show, possibly by restoring the relative levels of the two products (WU and HOW 1995). Also, one loss-of-function Su (z) 2 allele “rescues” another PcG phenotype, the embryonic lethality of l ( 4 ) 29 (AD-
LER et al. 1989).
The strong Abd-B misexpression of zygotic- mutants for the deficiency is in agreement with the known cutic- ular phenotype. The cuticles of zygotic- mutants look quite abnormal, most like Psc maternal- zygotic- cuti- cles (Wu and H o w 1995). There are strong defects in head formation and involution, holes in the dorsal epidermis, especially in the anterior half of the embryo, and laterally narrow denticle bands. This observation suggests that Psc and Su(z) 2 are indeed redundant, since cuticles of either zygotic mutant alone are close to wild type. Alternatively, a gene just distal to Su ( z ) 2 may play a role. The cuticles of maternal- zygotic- mutants of the deficiency had not been described. We find they are no more abnormal than the cuticles of zygotic- mutants, in agreement with the Abd-B misex- pression we describe above. One wild-type paternal copy rescues the maternal- zygotic- mutants. These embryos show wild-type homeotic expression and sur- vive to adulthood.
Psc effects on other homeotic loci: We examined al- most all genotypes with antibodies to abd-A, Ubx, An- tennapedia (Antp)
,
and Sex combs reduced (Scr) , and compared them with Abd-B. We omitted Antp and Scr staining of E(Pc) due to the low numbers of maternally mutant embryos. For most of the PcG mutations, the misexpression patterns of abd-A, Ubx, Antp, and Scr resemble those of Abd-B in tissue specificity and in ex- tent of misexpression. The abd-A and Ubx protein pat- terns for Psc were clearly distinctive. Figure 3 compares the protein patterns of Psc maternal - zygotic - embryos with those of deficiency for Psc and Su ( z ) 2 maternal - zygotic - embryos, PC’ zygotic - embryos, and Canton S wild-type embryos.abd-A expression: In Psc maternal- zygotic- mutants abd-A misexpression is much weaker and more limited than Abd-B misexpression (compare Figure 3C with Figure 1J). There is ectopic expression of abd-A ahead of the wild-type PS7 anterior boundary (Figure 3A)
( KARCH et al. 1990), but it is faint, and is not present
in the CNS. There is ectopic epidermal expression ante- riorly up to PS4, with no expression in the head seg- ments. The deficiency for Psc and S u ( z ) 2 gives the
same abd-A result as does Psc (Figure 3D)
.
As with esc,the monoclonal antibody for abd-A gave fainter staining than the polyclonal, specifically in the mutant embryos. The lack of Psc may also be altering the abd-A epitope recognized by the monoclonal antibody.
Ubx expression: The Ubx misexpression for maternal
Psc mutants is similar in extent to the abd-A misexpres- sion, and different from the Abd-B misexpression. In
Psc maternal- zygotic- mutants, by the time of CNS condensation, we see ectopic anterior spread in the epidermis through PS4 or PS3, and the overall level of Ubx is much lower than in wild type (Figure 3G)
.
The CNS does stain, but only to its proper anterior boundary (PS5).
The deficiency for Psc and Su ( z ) 2, when re- moved maternally, gives a similar result (Figure 3 H ) . In contrast,PC
shows strong Ubx misexpression throughout the head segments (Figure 3F) (WHITE and WILCOX 198513; MCKEON and BROCK 1991 ).
Antp expression: The Antp misexpression in Psc back-
grounds looks similar in extent to the Abd-B result, and resembles the Antp misexpression in PC mutants (Figure 3, J and K)
.
In wild-type embryos, Antp epider- mal and CNS expression is in many cells within PS4 and PS5 and in fewer cells in PS3 and PS6-13 (CARROLL et al. 1986). Psc maternal- zygotic- mutants show high levels of Antp staining all along the CNS, including PS14 and the brain lobes. The deficiency for Psc and Su ( z ) 2 also shows early and extensive CNS misexpres- sion (Figure 3L). One subtle difference is seen at germ band retraction, stage 13. The 1.68 deficiency embryos and PC embryos show enhanced epidermal and CNS staining in PS4 and 5, as in wild type. Psc maternal- zygotic- mutants show nearly uniform expression throughout the epidermis and CNS.Scr expression: As with Antp, the Scr misexpression patterns are similar in Psc maternal - zygotic - mutants and in PC (Figure 3, N and 0 ) . In wild-type embryos, Scr antibody detects expression in PS3 and the labial lobe in the epidermis, in the third ganglia in the CNS, and in the visceral mesoderm just posterior to where the gastric caeca form (Figure 3M) (CARROLL et al. 1988; MAHAFFEY and KAUFMAN 1987; RILEY et al. 1987). Misexpression in Psc maternal - zygotic - mutants begins at early germ band retraction, early stage 12, in the epidermis, when the maxillary lobe and PS4 show ec- topic staining. After germ band retraction, the mandib- ular lobe also expresses Scr, there are faint spots in the clypeolabrum, and there are lateral epidermal stripes spreading posteriorly to PS15, but Scr never appears in the CNS.
The deficiency for Psc and Su (z) 2 shows a slightly differ- ent pattern of Scr misexpression from Psc (Figure 3P), although it is still similar to PC. In maternal- zygotic- mu-
tants, the deficiency shows earlier misexpression (stage 9
238 M. C. Soto, T.-B. Chou and W. Bender
a-a bd-A
a-U
bx
a-Antp
a-Scr
PC
Psc
Df
Psc
&
Su(z)2
D
- f
7
H
PC. The two exceptions are Ubx and abd-A, which do not show strong misexpression as one might predict.
Abd-B transrepression in Psc mutants: Abd-B is
known to repress Ubx and abd-A in PS13 and PS14
( KARCH et al. 1990)
.
It is possible that such transrepres- sion explains the reduced Ubx and abd-A patterns in Psc mutant embryos. To test this, we constructed zygotic- mutants of the 1.b8deficiency for Pscand Su(z)2, which also lacked Abd-B. Zygotic- embryos of the 1.68 defi- ciency show the reduced misexpression of abd-A and Ubx, and it is much less complicated to remove Abd-B from the zygotic- embryos than from the maternal- zygotic- embryos. The dtficiency l.b8-; Abd-B- embryos did show significant ectopic abd-A expression when stained with the abd-A polyclonal antibody. Thus, the presence of Abd-B prevents abd-A (and presumably Ubx) from being misexpressed as strongly as the other homeotics. This result was unexpected because Abd-B does not prevent the ectopic expression of Ubx and abd-A in PC or esc mutants.Effects on a BX-C regulatory element: Each home-
FIGURE 3.-Comparison of the protein expression of Ubx, abd-A, Antp and Scr in Psc and control backgrounds. (A-D) abd-A; (E- H ) Ubx; (I-L) Antp; ( M - P ) Scr. The successive rows show staining in: wild-type Canton Sembryos, zy- gotically mutant Pc3 embryos, ma- ternal - zygotic - PschZ7 embryos, and embryos maternal- ,,ti, - for the deficiency Su(z)2', , whlch removes Psc and Su ( 2 ) 2. The em- bryos stained with Scr are shown at an earlier stage than most oth- ers in Figure 3, so that the head has not involuted and its mor- phology is more obvious. The head expression of the embryo in H is nonnuclear and appears to be background staining. The em- bryos are dissected and shown with the anterior end up.
otic gene could have multiple sites for PcC regulation. Partially redundant regulation might explain the tissue specificity seen in some PcG mutant backgrounds (see
DISCUSSION). We tested several of the mutations with
bxdl4, a PcG response site, carried on a Pelement out- side the complex. This 14.5-kb fragment from the bxd
(PS6) regulatory region of Ubx drives a l a d pattern in PS6 and more posterior parasegments. The pattern is faithfully maintained until late embryogenesis (Figure 4, left panel) (SIMON et al. 1993). The
l
a
d
pattern spreads into more anterior segments in embryos lacking zygotic contributions of any of the PcG genes tested sofar (SIMON et al. 1993). This P element also binds ph and PC proteins on polytene chromosomes (DECA- MILLIS et dl. 1992; CHIANG et al. 1995). For all these reasons, the bxdl4
l
a
d
construct appears a reliable re- porter of PcC function.Germline Mosaics of Polycomb Group 239
WT
Asx
m=,
2-
FIGURE 4.- Asx affects the lncZ
expression of a bithorax complex reporter construct. ladpattern of the Pelement bxdl4 in: wild-type,
Canton S embryo (left) and
maternal- zygotic- em- bryo (right). Note that the mu- tant embryo shows ectopic ladex- a pression ahead of PS6 (arrows) in
the epidermis and the CNS.
Asxx"/2"
affect regulation in all tissues. Asx must be available and functional in the CNS, although it is not needed there for regulation of the homeotics (Figure 1, E and F)
.
engrailed expression: MOAZED and O'FARRELL
( 1992) reported that the engrailed ( e n ) protein is not misexpressed when many of the PcG products are re- moved one at a time, but that double mutants do give en misexpression in many cases. One explanation for lack of misexpression in single mutants could be the presence of maternal product. As we made our mater- nal mutants, we examined en patterns.
There is no enhanced en misexpression in embryos lacking both maternal and zygotic contributions of Asx,
Pcl, or Su (z) 2. There is a difference in the en pattern when the maternal contribution is removed for Psc, or for the deficiency that removes Psc and Su(z)2. In Psc
maternal- zygotic- embryos, the lateral stripes of en in PS3 to PS15 are wider than in wild type, and the stripes in the head expand to include the anterior as well as posterior portions of the lobes (Figure 5 , right panel). This amount of misexpression is more than was seen for PC zygotic- embryos, but less than was seen for ph
zygotic- embryos (DURA and INCHAM 1988; MOAZED and O'FARRELL 1992).
DISCUSSION
Maternal contributions of the PcG genes on
2 R
The FLP/FRT/DFS system made it possible to remove the maternal contribution for several PcG genes. It is clear that for some of the PcC genes, the mother puts abun- dant product into the egg. Thus for Psc and esc the zygotic- embryos are almost wild type, but the mater- nal- zygotic- embryos show universal and strong misex- pression of the homeotics. Other members of the PcChave only modest maternal contributions. Pcl and Asx
maternal- zygotic- embryos look much like the
zy-
gotic- embryos, although the misexpression of the ho- meotics is earlier and slightly stronger. Two PcC genes had not been examined before in mutant germline clones. E(Pc) and Su(z)2 maternal- zygotic- embryos show near normal expression of the homeotics. They have no cuticular defects that can be explained as gain of function, homeotic transformations, although the cuticles of Su(z) 2 embryos have defects. These two
genes might still be included in the PcC because they enhance the phenotypes of other members. But be- cause they lack obvious homeotic phenotypes even when the maternal contributions are removed, their role is probably different from that of other PcG genes. It is possible that the enhancements are an indirect effect, but it is also possible that genes like Su(z)2 are integral members of a PcC complex or pathway with minor or redundant functions. We choose to define the PcC broadly until a more biochemical assay for its function becomes available.
Germline Mosaics of Polycomb Group 241
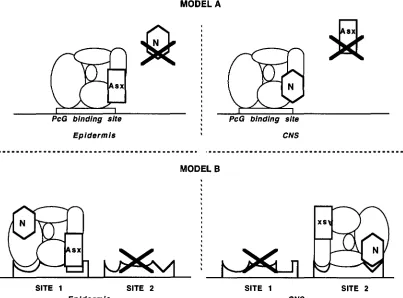
MODEL A
-
MODEL A
sx
PcG binding site
:
PcG binding siteEpidermis I CNS
I
"""""""""""""""""""""~.
~~"""""~""""""~"~"""~~"".
I
I I \ I I
PcG binding site
:
PcG binding siteEpidermis I CNS
I
"""""""""""""""""""""~.
~~"""""~""""""~"~"""~~"".
\I
I
"1.
MODEL B
I
,
SITE 1 SITE 2
Epidermis
SITE 1 CNS
SITE 2
FIGURE 6.-Models for the mechanism of repression of the PcG. (A) One type of PcG DNA binding site, different types of PcG protein complexes. ( B ) Different binding sites, one PcG complex. Gene N is some other member of the PcG. The "X"
indicates that sites or proteins are not functional in certain cell types. We show Asx interacting with the PcG binding site only for the clarity of the model. The complex is shown in two orientations to illustrate simply how one complex could affect two
different sites.
by examining the Ubx transcript pattern in maternal - zygotic - mutants of the deficiency for Psc and Su
(z)
2. We found that in the mutants, Ubx levels are normal at early stages, and drop at later stages. This is the pattern we expect if Ubx is turned on normally but is later trans- repressed by Abd-B protein. Because the loss of Ubx RNA occurs before the CNS condenses, the late CNS pattern is never set up in these embryos. This explains the apparent tissue specific (not in the CNS) ectopic expression of Ubx in Psc embryos. The slight amount of late epidermal misexpression detected by antibody probes is probably due to the perdurance of homeotic proteins.The long-standing result, that Ubx and abd-A are not repressed in e x and PC mutants, remains difficult to explain. There may be different Abd-B isoforms ex- pressed in the different PcG mutations. The different isoforms have been suggested to have functional differ- ences ( KUZIORA 1993). However, examination of the abd-A pattern in various Abd-B mutations shows that abd-A is repressed in PS13 by both of the known forms of Abd-B protein (called I and 11, or m and r ) ( KARCH
et al. 1990; MACIAS et al. 1990; LAMKA et al. 1992 )
.
An alternative explanation is that the overall level of Abd- B expression is higher in Psc mutants, high enough to repress Ubx and abd-A. In support of this explanation,Psc maternal zygotic - embryos have more ectopic Filz-
k@er than esc maternal- zygotic- embryos or PC zy-
gotic- embryos. The number of anterior Filzkoqer can be increased in esc maternal - zygotic - embryos if extra copies of the bithorax complex are added ( STRUHL 1983) or in wild-type embryos when high levels of Abd- B are expressed under control of the heat shock pro- moter ( LAMKA et al. 1992). Finally, the Abd-B protein could have different posttranslational modifications in the different mutant backgrounds. All of these explana- tions suggest distinctive functions for different mem- bers of the PcG.
Tissue specificity of the PcG: Prior work with zygoti- cally mutant embryos had suggested tissue specific regu- lation of the homeotics by some members of the PcG, and differences in the timing of their action ( MCKEON and BROCK 1991; SIMON et al. 1992). The experiments in this paper prove that tissue specificity of the PcG is real and not an artifact of differential maternal perdu- rance. For Asx, removal of the maternal and zygotic products gives an enhanced mutant phenotype (Figure 1, E and F) but this enhancement never includes CNS misexpression.
242 M. C. Soto, T.-B. Chou and W. Bender
matic misexpression is at late times, in the CNS. This change occurs between stage 12 and 14, as the CNS matures. One can describe the PcZ misexpression pat- terns as preferentially affecting the CNS.
Another form of tissue specificity is illustrated by the visceral mesoderm patterns. When we compare the tis- sues affected by the elimination of the various PcG products, the visceral mesoderm appears particularly sensitive. Even in the mutant backgrounds that cause the least dramatic misexpression, like Asx and Pcl, there is early and complete derepression of the visceral meso- derm staining of Abd-B and abd-A. Unlike the epider- mal and CNS misexpression, which was often graded, the anterior spread of Abd-B and abd-A visceral meso- derm expression was all or nothing. Once misexpres- sion was seen, at earliest times, it extended to the ante- rior end of the visceral mesoderm. This sensitivity of the visceral mesoderm to the presence of the PcG mem- bers is not apparent when zygotic mutants of the PcG genes are examined. For many loci, if only the zygotic contribution is removed, the first tissue to be affected is the CNS (Table 1 )
.
Therefore, the requirement for the PcG products in the visceral mesoderm may be re- stricted to earlier developmental times.Possible mechanisms for tissue specificity: Formally, there are two ways in which tissue specificity of the PcG might be explained. Either the components of the PcG complex are different in different tissues, or the sites at which the PcG acts are different in different tissues
(Figure 6 )
.
The simplest realization of the first alternative would be that some genes of the PcG are only present in some tissues. This is not the case for Asx; it is expressed in most embryonic tissues, and is highly expressed in the CNS (H. BROCK, personal communication)
.
It is possi- ble that Asx is modified or displaced from the complex in a tissue specific way. However, it is still difficult to explain the observation that Asx regulates the b x d l 4 construct in all tissues (Figure 5 ).
Asx must be present and capable of working together with other PcG mem- bers in all tissues.In the second alternative, the PcG complex would recognize and act at multiple sites near each target gene. Different sites might each require a different sub- set of the PcG members to achieve repression. Sites would only be functional in certain cell types, perhaps due to tissue specific factors that are not members of the PcG. By this model, the b x d l 4 lac2 construct would contain a site that requires Asx, but in the bithorax complex there might be other sites which do not re- quire Asx. These non-Asx sites might be tissue specific, so that they would maintain repression in certain cell types when Asx is removed. One difficulty with this model is that there exist few PcG binding sites in the 150 kb of DNA from the bithorax complex that have been surveyed ( CHIANG et al. 1995)
.
It is likely that reality is even more complicated than either model. It may be that the tissue specificity arises
from interaction among PcG binding sites or between PcG sites and other regulatory elements. Thus the cell specificity might be lost when PcG binding sites are studied in isolation, for example, outside the bithorax complex. These issues will be clarified when more PcG binding sites are found, when they are better defined and are tested in PcG mutant backgrounds.
We thank KATHY MATTHEWS and the Indiana Stock Center, the Bowling Green Stock Center, NORBERT PERRIMON, DONALD SINCWR and C.-TING Wu for fly stocks. Antibodies were generously provided by SUSAN CELNIKER ( Abd-B) , IAN DUNCAN (abd-A and Ubx) , MIKE HORNER (Scr) , FRANCOIS KARCH (abd-A) , ALLISON MCCORMICK (Antp) , and NIPAM PATEI. ( e n ) . H U G H BROCK, RENATO PARO, DON- AID SINGIAIR and C.-TING WU shared results before publication. C.-TING Wu, DONALD MORISATO, and the members of the BENDER lab provided helpful comments. This research was supported by a grant to W.B. from the National Institutes of Health.
LITERATURE CITED
ADI.ER, P. N., J. CHARLTON and B. BRUNK, 1989 Genetic interactions of the Suppressor 2 of zeste region genes. Dev. Genet. 10: 249-260. ADLER, P. N., E. C. MARTIN, J. CHARLTON and IC JONES, 1991 Phenc- typic consequences and genetic interactions of a null mutation in the Drosophila Posterior Sex Combs gene. Dev. Genet. 1 2 349-361. BEACHY, P. A,, S. L. HELFAND and D. S. HOGNESS, 1985 Segmental
distribution of bithorax complex proteins during Drosophila de- velopment. Nature 313: 545-551.
BREEN, T. R., and I. M. DUNCAN, 1986 Maternal expression of genes that regulate the bithorax complex of Drosophila mlanogaster.
Dev. Biol. 118: 442-456.
BRUNK, B. P., E. C. MARTIN and P. N. ADLER, 1991a Drosophila genes
Posterior Sex Combs and Suppressor two of zesie encode proteins with homology to the murine hi-1 oncogene. Nature 353 351-355. BRUNK, B. P., E. C. MARTIN and P. N. ADLER, 1991b Molecular genet-
ics of the Posterior Sex Combs/Suppressm 2 of zeste region of Dro- sophila: aberrant expression of the Suppressor 2 of zeste gene re- sults in aberrant bristle development. Genetics 128: 119-132. C~MPOSORTEGA, J. A,, and V. HARTENSTEIN, 1985 TheEminyonic De-
velopment of Drosophila melanogaster. Springer-Verlag, New York. CARROL.L, S. B., R. A. LAWON, M. A. MCCUTCHEIN, P. D. RILEY and
M. P. SCOTT, 1986 The localization and regulation of Antennapedia
protein expression in Drosophila embryos. Cell 47: 113-122.
CARROL.L, S. B., S. DINARDO, P. H. O’FARRELI., R. H. A. WHITE and M. P. Scorr, 1988 Temporal and spatial relationships between segmentation and homeotic gene expression in Drosophila em- bryos: distributions of the fushi tarazu, engrailed, Sex combs reduced, Antennapedia, and Ultrabithorax proteins. Genes Dev. 2: 350-360. CELNIKER, S. E., D. J. KEELAN and E. B. LEWIS, 1989 The molecular
genetics of the bithorax complex of Drosophila: characterization of the products of the Abdominal-B domain. Genes Dev. 3: 1424- 1436.
CHIANG, A,, M. O’CONNOR, R. PARO, J. SIMON and W. BENDER, 1995 Discrete Polycomb binding sites in each parasegmental domain of the bithorax complex. Development (in press).
CHOU, T. B., and N. PERRIMON, 1992 Use of a yeast site-specific recombinase to produce female germline chimeras in Drosoph- ila. Genetics 131: 643-653.
CHOU, T. B., E. NOLI. and N. PERRIMON, 1993 Autosomal P[ovo”’] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development 119: 1359-1369. D E W I L L I S , M., N. CHENG, P. PIERRE and H. W. BROCK, 1992 The
polyhomeotic gene of Drosophila encodes a chromatin protein that shares polytene chromosome-binding sites with Polycomb. Genes Dev. 6 223-232.
DENELI., R. E., and R. D. FREDERICK, 1983 Homeosis in Drosophila:
a description of the Polycomb lethal syndrome. Dev. Biol. 97:
34-47.
DUNCAN, I. M., 1982 Polycomblike: a gene that appears to be re- quired for the normal expression of the bithorax and Anten- napedia gene complexes of Drosophila melanoguster. Genetics 1 0 2
Germline Mosaics of Polycomb Group 243
DUNCAN, I., and E. B. LEWIS, 1982 Genetic control of body segment differentiation in Drosophila, pp. 533-554 in Developmental Order:
Its origin and Regulation, edited by S. SUBTELNY. Liss, New York. DURA, J.-M., and P. INGHAM, 1988 Tissue- and stage-specific control of homeotic and segmentation gene expression in Drosophila em- bryos by the polyhomeotic gene. Development 103: 733-744.
DURA, J.-M., J. DEATRICK, N. B. RANDSHOLT, H. W. BROCK and P. SANTAMARIA, 1987 A complex genetic locus, polyhomeotic, is re- quired for segmental specification and epidermal development in D. melanogaster. Cell 51: 829-839.
DURA, J.-M., J. DEATRICK, N. B. W D S H O L T , H. W. BROCK and P. SANTAMARIA, 1988 Maternal and zygotic requirement for the polyhomeotic complex genetic locus in Drosophila. Roux's Arch. Dev. Biol. 197: 239-246.
FRANKE, A,, M. DECAMILLIS, D. ZINK, N. CHENG, H. W. BROCK et al., 1992 Polycomb and polyhomeotic are constituents of a multimeric protein complex in chromatin of Drosophila melanogaster. EMBO J. 11: 2941-2950.
GALLONI, M., H. GWRKOVI~S, P. SCHEDL and F. KARCH, 1993 The bluetail transposon: evidence for independent cisregulatory do- mains and domain boundaries in the bithorax complex. EMBO J. 12: 1087-1097.
GLICKSMAN, M. A,, and D. L. BROWER, 1990 Persistent ectopic ex- pression of Drosophila homeotic genes resulting from maternal deficiency of the extra sex combs gene product. Dev. Biol. 1 4 2
GOLIC, K G., and S. LINDQUIST, 1989 The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome.
Cell 59: 499-509.
HAWIE, J. L., 1983 The maternal and zygotic roles of the gene
Polycomb in embryonic determination in Drosophila melanogaster. Dev. Biol. 100: 399-411.
INGHAM, P. W., 1984 Agene that regulates the bithorax complex differ- entially in larval and adult cells of Drosophila. Cell 37: 815-823.
JONES, R. S., and W. M. GELBART, 1990 Genetic analysis of the En- hancer of zeste locus and its role in genetic regulation in Drosophila melanogaster. Genetics 126 185-199.
JONES, R. S., and W. M. GELBART, 1993 The Drosophila Polycomb group gene Enhancer of zeste contains a region with sequence similarity to trithorax. Mol. Cell. Biol. 13: 6357-6366.
JURGENS, G., 1985 A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature 3 1 6 153-155.
KARCH, F., W. BENDER and B. WEIFFENBACH, 1990 abdA expression in Drosophila embryos. Genes Dev. 4 1573-1587.
KELLERMAN, K A,, D. M. MATTSON and I. DUNCAN, 1990 Mutations affecting the stability of the fushi tarazu protein of Drosophila. Genes Dev. 4 1936-1950.
KUZIORA, M., 1993 Abdominal-B protein isoforms exhibit distinct cu- ticular transformations and regulatory activities when ectopically expressed in Drosophila embryos. Mech. Dev. 4 2 125-137.
UMKA, M. L., A. M. BOUI.ET and S. SAKONJU, 1992 Ectopic expres- sion of UBX and ABD-B proteins during Drosophila embryogene- sis: competition, not a functional hierarchy, explains phenotypic suppression. Development 116: 841 -854.
LASKO, P. F., and M. L. PARDUE, 1988 Studies of the genetic organi- zation of the vestigial microregion of Drosophila melanogaster. Ge- netics 120: 495-502.
LAWRENCE, P. A,, P. JOHNSTON and G. STRUHI., 1983 Different re- quirements for homeotic genes in the soma and germ line of
Drosophila. Cell 35: 27-34.
LEWIS, E. B., 1978 A gene complex controlling segmentation in Drosophila. Nature 276: 565-570.
LONIE, A., R. D'ANDREA, R. PARO and R. SAINT, 1994 Molecular characterisation of the Polycomblike gene of Drosophila melanogas- ter, a transacting negative regulator of homeotic gene expres- sion. Development 1 2 0 2629-2636.
MACIAS, A,, J. CASANOVA and G. MORATA, 1990 Expression and regu- lation of the abd-A gene of Drosophila. Development 110: 1197-
1207.
" F E Y , J. W., and T. C. KAUFMAN, 1987 Distribution of the Sex
422-431.
combs reduced Gene Products in Drosophila melanogaster. Genetics
117: 51-60.
MARTIN, E. C., and P. N. ADLER, 1993 The Polycomb group gene Posterior Sex Combs encodes a chromosomal protein. Develop ment 117: 641-655.
MCCALL, K, M. B. O'CONNOR and W. BENDER, 1994 Enhancer traps in the Drosophila bithorax complex mark parasegmental do- mains. Genetics 138: 387-399.
MCKEON, J., and H. W. BROCK, 1991 Interactions of the Polycomb group of genes with homeotic loci of Drosophila. Roux's Arch. Dev. Biol. 199: 387-396.
MOAZED, D., and P. H. O'FARRELL, 1992 Maintenance of the en-
grailed expression pattern by Polycomb group genes in Drosophila. Development 116: 805-810.
PATEL, N. H., E. MARTIN-BIANCO, K G. COLEMAN, S. J. POOLE, M. C.
ELLIS et al., 1989 Expression of engrailed proteins in arthro- pods, annelids, and chordates. Cell 5 8 955-968.
PELEGRI, F., and R. LEHMANN, 1994 A role of Polycomb group genes in the regulation of gap gene expression in Drosophila. Genetics
136: 1341-1353.
RILEY, P. D., S. B. CARROI.I. and M. P. SCOTT, 1987 The expression and regulation of Sex combs reduced protein in Drosophila embryos. Genes Dev. 1: 716-730.
SATO, T., M. A. RUSSELL and R. E. DENELL, 1983 Homeosis in Dro- sophila: a new enhancer of Polycomb and related homeotic mu- tations. Genetics 105: 357-370.
SATO, T., P. H. HAYES and R. E. DENELL, 1984 Homeosis in Drosoph- ila: maternal effect of the Enhancer of Polycomb locus and its interaction with Polycomb and related loci. Dev. Genet. 4: 185-
198.
SIMON, J., M. PEIFER, W. BENDER and M. O'CONNOR, 1990 Regula- tory elements of the bithorax complex that control expression along the anterior-posterior axis. EMBO J. 9: 3945-3956.
SIMON, J., A. CHIANG and W. BENDER, 1992 Ten different Polycomb group genes are required for spatial control of the abdA and
AbdB homeotic products. Development 114: 493-505.
SIMON, J., A. CHIANG, W. BENDER, M. J. SHIMELL and M. O'CONNOR,
1993 Elements of the Drosophila bithorax complex that mediate repression by Polycombgroup products. Dev. Biol. 158: 131-144.
SMOUSE, D., C. GOODMAN, A. MAHOWALD and N. PERRIMON, 1988
polyhomeotic: a gene required for the development of axon path- ways in the central nervous system of Drosophila. Genes Dev. 2
830-842.
STRUHL, G., 1981 A gene product required for correct initiation of segmental determination in Drosophila. Nature 293: 36-41.
STRUHL, G., 1983 Role of the esc' gene product in ensuring the selective expression of segment-specific homeotic genes in Dro- sophila. J. Embryol. Exp. Morphol. 76: 297-331.
STRUHL, G., and M. AKAM, 1985 Altered distributions of Ultrabithorax
J. 4: 3259-3264.
transcripts in extra sex combs mutant embryos of Drosophila. EMBO
STRUHL, G., and R. A. H. WHITE, 1985 Regulation of the Ultrabithorax gene of Drosophila by other bithorax complex genes. Cell 43:
507-519.
VAN LOHUIZEN, M., M. FRASCH, E. WIENTJENS and A. BERNS, 1991
Sequence similarity between the mammalian bmi-l proto-onco- gene and the Drosophila regulatory genes Psc and Su(z)2. Na- ture 353: 353-355.
WHITE, R. A. H., and M. WILCOX, 1985a Distribution of Ultrabithorax proteins in Drosophila. EMBO J. 4: 2035-2043.
WHITE, R. A. H., and M. WILCOX, 1985b Regulation of the distribu- tion of Ultrabithoraxproteins in Drosophila. Nature 318: 563-567.
WU, C.-T., 1984 A genetic analysis of transvection in Drosophila mela- nogmter. Ph.D. Thesis, Haward.
WU, C.-T., and M. HOW, 1995 A genetic analysis of the Suppressor
of zeste 2-complex of Drosophila melanogaster. Genetics 140: 139- 181.
ZINK, B., and R. PARO, 1989 In vivobhding pattern of a transregula- tor of homeotic genes in Drosophila melanogaster. Nature 337:
468-471.