Medicare Coverage Guidebook
Using this Guidebook
Clinical Laboratories are required by law to inform clients annually about Medicare compliance policies pertaining to the use of laboratory testing for Medicare beneficiaries. This booklet contains a summary of these policies, and the medical necessity requirements for laboratory tests that have National and/or Local Coverage limitations.
The responsibility for complying with Medicare when ordering laboratory testing lies with the ordering physician. The coding information contained in this booklet was obtained from the Centers for Medicare & Medicaid Services website, coverage index. This booklet should not be used as a sole or final reference or interpretation of Medicare law. For more complete information, please refer to a current CMS ICD-9-CM Coding Manual or the CMS website resources.
Resources
The CMS website:www.cms.gov/center/coverage.asp
The CMS Guide to Medicare Preventative Services:
www.cms.gov/MLNProducts/downloads/mps_guide_web-061305.pdf
The CMS Laboratory Fee Schedule:
www.cms.gov/ClinicalLabFeeSched
The CMS Medicare Coverage database LCD/NCD Index:
http://www.cms.gov/medicare-coverage-database/overview-and-quick-search.aspx
Medicare Fiscal Intermediary for EMH Reference Laboratory: National Government Services, Inc (00131, FI)
http://www.ngsmedicare.com/wps/portal/ngsmedicare The EMH Reference Laboratory website: www.emhreflab.org.
Table of Contents pg #
How to Use the Advanced Beneficiary Notice (ABN) 1
Price Estimates for Medicare Limited Tests on ABN 2
Laws and Regulations Governing Laboratories 4
Medicare Coverage for Laboratory Services 6
Medicare Coding, and Billing for Laboratory Services 7
Medicare Rules for Test Ordering 9
EMHRL Reflexed Tests 10
Acid Phosphatase LCD 11
Allergen Testing LCD 13
Alpha-fetoprotein (AFP) Tumor Marker NCD 15 Beta-Natriuretic Peptide (BNP) LCD 18 CA 125 Tumor Marker NCD 20 CA 15-3 / CA 27.29 Tumor Markers NCD 22 CA 19-9 Tumor Marker NCD 23 Carcinoembryonic Antigen (CEA) NCD 24 Cell Counts, Blood (CBC) NCD 28 Circulating Tumor Cell Assay (CTC) NON-COVERED TEST 41
Collagen Crosslinks (N-Telopeptide) NCD 42 Digoxin Therapeutic Drug Assay NCD 44
Drug Screen, Qualitative LCD 48
Fecal Occult Blood Test, Diagnostic NCD 54 Fecal Occult Blood Test, Colorectal Cancer Screening Prevention 61 Gamma Glutamyl Transferase (GGT) NCD 64
Galectin-3 NON-COVERED TEST 77 Glucose, Diagnostic NCD 78
Glucose, Diabetes Screening Prevention 95 Glycated Hemoglobin/Glycated Protein NCD 96 Hepatitis Panel/Acute Hepatitis Panel NCD 99 Human Chorionic Gonadotropin (hCG) NCD 103 Human Immunodeficiency Virus (HIV) Diagnostic NCD 106 Human Immunodeficiency Virus (HIV) Prognosis / Monitoring NCD 114 Human Immunodeficiency Virus (HIV) Screening Prevention 116 Initial Preventive Physical Examination (IPPE) Prevention 118
Iron Studies NCD 120
Table of Contents pg #
Lipids, Cardiovascular Screening Prevention 146
Pap Test, Diagnostic NCD/LCD 147
Pap Test, Screening Prevention 159
Prostate Specific Antigen (PSA), Diagnostic NCD 161
Prostate Specific Antigen (PSA) Cancer Screening Prevention 163
Prothrombin Time (PT) NCD 164
Partial Thromboplastin Time (PTT) NCD 182
Thyroid Testing NCD 194
Urine Culture, Bacterial NCD 202
Vitamin D Assay LCD 207
Medicare Preventative Services
Screening Tests
Cardiovascular
Screening page 146
Cervical
Cancer
Prevention
page 159
Colorectal
Cancer
Screening
page 61
Diabetes Screening
page 95
HIV Screening
page 116
Initial Preventive Physical Examination
page 118
How to Use Advance Beneficiary Notices (ABNs)
For all tests listed in this booklet, you must obtain a signed ABN from the patient
before ordering the test for payment by Medicare; these tests may not be
covered if medical necessity requirements are not met. The test(s) should not be
ordered if the beneficiary is unwilling to sign an ABN.
The ABN must contain the specific name of the test, and an estimate of the cost
(see following pages for estimated costs).
The beneficiary must be given a reason why Medicare may not cover the test, such
as frequency limitations or noncovered service.
The beneficiary must sign the ABN and receive a copy. A duplicate copy must
accompany the specimen and test order to the laboratory.
For more information about ABN’s, see page 6.
NEED AN ABN FORM?
EMH Reference Laboratory provides ABN forms to clients at no charge. ABN forms
are available in English and Spanish. To request ABN forms, you can go to
Medicare Limited Tests - Estimate of Costs if Not Covered
CPT TEST NAME $ Est. Bill*
84060 Acid Phosphatase, Total 86.00 84066 Acid Phosphatase, Prostatic 96.00 86003 Allergen Tests, specific IgE (per allergen) 59.00 82105 Alpha Fetal Protein (AFP),Tumor Marker 252.00 83880 Beta Natriuretic Peptide (BNP) 237.00 85027 Blood Count, CBC Only 104.00 85025 Blood Count, CBC/Diff 75.00 85014 Blood Count, Hemoglobin 36.00 85018 Blood Count, Hematocrit 40.00 85595 Blood Count, Platelets (automated) 56.00 85048 Blood Count, WBC (automated) 82.00 85007 Blood Count, manual Differential 42.00
86304 CA 125 243.00
86300 CA 15-3 (CA 27.29) 82.00
86301 CA 19-9 86.00
82378 CEA 272.00
82523 Collagen Crosslinks, NTX (urine) 216.00 82523 Collagen Crosslinks (N-Telopeptides), serum 198.00
80162 Digoxin (Lanoxin) 184.00
G0431 Drug Screen, Qualitative (per drug class) 23.00 80102 Drug Confirmation (per drug class) 56.00 82272 Fecal Occult Blood, guaiac diagnostic 89.00 82270 Fecal Occult Blood, guaiac screen 89.00 82977 Gamma Glutamyl Transferase (GGT) 75.00 82950 Glucose, 2-hour post 75gm 185.00 82951 Glucose Tolerance Test, 2hour 227.00 82947 Glucose, quantitative blood 44.00 83036 Glycated hemoglobin (HgbA1c) 68.00 82985 Glycated protein, fructosamine 54.00 84702 HCG (Pregnancy), serum quantitative 238.00 80074 Hepatitis Panel, Acute 569.00 86689 HIV-1+2 Antibody Confirmation Evaluation (Western Blot) 184.00 87389 HIV-1+2 Antibody Screen 125.00 87536 HIV-1, Quantitative by amplified probe 304.00 82728 Iron Studies, Ferritin 231.00 83540 Iron Studies, Total Iron 54.00
Medicare Limited Tests - Estimate of Costs if Not Covered
CPT TEST NAME $ Est. Bill*
83550 Iron studies, Binding Capacity 105.00 84466 Iron Studies, Transferrin 78.00
80061 Lipids, Lipid Panel 174.00
82465 Lipids, Total Cholesterol 39.00
84478 Lipids, Triglycerides 77.00
83721 Lipoprotein, direct LDL cholesterol 82.00 83718 Lipoprotein, HDL cholesterol 87.00 88142
P3000 G0123
Pap test, liquid specimen 158.00 88164
P3000 G0123
Pap test, conventional smear specimen 91.00 84153 Prostate Specific Antigen (PSA) Diagnostic 130.00 G0103 Prostate Specific Antigen (PSA) Screen 130.00 85610 PT/INR (Prothrombin time ) 89.00
85730 PTT (APTT) 81.00
84439 Thyroid testing, Free T4 137.00 84436 Thyroid testing, Total T4 94.00
84443 Thyroid testing, TSH 140.00
87086 Urine Culture 63.00
82306 Vitamin D, 25 Hydroxy 172.00
*Prices listed here are to be used to estimate costs and may not include all charges actually billed. Prices may change without notice.
The Social Security Act
Medicare and Medicaid laws, rules and regulations come under this act.
Anti-Kickback Laws
Federal and state law provides criminal penalties for individuals or entities that knowingly and willfully offer, pay, solicit or receive money or favors for referrals of tests or services that will be paid for by the Medicare or Medicaid programs. This prohibits laboratories from offering inducements to physicians in order to gain their business. To comply with the law, the following rules apply to the offering of laboratory services:
Supplies
Laboratories may only give supplies to a physician for the drawing, processing, storing or transporting of specimens to the laboratory, and cannot provide supplies for physicians to use for their own purposes. The laboratory must monitor the amount of supplies provided to ensure that it matches the number of tests
sent to the laboratory.
Discounts, Gifts or Billing Adjustments
The lab can give discounts, but the price must be above cost and at "fair market value." The lab cannot give excessive or expensive gifts or entertainment to physicians.
The laboratory may write off charges only when laboratory errors in billing or testing occur.
Phlebotomy Service
Laboratories may place phlebotomists or other employees in a client’s office only if all of the following conditions are met:
The laboratory employee may only perform laboratory related tasks.
There is a written understanding given to the physician about what the employee can and cannot do. Periodic audits are done to ensure the employee is following these policies.
Equipment
Likewise, laboratories may place printers, computers, fax machines or other equipment or products in client offices as long as they ensure that:
The physician understands that the equipment belongs to the laboratory.
The equipment is used for laboratory purposes, like receiving reports or ordering tests.
Periodic audits are done to ensure that the client is using the equipment only for laboratory related tasks.
Office Space
Laboratories may only lease space from physicians who refer Medicare patients to them under certain circumstances:
There must be a written lease for at least one year. Lease price must be at "fair market value."
Couriers
The laboratory's couriers may not transport items except those related to the testing services offered by the laboratory.
Laws and Regulations Governing Laboratories
Anti-Trust Laws
Federal laws prohibit unfair pricing practices.
Most laboratories have one fee schedule for customers that must be billed individually (patients, insurance, Medicare) and one for customers billed monthly on an invoice type of statement (client or doctor billing). The difference in price between the two schedules should be a reflection of the financial benefits of direct client billing.
Test prices should be determined by means of a financial analysis that include such factors as cost, market value and reasonable profit.
Contractually arranged pricing that results from negotiations with insurance and managed care companies should at least cover costs of testing.
Laboratories may not work together to fix or set prices in the market place.
False Claims Act
Provides criminal penalties for knowingly or willingly filing a false claim to a government program. ICD-9 codes can only be supplied by the ordering physician or a representative of that physician.
It is against the law for a laboratory to change or supply an ICD-9-CM code to a test order submitted by a physician.
Code steering means to steer or direct a physician to supply an ICD-9 code that is payable. Code Steering is illegal. The code must come from the patient's medical record.
Missing ICD-9 codes cannot be obtained by copying them from a previous laboratory order.
It is against the law to use the wrong ICD-9-CM code for the purpose of causing or increasing payment for a test.
Health Insurance Portability and Accountability Act (HIPAA)
HIPAA provides protection for the privacy of an individual’s health information.
When releasing test results by phone, fax and other non-routine methods, the laboratory may only release test results to physicians (or authorized representatives) who are involved in the patient’s care, or to a patient who is involved in their own medical treatment as directed by their physician.
HIPAA regulations prohibit facsimile transmission of confidential records without documented verification of the fax number transmitted from the authorized recipient’s fax machine.
For a copy of our Protected Health Information (PHI) policy, please call EMH Reference Laboratory at 866-941-4542.
Federal Self-Referral Laws (STARK)
STARK laws apply to financial relationships that have the potential to result in directed referrals to the individuals or entities involved.
Prohibits the referral of patients or tests between related entities unless certain conditions are met.
Stark safe harbors allow hospitals to support up to 85% of EMR startup and implementation costs, excluding physician office hardware.
Part B of title XVIII of the Social Security Act provides for Supplementary Medical Insurance for certain Medicare Beneficiaries, specifying what health care items or services will be covered by the Medicare Part B program. Diagnostic laboratory tests are generally covered by Part B under the following rules:
Medical Necessity
According to the statute, testing must be reasonable and necessary for the diagnosis or treatment of an illness or injury in order to be covered. Tests performed in the absence of signs, symptoms, complaints, or personal history of disease or injury are not covered except as explicitly authorized by statute. These include exams required by insurance companies, business establishments, government agencies, or other third parties.
National and Local Coverage Limitations
A National Coverage Determination (NCD) for a diagnostic laboratory test is a document stating CMS policy with respect to the circumstances under which the test will be considered reasonable and necessary for Medicare to cover it. Such a policy applies nationwide. It is neither a practice parameter nor a statement of the accepted standard of medical practice. Claims for tests for which there is a national coverage policy will be denied if submitted without an ICD-9-CM code or narrative diagnosis listed as covered in the policy, unless
documentation justifying the necessity is submitted with the claim.
Local Medicare contractors are authorized by CMS to develop coverage policies for laboratory tests as
necessary for their respective regions. These policies are referred to as Local Coverage Determinations (LCD). Claims for a test for which an LCD exists that does not fulfill the coverage requirements described may be denied. Denied claims may be given individual consideration based on a review of all pertinent medical information.
Local contractors may also develop an LCD to clarify or supplement, but not conflict with, and NCD. If a national or local policy identifies a frequency expectation, a claim for a test that exceeds that expectation may be denied as not reasonable and necessary, unless it is submitted with documentation justifying increased frequency.
Advanced Beneficiary Notice (ABN)
A test may be considered medically appropriate, but nonetheless be excluded from Medicare coverage by statute. The provider must notify the beneficiary in writing if the provider is aware that Medicare may not cover the test, item or procedure. All the tests included in this booklet have limited coverage based on an existing NCD, LCD, or both. If the patient’s diagnosis is not supported by the ICD-9-CM codes listed, or if frequency limitations are exceeded, an ABN must be signed before ordering the test (see page 1). The test order should not be placed if the patient is unwilling to sign the ABN. However, if the physician feels obligated to order the test, the ABN may be submitted by a third party witness, documenting the beneficiary’s refusal to sign the ABN.
Required Documentation
Failure to provide documentation of the medical necessity of tests may result in denial of claims. The patient’s medical record must contain documentation that fully supports the medical necessity for the test as Medicare covers it. This documentation includes, but is not limited to, relevant medical history, physical examination, results of pertinent diagnostic tests or procedures, and signed copies of any Advanced Beneficiary Notices. In addition, failure to provide independent verification that the test was ordered by the treating physician (or qualified nonphysical practitioner) through documentation in the physician’s office may result in denial.
Qualified Practitioners
Tests that are not ordered by a treating physician or other qualified treating non-physician practitioner acting within the scope of their license and in compliance with Medicare requirements will be denied as not reasonable and necessary. A “treating” physician or practitioner is defined as someone who is fully knowledgeable about the beneficiary’s medical condition, and who would be responsible for using the results of any examination performed in the overall management of the beneficiary’s specific medical problem.
Qualified Laboratories
Failure of the laboratory performing the test to have the appropriate Clinical Laboratory Improvement Amendments of 1988 (CLIA) certificate for the testing performed will result in denial of claims.
Medicare Coding and Billing for Laboratory Services
HCPCS and CPT Codes
CPT (Current Procedural Terminology) codes are used to describe specific tests or services. The amount of payment for a test is dependent on the CPT code. It is against the law to use the wrong CPT code for a test for the purpose of causing or increasing payment for a test.
CPT or HCPCS (HICFA Common Procedure Coding System) descriptors are used in this booklet. CPT codes and their descriptors are developed and copyrighted by the American Medical Association (AMA). If a
descriptor does not accurately or fully describe the test, a more complete description may be included elsewhere in the policy, such as in the Indications section.
ICD-9-CM Codes
ICD-9-CM (International Classification of Disease, 9th Edition, Clinical Modification) codes are used to classify diseases and conditions, and describe signs, symptoms and medical circumstances. ICD-9-CM codes are submitted to indicate the medical necessity of a particular test, and determine when coverage is allowed.
The correct use of an ICD-9-CM code does not assure coverage of a service. The service must be reasonable and necessary in the specific case and must meet the criteria specified in coverage determinations.
For all tests listed in this booklet, an appropriate ICD-9-CM code (or equivalent verbiage) must be given to the laboratory at the time it is ordered. The Balanced Budget Act of 1997 made it illegal for physicians to
order limited coverage tests without supplying an ICD-9-CM code with the order.
It is the responsibility of the provider to code to the highest level specified in the 2012 ICD-9-CM Coding Manual (e.g., to the fourth or fifth digit). A three-digit ICD-9-CM code is to be used only if it is not further subdivided. Where fourth-digit and/or fifth-digit sub-classifications are provided, they must be assigned. A code is invalid if it has not been coded to the full number of digits required for that code.
Diagnostic vs. Screening Tests
Screening is the testing for disease or disease precursors so that early detection and treatment can be provided
for those who test positive for the disease. Screeningtests are performed when no specific sign, symptom or diagnosis is present and the patient has not been exposed to a disease. Screening tests are not covered by Medicare except those provided under specific statutes.
Diagnostic tests are performed to rule out or confirm a suspected diagnosis when a patient has signs and/or
symptoms related to the suspected diagnosis.
Unconfirmed or Underlying Conditions
Codes that describe symptoms and signs, as opposed to diagnosis, should be provided for reporting purposes when the physician has not established a diagnosis. Diagnoses documented as “probable”, “suspected,” “questionable,” “rule-out,” or “working diagnosis” should not be coded as though they exist. Rather, code the condition(s) to the highest degree of certainty for that encounter/visit, such as signs, symptoms, abnormal test results, or other reasons for the visit. The ICD-9 code submitted for the underlying sign, symptom, or condition must be related to the indications for the test.
When the reason for performing the test is because the patient has been exposed to a communicable disease, the appropriate code from category V01 “Contact with or exposure to communicable diseases,” should be assigned, not a screening code; however, the test may still be considered screening and not covered by Medicare.
A diagnostic statement that is listed as a manifestation of an underlying condition in ICD-9-CM must be expanded to include the underlying disease in order to accurately code the condition.
Elmhurst Memorial Reference Laboratory - Medicare Billing
Billing department employees must ensure that complete records and documentation exist for all Medicare billing transactions. It is unlawful for anyone other than the licensed ordering physician to change or add any information on a physician’s signed order for medical services.
When a client assigns Medicare billing to EMRL, tests will be billed using the CPT codes given in the Elmhurst Memorial Reference Laboratory electronic test menu (www.emhreflab.org) or the Client User’s Manual. When the licensed ordering physician provides all required information on the service requisition at the time of service, Elmhurst Memorial Reference Laboratory will accept assignment for laboratory services rendered to your patients with Medicare insurance, and bill the appropriate contractor. Payment received from this contractor will be accepted as payment in full for the laboratory services billed.
The required information to be provided with the test order includes:
1.
Patient name, date of birth, and complete address
2.Medicare/Medicaid number (or a copy of card)
3.Primary or Secondary Payer information
4.Diagnosis (ICD-9 Codes)
5.
Physicians name, signature, UPIN, and NPI# (if not on file with us)
6.Copy of ABN (if indicated for the test ordered)
Top Five Reasons for Denial of Claims
1. Diagnosis does not support medical necessity or a covered service 2. Service may be covered by a Primary or Secondary Payer
3. Duplicate claims
4. Patient not identified as a Medicare recipient
5. Expenses were incurred after coverage was terminated
Top Tests Denied Payment due to Inappropriate ICD-9-CM Codes
1. Vitamin D, 25 hydroxy2. Lipid Panel
3. Prothrombin Time (PT)
Medicare Rules for Test Ordering
Date of Service
During the clinical diagnostic laboratory services negotiated rulemaking, CMS learned that there was
considerable variability regarding the date of service on laboratory claims. In order to promote uniformity, the committee recommended a national policy related to the date of service on laboratory claims. CMS published the rule final on November 23, 2001 (66 FR 58788). The final rule states:
The date of service for laboratory tests that is reported on the claim is to be the date the tested specimen was collected; and
The person obtaining the specimen must furnish the date of collection of the specimen to the entity billing Medicare.
Physicians or their staff who draw specimens for testing must report the date of collection of the specimen on
orders for laboratory tests. Laboratories may refuse to perform tests on orders for laboratory tests that do not include the information they need in order to seek payment for services performed, i.e., the date of collection of the specimen.
Ambiguous or Unclear Test Orders
By law, the laboratory cannot perform and bill for tests that are not specifically ordered by the treating physician. When the orders for a test are not absolutely clear, laboratory personnel may not use their own judgment or information supplied by the patient to change or clarify the test order; they must contact the
ordering physician.
Custom Panels
A custom panel is a test grouping that is created by the physician for the physician’s ordering convenience. This does not include “common practice” panels.
Our requisitions are designed so that all tests included in custom panels are listed, to facilitate individual test ordering if necessary. However, if you use a paper requisition containing a custom panel that does not indicate individual test components due to space limitations, Medicare requires that you sign a “Physician
Acknowledgement” form annually, indicating that you are aware of any reimbursement limitations your profile or reflex test may have for beneficiaries. Physicians may choose not to use their custom panel for Medicare patients, but instead order individual tests when clinically indicated. If the physician feels that the patient will benefit from tests not covered by Medicare, the patient must be asked to sign an Advance Beneficiary Notice (ABN), advising them that they will be financially responsible for services not covered by Medicare.
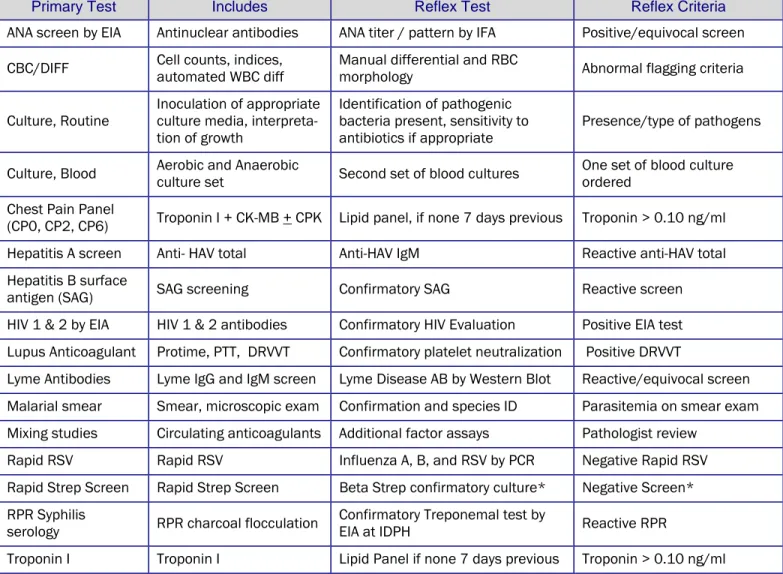
Reflexed Tests
A reflexed test is a secondary test performed after an initial test result is outside of established parameters. These secondary “reflex” tests further enhance the clinical picture or confirm the initial results, facilitating patient care. Types of reflex testing;
Automatic Reflex –Considered common laboratory practice, the secondary test is performed and billed automatically, if appropriate,without additional orders from a physician (Table 1).
Ordered Reflex –The secondary test is not performed automatically. The physician must choose the
“reflex” alternative of the primary test on the test order, or specify the secondary test and reflex criteria on the test order (Table 2). A physician acknowledgement is not needed.
Custom Reflex -Reflex tests not available as described above may be created for the individual physician upon request. A physician acknowledgement form must be signed annually in order for the physician to use a custom reflex test for Medicare patients.
Primary Test Includes Reflex Test Reflex Criteria
ANA screen by EIA Antinuclear antibodies ANA titer / pattern by IFA Positive/equivocal screen CBC/DIFF Cell counts, indices, automated WBC diff Manual differential and RBC morphology Abnormal flagging criteria Culture, Routine Inoculation of appropriate culture media,
interpreta-tion of growth
Identification of pathogenic bacteria present, sensitivity to
antibiotics if appropriate Presence/type of pathogens Culture, Blood Aerobic and Anaerobic culture set Second set of blood cultures One set of blood culture ordered Chest Pain Panel
(CP0, CP2, CP6) Troponin I + CK-MB + CPK Lipid panel, if none 7 days previous Troponin > 0.10 ng/ml Hepatitis A screen Anti- HAV total Anti-HAV IgM Reactive anti-HAV total Hepatitis B surface
antigen (SAG) SAG screening Confirmatory SAG Reactive screen HIV 1 & 2 by EIA HIV 1 & 2 antibodies Confirmatory HIV Evaluation Positive EIA test Lupus Anticoagulant Protime, PTT, DRVVT Confirmatory platelet neutralization Positive DRVVT
Lyme Antibodies Lyme IgG and IgM screen Lyme Disease AB by Western Blot Reactive/equivocal screen Malarial smear Smear, microscopic exam Confirmation and species ID Parasitemia on smear exam Mixing studies Circulating anticoagulants Additional factor assays Pathologist review
Rapid RSV Rapid RSV Influenza A, B, and RSV by PCR Negative Rapid RSV Rapid Strep Screen Rapid Strep Screen Beta Strep confirmatory culture* Negative Screen* RPR Syphilis
serology RPR charcoal flocculation Confirmatory Treponemal test by EIA at IDPH Reactive RPR
Troponin I Troponin I Lipid Panel if none 7 days previous Troponin > 0.10 ng/ml *Only on children under 18 yrs old
Table 2. Ordered Reflex Testing (Physician must indicate Reflex Test when ordering)
Primary Test Includes Secondary Test Reflex Criteria
ANA Reflex Antinuclear antibodies, titer and pattern anti ENA, anti-DNA, anti-SSA, anti-SSB Positive ANA screen PAP w HPV Reflex Liquid-based Pap Test High-Risk HPV DNA testing PAP interpretation = ASCUS PSA Free Reflex Total PSA % Free PSA Total PSA = 4-10 ng/ml. Thyroid Function TSH T4, possible T3 (per algorithm) TSH is abnormal Serum Protein
Electrophoresis Reflex
Total protein, albumin, alpha, beta & gamma
globulin, A/G ratio Immunofixation Abnormal band on SPE UA Dip Reflex Urine dipstick chemical analysis Microscopic analysis of urine Abnormal dipstick results Urine Drug Screen
ACID PHOSPHATASE
Acid Phosphatase
Local Coverage Determination, National Government Services, Inc. (00131, FI)
Acid phosphatase is present in highest concentrations in the prostate and in metastases to bone. It has also been detected in tissue of heart, muscle, liver, testicles, spleen, skin, and hemopoietic cells. The enzyme hydrolyzes esters to inorganic phosphate when at an acid pH and can be measured by enzymatic analysis (Roy method) or immunoassay technique. Prostatic acid phosphatase increases with advanced prostate cancer and is consistent with extracapsular disease or metastases. Acid phosphatase has also been clinically relevant in the diagnosis and follow-up of patients with Gaucher’s disease.
Indications
The prostatic acid phosphatase is mostly a tartrate sensitive isomer, whereas the isomer associated with Gaucher's disease and other entities is tartrate resistant. In patients suspected of having Gaucher's disease the correct test to perform is total acid phosphatase.
Since the introduction of prostate specific antigen (PSA), the use of prostatic acid phosphatase has declined and is no longer routinely used for screening or staging of prostate cancer as it seldom provides additional useful
information. The American Urological Association states that PSA is the best predictor of skeletal metastases found on radionuclide bone scan. Additionally, the standard for defining response to drugs in clinical trials is the change in PSA. New biochemical markers (e.g., IL-6, TGF-β1) are being investigated for the staging of prostate cancer.
The clinical accuracy of prostatic acid phosphatase assay is problematic. The assay is not organ specific, and levels measured are influenced by diurnal fluctuations, prostate examinations prior to blood sampling, and enzyme instability (due to pH, temperature and time since blood-drawing) if not handled properly prior to testing. Furthermore, elevated values of radioimmunoassays may not be as interpretable as results when the test is
performed by the Roy enzymatic test.
Limitations of Coverage
Prostatic acid phosphatase (CPT code 84066) is not covered for any indication, and will be denied as not
medically necessary for all diagnosis including Gaucher’s disease and osteoporosis. Total acid phosphatase (CPT code 84060) will be denied as not medically necessary for a diagnosis of prostate disease or osteoporosis.
Total acid phosphatase (84060) will be covered for the ICD-9-CM codes listed below.
Covered Tests
CPT/HCPCS Codes Descriptor
84060 Phosphatase, Acid Total
Covered Diagnosis Codes for CPT 84060
Phosphatase, Acid Total ICD-9-CM codes
198.5 Secondary malignant neoplasm of bone and bone marrow
205.00 Acute myeloid leukemia, without mention of having achieved remission 205.01 Myeloid leukemia acute in remission
205.02 Acute myeloid leukemia, in relapse
205.10 Chronic myeloid leukemia, without mention of having achieved remission 205.11 Myeloid leukemia chronic in remission
205.12 Chronic myeloid leukemia, in relapse
205.20 Subacute myeloid leukemia, without mention of having achieved remission 205.21 Myeloid leukemia subacute in remission
Phosphatase, Acid Total ICD-9-CM codes
205.22 Subacute myeloid leukemia, in relapse
205.30 Myeloid sarcoma, without mention of having achieved remission 205.31 Myeloid sarcoma in remission
205.32 Myeloid sarcoma, in relapse
205.80 Other myeloid leukemia, without mention of having achieved remission 205.81 Other myeloid leukemia in remission
205.82 Other myeloid leukemia, in relapse
205.90 Unspecified myeloid leukemia, without mention of having achieved remission 205.91 Unspecified myeloid leukemia in remission
205.92 Unspecified myeloid leukemia, in relapse 252.00 Hyperparathyroidism, unspecified 252.01 Primary hyperparathyroidism 252.02 Secondary hyperparathyroidism, non-renal 252.08 Other hyperparathyroidism 272.7 Lipidoses
ALLERGEN TESTING
Allergen Testing
Local Coverage Determination, National Government Services, Inc. (00131,FI)
Radioallergosorbent test (RAST), fluoroallergosorbent test (FAST), and multiple antigen simultaneous tests are in vitro techniques for determining whether a patient's serum contains IgE antibodies against specific allergens of clinical importance. As with any allergy testing, the need for such tests is based on the findings during a complete history and physical examination of the patient.
The multiple antigen simultaneous testing technique is similar to the RAST/FAST techniques in that it depends upon the existence of allergic antibodies in the blood of the patient being tested. With the multiple antigen simultaneous test system, several antigens may be used to test for specific IgE simultaneously.
ELISA (enzyme-linked immunosorbent assay) is another in vitro method of allergy testing for specific IgE antibodies against allergens. This method is also a variation of RAST.
Limitations
It is expected that these services would be performed as indicated by current medical literature and/or standards of practice. When services are performed in excess of established parameters, they may be subject to review for medical necessity.
The following tests are considered to be NOT medically necessary and will be denied. ELISA/Act qualitative antibody testing
This testing is used to determine in vitro reaction to various foods and relies on lymphocyte blastogenesis in response to certain food antigens.
LMRA (Lymphocyte Mitogen Response Assays) by ELISA/Act IgG ELISA, indirect method (CPT code 86001)
Qualitative multi-allergen screen (CPT code 86005)
This is a non-specific test that does not identify a specific antigen.
IgG and IgG subclass antibody tests for food allergy do not have clinical relevance, are not validated, lack sufficient quality control, and should not be performed.
Covered Tests
CPT/HCPCS Codes Descriptor
86003 Allergen specific IGE; quantitative or semi-quantitative, each allergen
Covered Diagnosis Codes
Allergen Tests ICD-9 Codes Covered
The following ICD-9 Codes apply only to CPT code 86003: 477.0 Allergic rhinitis due to pollen
477.1 Allergic rhinitis due to food
477.2 Allergic rhinitis, due to animal (cat) (dog) hair and dander 477.8 Allergic rhinitis due to other allergen
477.9 Allergic rhinitis cause unspecified 493.00 Extrinsic asthma unspecified
493.01 Extrinsic asthma with status asthmaticus 493.02 Extrinsic asthma with (acute) exacerbation 493.82 Cough variant asthma
Allergen Tests ICD-9 Codes Covered
493.91 Asthma unspecified type with status asthmaticus 493.92 Asthma unspecified with (acute) exacerbation 691.8 Other atopic dermatitis and related conditions 708.0 Allergic urticaria
708.8 Other specified urticaria 708.9 Unspecified urticaria 786.07 Wheezing
989.5* Toxic effect of venom 995.0 Other anaphylactic reaction
995.1 Angioneurotic edema not elsewhere classified
995.20 Unspecified adverse effect of unspecified drug, medicinal and biological substance 995.22 Unspecified adverse effect of anesthesia
995.27 Other drug allergy
995.29 Unspecified adverse effect of other drug, medicinal and biological substance 995.3 Allergy unspecified not elsewhere classified
995.60 Anaphylactic reaction due to unspecified food 995.61 Anaphylactic reaction due to peanuts
995.62 Anaphylactic reaction due to crustaceans
995.63 Anaphylactic reaction due to fruits and vegetables 995.64 Anaphylactic reaction due to tree nuts and seeds 995.65 Anaphylactic reaction due to fish
995.66 Anaphylactic reaction due to food additives 995.67 Anaphylactic reaction due to milk products 995.68 Anaphylactic reaction due to eggs
995.69 Anaphylactic reaction due to other specified food
V15.09 Personal history of other allergy other than to medicinal agents *ICD-9-CM code 989.5 should be reported for venom hypersensitivity.
ALPHA FETOPROTEIN TUMOR MARKER
Alpha Fetoprotein, Tumor Marker
National Coverage Determination, Center for Medicare & Medicaid Services
Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain malignancies to therapy.
Indications
AFP is useful for the diagnosis of hepatocellular carcinoma in high-risk patients (such as alcoholic cirrhosis, cirrhosis of viral etiology, hemochromatosis, and alpha 1-antitrypsin deficiency) and in separating patients with benign hepatocellular neoplasms or metastases from those with hepatocellular carcinoma and, as a non-specific tumor associated antigen, serves in marking germ cell neoplasms of the testis, ovary, retro peritoneum, and mediastinum.
Covered Tests
CPT/HCPCS Codes Descriptor
82105 Alpha-Fetoprotein; Serum
Covered Diagnosis Codes
Alpha-fetoprotein, Serum ICD-9 Codes Covered
070.22 Chronic viral hepatitis B with hepatic coma without hepatitis delta 070.23 Chronic viral hepatitis B with hepatic coma with hepatitis delta 070.32 Chronic viral hepatitis B without hepatic coma without hepatitis delta 070.33 Chronic viral hepatitis B without hepatic coma with hepatitis delta 070.44 Chronic hepatitis C with hepatic coma
070.54 Chronic hepatitis C without hepatic coma 095.3 Syphilis of liver
121.1 Clonorchiasis 121.3 Fascioliasis
155.0 Malignant neoplasm of liver primary
155.1 Malignant neoplasm of intrahepatic bile ducts
155.2 Malignant neoplasm of liver not specified as primary or secondary 164.2 Malignant neoplasm of anterior mediastinum
164.3 Malignant neoplasm of posterior mediastinum 164.8 Malignant neoplasm of other parts of mediastinum 164.9 Malignant neoplasm of mediastinum part unspecified
165.0 Malignant neoplasm of upper respiratory tract part unspecified
165.8 Malignant neoplasm of other sites within the respiratory system and intrathoracic organs 165.9 Malignant neoplasm of ill-defined sites within the respiratory system
183.0 Malignant neoplasm of ovary
186.0 Malignant neoplasm of undescended testis
186.9 Malignant neoplasm of other and unspecified testis 197.1 Secondary malignant neoplasm of mediastinum
Alpha-fetoprotein, Serum ICD-9 Codes Covered 197.7 Secondary malignant neoplasm of liver 198.6 Secondary malignant neoplasm of ovary 198.82 Secondary malignant neoplasm, genital organs
209.20-209.29 Malignant carcinoid tumors of other and unspecified sites 209.70 Secondary neuroendocrine tumor, unspecified site 209.71 Secondary neuroendocrine tumor of distant lymph nodes 209.72 Secondary neuroendocrine tumor of liver
209.73 Secondary neuroendocrine tumor of bone 209.74 Secondary neuroendocrine tumor of peritoneum 209.75 Secondary Merkel cell carcinoma
209.79 Secondary neuroendocrine tumor of other sites 211.5 Benign neoplasm of liver and biliary passages
235.3 Neoplasm of uncertain behavior of liver and biliary passages 272.2 Mixed hyperlipidemia
273.4 Alpha-1-antitrypsin deficiency 275.01 Hereditary hemochromatosis
275.02 Hemochromatosis due to repeated red blood cell transfusions 275.03 Other hemochromatosis
275.09 Other disorders of iron metabolism 275.1 Disorders of copper metabolism 277.00 Cystic fibrosis without meconium ileus
277.03 Cystic fibrosis with gastrointestinal manifestations 277.6 Other deficiencies of circulating enzymes
285.0 Sideroblastic anemia 338.3 Neoplasm related pain, acute or chronic
414.4 Coronary atherosclerosis due to calcified coronary lesion 444.01 Saddle embolus of abdominal aorta
444.09 Other arterial embolism and thrombosis of abdominal aorta 571.2 Alcoholic cirrhosis of liver
571.40 Chronic hepatitis unspecified 571.41 Chronic persistent hepatitis 571.42 Autoimmune hepatitis 571.49 Other chronic hepatitis 571.5 Cirrhosis of liver without alcohol
573.5 Hepatopulmonary syndrome 608.89 Other specified disorders of male genital organs 793.11 Solitary pulmonary nodule
ALPHA FETOPROTEIN TUMOR MARKER Alpha-fetoprotein, Serum ICD-9 Codes Covered
793.19 Other nonspecific abnormal finding of lung field
793.2 Nonspecific abnormal findings of other intrathoracic organs 793.3 Nonspecific abnormal findings of biliary tract
793.6 Nonspecific abnormal findings of abdominal area including retroperitoneum 795.89 Other abnormal tumor markers
V10.07 Personal history of malignant neoplasm of liver V10.43 Personal history of malignant neoplasm of ovary V10.47 Personal history of malignant neoplasm of testis V86.0 Estrogen receptor positive status [ER+]
Beta-Natriuretic Peptide (BNP)
Local Coverage Determination, National Government Services, Inc. (00131, FI)
B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It is secreted in response to ventricular volume expansion and pressure overload, factors often found in congestive heart failure (CHF). Used in conjunction with other clinical information, rapid measurement of BNP is useful in establishing or excluding the diagnosis and assessing the severity of CHF in patients with acute dyspnea so that appropriate and timely treatment can be initiated. This test is also used to predict the long-term risk of cardiac events or death across the spectrum of acute coronary syndromes when measured in the first few days after an acute coronary event. For the purposes of this policy, either total or N-terminal assays are acceptable.
Indications
BNP measurements may be considered reasonable and necessary when used in combination with other medical data such as medical history, physical examination, laboratory studies, chest x-ray, and electrocardiography:
To distinguish cardiac cause of acute dyspnea from pulmonary or other non-cardiac causes. Plasma BNP
levels are significantly increased in patients with CHF presenting with acute dyspnea compared with patients presenting with acute dyspnea due to other causes.
To distinguish decompensated CHF from exacerbated chronic obstructive pulmonary disease (COPD) in a symptomatic patient with combined chronic CHF and COPD. Plasma BNP levels are significantly increased in patients with CHF with or without concurrent lung disease compared with patients primary lung disease.
Limitations
BNP measurements must be analyzed in conjunction with standard diagnostic tests, the medical history and clinical findings. The efficacy of BNPmeasurement as a stand-alone test has not yet been established. Clinicians should be aware that certain conditions such as ischemia, infarction and renal insufficiency, may cause elevation of circulating BNP concentration and require alterations of the interpretation of BNP results.
Additional investigation is required to further define the diagnostic value of plasma BNP in monitoring the efficiency of treatment for CHF and in tailoring the therapy for heart failure. Therefore, BNP measurements for monitoring and management of CHF are not a covered service.
Although a correlation between serum BNPlevels and the clinical severity of HF has been shown in broad populations, “it cannot be assumed that BNP levels can be used effectively as targets for adjustment of therapy in individual patients… BNPmeasurement has not been clearly shown to supplement careful clinical assessment.” (Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A Report of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines, pgs. 14-15) . The 2009 Guidelines stated, “The value of serial measurements of BNP to guide therapy for patient with HF is not well established.
Covered Tests
CPT/HCPCS Codes Descriptor
83880 Natriuretic Peptide
Covered Diagnosis Codes
When billed in either an office or outpatient setting. CMS does not support medical necessity for BNP in hospital settings Natriuretic Peptide ICD-9 Codes Covered
402.01 Malignant hypertensive heart disease with heart failure 402.11 Benign hypertensive heart disease with heart failure 402.91 Unspecified hypertensive heart disease with heart failure
BETA-NATRIURETIC PEPTIDE Natriuretic Peptide ICD-9 Codes Covered
404.03 Hypertensive heart and chronic kidney disease, malignant, with heart failure and with chronic kidney disease stage V or end stage renal disease 404.11 Hypertensive heart and chronic kidney disease, benign, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified 404.13 Hypertensive heart and chronic kidney disease, benign, with heart failure and chronic kidney disease stage V or end stage renal disease 404.91 Hypertensive heart and chronic kidney disease, unspecified, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified 404.93 Hypertensive heart and chronic kidney disease, unspecified, with heart failure and chronic kidney disease stage V or end stage renal disease 428.0 Congestive heart failure unspecified
428.1 Left heart failure
428.20 Unspecified systolic heart failure 428.21 Acute systolic heart failure 428.22 Chronic systolic heart failure
428.23 Acute or chronic systolic heart failure 428.30 Unspecified diastolic heart failure 428.31 Acute diastolic heart failure 428.32 Chronic diastolic heart failure
428.33 Acute or chronic diastolic heart failure
428.40 Unspecified combined systolic and diastolic heart failure 428.41 Acute combined systolic and diastolic heart failure 428.42 Chronic combined systolic and diastolic heart failure
428.43 Acute or chronic combined systolic and diastolic heart failure 428.9 Heart failure unspecified
491.21 Obstructive chronic bronchitis with (acute) exacerbation 491.22 Obstructive chronic bronchitis with acute bronchitis 493.22 Chronic obstructive asthma with (acute) exacerbation 493.92 Asthma unspecified with (acute) exacerbation 519.11 Acute bronchospasm
786.00 Respiratory abnormality unspecified 786.02 Orthopnea
786.05 Shortness of breath 786.06 Tachypnea 786.07 Wheezing
CA 125
National Coverage Determination, Center for Medicare & Medicaid Services
Immunoassay determinations of the serum levels of certain proteins or carbohydrates, such as CA 125, serve as tumor markers. When elevated, serum concentration of these markers may reflect tumor size and grade.
Indications
CA 125 is a high molecular weight serum tumor marker elevated in 80% of patients who present with epithelial ovarian carcinoma. It is also elevated in carcinomas of the fallopian tube, endometrium, and endocervix. An elevated level may also be associated with the presence of a malignant mesothelioma or primaryperitoneal carcinoma.
A CA 125 level may be obtained as part of the initial pre-operative work-up for women presenting with a suspicious pelvic mass to be used as a baseline for purposes of post-operative monitoring. Initial declines in CA125 after initial surgery and/or chemotherapy for ovarian carcinoma are also measured by obtaining three serum levels during the first month post treatment to determine the patient's CA-125 half-life, which has significant prognostic implications. CA 125 levels are again obtained at the completion of chemotherapy as an index of residual disease. Surveillance measurements are generally obtained every 3 months for 2 years, every 6 months for the next 3 years, and yearly thereafter.
CA 125 levels are also an important indicator of a patient's response to therapy in the presence of advanced or recurrent disease. In this setting, CA 125 levels may be obtained prior to each treatment cycle.
Limitations
These services are not covered for the evaluation of patients with signs or symptoms suggestive of malignancy. The service may be ordered at times necessary to assess either the presence of recurrent disease or the patient's response to treatment with subsequent treatment cycles.
CA 125 is specifically not covered for aiding in the differential diagnosis of patients with a pelvic mass as the sensitivity and specificity of the test is not sufficient. In general, a single "tumor marker" will suffice in following a patient with one of these malignancies.
Covered Tests
CPT/HCPCS Codes Descriptor
86304 Immunoassay For Tumor Antigen, Quantitative; CA 125
Covered Diagnosis Codes
Immunoassay for Tumor Antigen CA- 125 ICD-9 Codes Covered 158.8 Malignant neoplasm, specified parts of peritoneum
158.9 Malignant neoplasm, peritoneum, unspecified 180.0 Malignant neoplasm, endocervix
182.0 Malignant neoplasm of corpus uteri, except isthmus 183.0 Malignant neoplasm, ovary
183.2 Malignant neoplasm, fallopian tube
183.8 Malignant neoplasm, other specified sites of uterine adnexa 184.8 Malignant neoplasm, other specified sites of female genital organs 198.6 Secondary malignant neoplasm, ovary
198.82 Secondary malignancy of genital organs 236.0 Neoplasm of uncertain behavior of uterus 236.1 Neoplasm of uncertain behavior of placenta
CA 125 TUMOR ANTIGEN ASSAY Immunoassay for Tumor Antigen CA- 125 ICD-9 Codes Covered
236.2 Neoplasm of uncertain behavior of ovary
236.3 Neoplasm of uncertain behavior of other and unspecified female genital organs 338.3 Neoplasm related pain, acute or chronic
789.39 Abdominal or pelvic swelling, mass or lump of other specified site 795.82 Elevated cancer antigen 125 [CA 125]
795.89 Other abnormal tumor markers
V10.41 Personal history of malignant neoplasm, cervix uteri
V10.42 Personal history of malignant neoplasm, other parts of the uterus V10.43 Personal history of malignant neoplasm of ovary
CA 15-3 / CA 27.29
National Coverage Determination, Center for Medicare & Medicaid Services
Immunoassay determinations of the serum levels of certain proteins or carbohydrates serve as tumor markers. When elevated, serum concentration of these markers may reflect tumor size and grade.
Indications
Multiple tumor markers are available for monitoring the response of certain malignancies to therapy and assessing whether residual tumor exists post-surgical therapy.
CA 15-3 is often medically necessary to aid in the management of patients with breast cancer. Serial testing must be used in conjunction with other clinical methods for monitoring breast cancer. For monitoring, if medically necessary, use consistently either CA 15-3 or CA 27.29, not both.
CA 27.29 is equivalent to CA 15-3 in its usage in management of patients with breast cancer.
Limitations
These services are not covered for the evaluation of patients with signs or symptoms suggestive of malignancy. The service may be ordered at times necessary to assess either the presence of recurrent disease or the patient's response to treatment with subsequent treatment cycles.
Covered Tests
CPT/HCPCS Codes Descriptor
86300 Immunoassay for Tumor Antigen, Quantitative; CA 15.3 (27.29)
Covered Diagnosis Codes
Immunoassay for Tumor AntigenCA-15.3 / CA-27.29 ICD-9 Codes Covered 174.0-174.9 Breast, primary (female) - malignant neoplasm of female breast 175.0-175.9 Breast, primary (male) - malignant neoplasm of male breast 198.2 Secondary malignant neoplasm (breast)
198.81 Secondary malignant neoplasm (breast) 338.3 Neoplasm related pain, acute or chronic 795.89 Other abnormal tumor markers
CA 19.9 TUMOR ANTIGEN ASSAY
CA 19-9
National Coverage Determination, Center for Medicare & Medicaid Services
Immunoassay determinations of the serum levels of certain proteins or carbohydrates serve as tumor markers. When elevated, serum concentration of these markers may reflect tumor size and grade.
Indications
Multiple tumor markers are available for monitoring the response of certain malignancies to therapy and assessing whether residual tumor exists post-surgical therapy.
Levels are useful in following the course of patients with established diagnosis of pancreatic and biliary ductal carcinoma. The test is not indicated for diagnosing these two diseases.
Limitations
These services are not covered for the evaluation of patients with signs or symptoms suggestive of malignancy. The service may be ordered at times necessary to assess either the presence of recurrent disease or the patient's response to treatment with subsequent treatment cycles.
Covered Tests
CPT/HCPCS Codes Descriptor
86301 Immunoassay For Tumor Antigen, Quantitative; CA 19-9
Covered Diagnosis Codes
Immunoassay for Tumor Antigen CA-19.9 ICD-9 Codes Covered 155.1 Malignant neoplasm of intrahepatic bile ducts
156.0 Malignant neoplasm of the gallbladder 156.1 Malignant neoplasm of extrahepatic bile ducts 156.2 Malignant neoplasm of the Ampulla of Vater
156.8 Malignant neoplasm of other specified sites of gallbladder and extrahepatic bile ducts 156.9 Malignant neoplasm of biliary tract part unspecified site
157.0 - 157.9 Malignant neoplasm, pancreas
197.8 Secondary malignant neoplasm of other digestive organs and spleen 235.3 Neoplasm of uncertain behavior of liver and biliary passages
235.5 Neoplasm of uncertain behavior of other and unspecified digestive organs 338.3 Neoplasm related pain, acute or chronic
795.89 Other abnormal tumor markers V10.09 Other personal history of cancer
Carcinoembryonic Antigen (CEA)
National Coverage Determination, Center for Medicare & Medicaid Services
Carcinoembryonic antigen is a protein polysaccaride found in some carcinomas. It is effective as a biochemical marker for monitoring the response to therapy.
Indications
CEA may be medically necessary for follow-up of patients with colorectal carcinoma. It would however only be medically necessary at treatment decision making points.
In some clinical situations (e.g. adenocarcinoma of the lung, small cell carcinoma of the lung, and some
gastrointestinal carcinomas) when a more specific marker is not expressed by the tumor, CEA may be a medically necessary alternative marker for monitoring.
Preoperative CEA may also be helpful in determining the post operative adequacy of surgical resection and subsequent medical management. In general, a single tumor marker will suffice in following patients with colorectal carcinoma or other malignancies that express such tumor markers.
In following patients who have had treatment for colorectal carcinoma, ASCO guideline suggests that if resection of liver metastasis would be indicated, it is recommended that post-operative CEA testing be performed every two to three months in patients with initial stage II or stage III disease for at least two years after diagnosis.
For patients with metastatic solid tumors which express CEA, CEA may be measured at the start of the treatment and with subsequent treatment cycles to assess the tumor's response to therapy.
Limitations
Serum CEA determinations are generally not indicated more frequently than once per chemotherapy treatment cycle for patients with metastatic solid tumors which express CEA or every two months post-surgical treatment for patients who have had colorectal carcinoma. However, it may be proper to order the test more frequently in certain situations, for example, when there has been a significant change from prior CEA level or a significant change in patient status which could reflect disease progression or recurrence.
Testing with a diagnosis of an in situ carcinoma is not reasonably done more frequently than once, unless the result is abnormal, in which case the test may be repeated once.
Specific Coding Guidelines
To show elevated CEA, use ICD-9-CM 790.99 (Other nonspecific findings on examination of blood) if a more specific diagnosis has not been made. If a more specific diagnosis has been made, use the code for that diagnosis.
Covered Tests
CPT/HCPCS Codes Descriptor
82378 Carcinoembryonic Antigen (CEA)
Covered Diagnosis Codes
CEA ICD-9 Codes Covered
150.0 Malignant neoplasm of cervical esophagus 150.1 Malignant neoplasm of thoracic esophagus 150.2 Malignant neoplasm of abdominal esophagus 150.3 Malignant neoplasm of upper third of esophagus 150.4 Malignant neoplasm of middle third of esophagus 150.5 Malignant neoplasm of lower third of esophagus
CEA CEA ICD-9 Codes Covered
150.8 Malignant neoplasm of other specified part of esophagus 150.9 Malignant neoplasm of esophagus unspecified site 151.0 Malignant neoplasm of cardia
151.1 Malignant neoplasm of pylorus 151.2 Malignant neoplasm of pyloric antrum 151.3 Malignant neoplasm of fundus of stomach 151.4 Malignant neoplasm of body of stomach
151.5 Malignant neoplasm of lesser curvature of stomach unspecified 151.6 Malignant neoplasm of greater curvature of stomach unspecified 151.8 Malignant neoplasm of other specified sites of stomach
151.9 Malignant neoplasm of stomach unspecified site 152.0 Malignant neoplasm of duodenum
152.1 Malignant neoplasm of jejunum 152.2 Malignant neoplasm of ileum
152.3 Malignant neoplasm of meckel's diverticulum
152.8 Malignant neoplasm of other specified sites of small intestine 152.9 Malignant neoplasm of small intestine unspecified site 153.0 Malignant neoplasm of hepatic flexure
153.1 Malignant neoplasm of transverse colon 153.2 Malignant neoplasm of descending colon 153.3 Malignant neoplasm of sigmoid colon 153.4 Malignant neoplasm of cecum
153.5 Malignant neoplasm of appendix vermiformis 153.6 Malignant neoplasm of ascending colon 153.7 Malignant neoplasm of splenic flexure
153.8 Malignant neoplasm of other specified sites of large intestine 153.9 Malignant neoplasm of colon unspecified site
154.0 Malignant neoplasm of rectosigmoid junction 154.1 Malignant neoplasm of rectum
154.2 Malignant neoplasm of anal canal
154.3 Malignant neoplasm of anus unspecified site
154.8 Malignant neoplasm of other sites of rectum rectosigmoid junction and anus 157.0 Malignant neoplasm of head of pancreas
157.1 Malignant neoplasm of body of pancreas 157.2 Malignant neoplasm of tail of pancreas 157.3 Malignant neoplasm of pancreatic duct 157.4 Malignant neoplasm of islets of langerhans
CEA ICD-9 Codes Covered
157.8 Malignant neoplasm of other specified sites of pancreas 157.9 Malignant neoplasm of pancreas part unspecified 159.0 Malignant neoplasm of intestinal tract, part unspecified
162.0 Malignant neoplasm of trachea 162.2 Malignant neoplasm of main bronchus
162.3 Malignant neoplasm of upper lobe bronchus or lung 162.4 Malignant neoplasm of middle lobe bronchus or lung 162.5 Malignant neoplasm of lower lobe bronchus or lung 162.8 Malignant neoplasm of other parts of bronchus or lung 162.9 Malignant neoplasm of bronchus and lung unspecified 174.0 Malignant neoplasm of nipple and areola of female breast 174.1 Malignant neoplasm of central portion of female breast 174.2 Malignant neoplasm of upper-inner quadrant of female breast 174.3 Malignant neoplasm of lower-inner quadrant of female breast 174.4 Malignant neoplasm of upper-outer quadrant of female breast 174.5 Malignant neoplasm of lower-outer quadrant of female breast 174.6 Malignant neoplasm of axillary tail of female breast
174.8 Malignant neoplasm of other specified sites of female breast 174.9 Malignant neoplasm of breast (female) unspecified site 175.0-175.9 Malignant neoplasm of male breast
183.0 Malignant neoplasm of ovary
197.0 Secondary malignant neoplasm of neoplasm of lung 197.4 Secondary malignant neoplasm of small intestine
197.5 Secondary malignant neoplasm of large intestine and rectum
209.00-209.03 Malignant carcinoid tumors of the small intestine
209.10-209.17 Malignant carcinoid tumors of the appendix, large intestine and rectum
209.20-209.29 Malignant carcinoid tumors of other and unspecified sites 209.70 Secondary neuroendocrine tumor, unspecified site 209.71 Secondary neuroendocrine tumor of distant lymph nodes 209.72 Secondary neuroendocrine tumor of liver
209.73 Secondary neuroendocrine tumor of bone 209.74 Secondary neuroendocrine tumor of peritoneum 209.75 Secondary Merkel cell carcinoma
209.79 Secondary neuroendocrine tumor of other sites 230.3 Carcinoma in situ of colon
CEA CEA ICD-9 Codes Covered
230.4 Carcinoma in situ of rectum
230.7 Carcinoma in situ of other/unspecified parts of intestine 230.9 Carcinoma in situ other and unspecified digestive organs 235.2 Neoplasm of uncertain behavior of stomach, intestines, rectum 338.3 Neoplasm related pain, acute or chronic
790.99 Other nonspecific findings on examination of blood 795.81 Elevated carcinoembryonic antigen [CEA]
795.89 Other abnormal tumor markers
V10.00 Personal history of malignant neoplasm of gastro-intestinal tract, unspecified V10.05 Personal history of malignant neoplasm, large intestine
V10.06 Personal history of malignant neoplasm, rectum, rectosigmoid junction, anus V10.11 Personal history of malignant neoplasm, bronchus, and lung
V10.3 Personal history of malignant neoplasm, breast V10.43 Personal history of malignant neoplasm, ovary V67.2 Follow-up examination following chemotherapy
Cell Count, Blood (CBC)
National Coverage Decision, Center for Medicare & Medicaid Services
Blood counts are used to evaluate and diagnose diseases relating to abnormalities of the blood or bone marrow. These include primary disorders such as anemia, leukemia, polycythemia, thrombocytosis and thrombocytopenia. Many other conditions secondarily affect the blood or bone marrow, including reaction to inflammation and infections, coagulopathies, neoplasms and exposure to toxic substances. Many treatments and therapies affect the blood or bone marrow, and blood counts may be used to monitor treatment affects.
The complete blood count (CBC) includes a hemogram and differential white blood count (WBC). The
hemogram includes enumeration of red blood cells, white blood cells, and platelets, as well as the determination of hemoglobin, hematocrit, and indices.
The symptoms of hematological disorders are often nonspecific, and are commonly encountered in patients who may or may not prove to have a disorder of the blood or bone marrow. Furthermore, many medical conditions that are not primarily due to abnormalities of blood or bone marrow may have hematological manifestations that result from the disease or its treatment. As a result the CBC is one of the most commonly indicated laboratory tests. In patients with possible hematological abnormalities, it may be necessary to determine the hemoglobin and hematocrit, to calculate the red cell indices, and to measure the concentration of white blood cells and platelets. These measurements are usually performed on a multichannel analyzer that measures all of the parameters on every sample. Therefore, laboratory assessments routinely include these measurements.
Indications
Indications for a CBC or hemogram include red cell, platelet, and white cell disorders. Examples are:
1. Indications for a CBC generally include the evaluation of bone marrow dysfunction as a result of neoplasms, therapeutic agents, exposure to toxic substances, or pregnancy. The CBC is also useful in assessing peripheral destruction of blood cells, suspected bone marrow failure or bone marrow infiltrate, suspected
myeloproliferative, myelodysplastic, or lymphoproliferative processes, and immune disorders.
2. Indications for hemogram or CBC related to red cell (RBC) parameters of the hemogram include signs, symptoms, test results, illness, or disease that can be associated with anemia or other red blood cell disorder (e.g., pallor, weakness, fatigue, weight loss, bleeding, acute injury associated with blood loss or suspected blood loss, abnormal menstrual bleeding, hematuria, hematemesis, hematochezia, positive fecal occult blood test, malnutrition, vitamin deficiency, malabsorption, neuropathy, known malignancy, presence of acute or chronic disease that may have associated anemia, coagulation or hemostatic disorders, postural dizziness, syncope, abdominal pain, change in bowel habits, chronic marrow hypoplasia or decreased RBC production, tachycardia, systolic heart murmur, congestive heart failure, dyspnea, angina, nailbed deformities, growth retardation, jaundice, hepatomegaly, splenomegaly, lymphadenopathy, ulcers on the lower extremities). 3. Indications for hemogram or CBC related to red cell (RBC) parameters of the hemogram include signs,
symptoms, test results, illness, or disease that can be associated with polycythemia (for example, fever, chills, ruddy skin, conjunctival redness, cough, wheezing, cyanosis, clubbing of the fingers, orthopnea, heart murmur, headache, vague cognitive changes including memory changes, sleep apnea, weakness, pruritus, dizziness, excessive sweating, visual symptoms, weight loss, massive obesity, gastrointestinal bleeding, paresthesias, dyspnea, joint symptoms, epigastric distress, pain and erythema of the fingers or toes, venous or arterial thrombosis, thromboembolism, myocardial infarction, stroke, transient ischemic attacks, congenital heart disease, chronic obstructive pulmonary disease, increased erythropoietin production associated with neoplastic, renal or hepatic disorders, androgen or diuretic use, splenomegaly, hepatomegaly, diastolic hypertension.) 4. Specific indications for CBC with differential count related to the WBC include signs, symptoms, test results,
illness, or disease associated with leukemia, infections or inflammatory processes, suspected bone marrow failure or bone marrow infiltrate, suspected myeloproliferative, myelodysplastic or lymphoproliferative disorder, use of drugs that may cause leukopenia, and immune disorders (e.g., fever, chills, sweats, shock, fatigue, malaise, tachycardia, tachypnea, heart murmur, seizures, alterations of consciousness, meningismus, pain such as headache, abdominal pain, arthralgia, odynophagia, or dysuria, redness or swelling of skin, soft
CELL COUNT, BLOOD (CBC) tissue bone, or joint, ulcers of the skin or mucous membranes, gangrene, mucous membrane discharge,
bleeding, thrombosis, respiratory failure, pulmonary infiltrate, jaundice, diarrhea, vomiting, hepatomegaly, splenomegaly, lymphadenopathy, opportunistic infection such as oral candidiasis.)
5. Specific indications for CBC related to the platelet count include signs, symptoms, test results, illness, or disease associated with increased or decreased platelet production and destruction, or platelet dysfunction (e.g., gastrointestinal bleeding, genitourinary tract bleeding, bilateral epistaxis, thrombosis, ecchymosis, purpura, jaundice, petechiae, fever, heparin therapy, suspected DIC, shock, pre-eclampsia, neonate with maternal ITP, massive transfusion, recent platelet transfusion, cardiopulmonary bypass, hemolytic uremic syndrome, renal diseases, lymphadenopathy, hepatomegaly, splenomegaly, hypersplenism, neurologic abnormalities, viral or other infection, myeloproliferative, myelodysplastic, or lymphoproliferative disorder, thrombosis, exposure to toxic agents, excessive alcohol ingestion, autoimmune disorders (SLE, RA and other).
6. Indications for hemogram or CBC related to red cell (RBC) parameters of the hemogram include, in addition to those already listed, thalassemia, suspected hemoglobinopathy, lead poisoning, arsenic poisoning, and spherocytosis.
7. Specific indications for CBC with differential count related to the WBC include, in addition to those already listed, storage diseases/mucopolysaccharidoses, and use of drugs that cause leukocytosis such as G-CSF or CM-CSF.
8. Specific indications for CBC related to platelet count include, in addition to those already listed, May-Hegglin syndrome and Wiskott-Aldrich syndrome.
Limitations
Testing of patients who are asymptomatic, or who do not have a condition that could be expected to result in a hematological abnormality, is screening and is not a covered service.
In some circumstances it may be appropriate to perform only a hemoglobin or hematocrit to assess the oxygen carrying capacity of the blood. When the ordering provider requests only a hemoglobin or hematocrit, the remaining components of the CBC are not covered.
When a blood count is performed for an end-stage renal disease (ESRD) patient, and is billed outside the ESRD rate, documentation of the medical necessity for the blood count must be submitted with the claim.
In some patients presenting with certain signs, symptoms or diseases, a single CBC may be appropriate. Repeat testing may not be indicated unless abnormal results are found, or unless there is a change in clinical condition. If repeat testing is performed, a more descriptive diagnosis code (e.g., anemia) should be reported to support medical necessity. However, repeat testing may be indicated where results are normal in patients with conditions where there is a continued risk for the development of hematologic abnormality.
Covered Tests
CPT/HCPCS Codes Descriptor
85004 Blood count, automated differential white blood cell (WBC) count
85007 Blood count; blood smear, microscopic examination with manual differential WBC count 85008 Blood count; blood smear, microscopic examination without manual differential WBC count 85013 Blood count, Spun microhematocrit
85014 Blood count, hematocrit (Hct) 85018 Blood count, Hemoglobin
85025 Blood count, complete (CBC), automated (Hgb, Hct, RBC, WBC and platelet count) and automated differential WBC count 85027 Blood count, complete (CBC), automated (Hgb, Hct, RBC, WBC and platelet count) 85032 Blood count; manual cell count (erythrocyte, leukocyte, or platelet) each
CPT/HCPCS Codes Descriptor
85048 Blood count, leukocyte (WBC), automated 85049 Blood count; platelet, automated
Covered Diagnosis Codes
Blood Count, blood ICD-9 Codes
Any code that DOES NOT APPEAR on the “ICD-9-CM CODES THAT DO NOT SUPPORT MEDICAL NECESSITY” below, and supports medical necessity as explained in the indications section above.
ICD-9-CM Codes That DO NOT Support Medical Necessity
Code Description
078.10-078.19 Viral warts
210.0-210.9 Benign neoplasm of lip, oral cavity, and pharynx 214.0 Lipoma, skin and subcutaneous tissue