PROCESS OPTIMIZATION FOR EXTRACTION OF NATURAL DYE
FROM
M. PHILIPPINENSIS
FRUITS AND ITS APPLICATION ON
DIFFERENT FABRICS
Rakesh Kumar* and Y. C. Tripathi and Prasoon K. Kaushik
Chemistry Division, Forest Research Institute, New Forest, Dehradun – 248006
(Uttarakhand) India.
ABSTRACT
The present study was aimed at optimizing conditions such as material
to liquor ratio (MLR), alkalinity of the extractant and extraction time
for maximum extraction of natural dye from M. philippinensis fruits.
The extracted dye was applied for dyeing different kinds of fabrics
including silk, wool and cotton and colourfastness of dyed fabrics
against various colour-fading factors including light, washing, rubbing,
perspiration, etc. was determined. Also, CIE L*, a*, b*, C*, h0 and K/S values were studied by standard methods. Based on the highest optical
density, the optimum conditions, MLR (8.5 g/100 mL), alkali content
(sodium carbonate 0.3 g/100 mL) and extraction time (70 min) for dye
extraction was determined. Extraction of dye with optimized
parameters resulted in significantly high i.e. 12.8% recovery of natural
dye from the plant as compared to conventional method. Further, different types of dyed
fabrics exhibited high fastness score in the range of 4-5 that signifies their very good to
excellent colourfastness property. The research outcome may contribute to the scale-up of M.
philippinensis fruits dye production and is useful for textile industries. It is also an efficient
approach towards natural dye extraction from the plant. M. philippinensis fruit dye having
high medicinal and antimicrobial activity can be used in production of textiles for medical
application and protective clothing.
KEYWORDS: M. philippinensis, Natural dye, Process optimization, Fabric dyeing, Colourfastness.
Volume 5, Issue 4, 927-945. Research Article ISSN 2277– 7105
*Correspondence for
Author
Rakesh Kumar
Chemistry Division,
Forest Research Institute,
New Forest, Dehradun –
248006 (Uttarakhand)
India.
Article Received on 25 Jan 2016,
Revised on 15 Feb 2016, Accepted on 07 Mar 2016
INTRODUCTION
Dyeing with natural colourants has been one of the oldest techniques practiced by the ancient
human civilization. However, advent of the first synthetic dye changed the situation and
synthetic dyes received faster acceptability due to ease in dyeing, high colourfastness, low
cost and wide range of applications. Nevertheless, production and use of synthetic dyes pose
serious problem to environment and living biota.[1] Water pollution due to discharge of non-biodegradable coloured effluents from dye manufacturing and textile-dyeing has become one
of the major environmental concerns.[2] During the last few decades, use of synthetic dyes is gradually decreasing due to their toxicity, non-biodegradability, associated serious health
hazards like allergic and carcinogenicity and increased environmental awareness. There is a
movement across the world to return to the natural dyes that offer much more advantages
including renewable sources, minimal health hazard, mild reaction conditions, no disposal
problems and harmonization with nature.[3] There is a wide spread interest in the natural dyeing because of their better biodegradability and high compatibility with the environment,
which has prompted researchers to look for eco-friendly dye products.[4] As a result, a number of plants have so far been investigated as a source of natural dyes.[5-9]
Despite several advantages of natural dyes, their world-wide consumption is just 1% of the
synthetic dye due to some technical limitations including low yield, complexity of dyeing
process, limited and non-reproducible shades, blending problems and inadequate fastness
properties.[10] Of which, the major challenge is to increase the yield of natural dyes through proper research intervention. Although the Indian subcontinent possesses large plant
resources, only little has been exploited so far.[11] The present work has been aimed to address this issue through optimization of extraction process to obtain maximum possible quantity of
dye from M. philippinensis.
M. philippinensis Muell. Arg. (Fig.1&2) commonly known as Kamala or Senduri is a large
woody multipurpose tree up to 10-12 meters in height under family Euphorbiaceae, naturally
found in Sal and certain mixed forests and widely distributed throughout tropical India along
with the Himalaya from Kashmir east wards up to 1500 m . It has traditionally been regarded
as a popular dye-yielding plant and a rich source of biologically active compounds.[12] Several medicinal properties are attributed to the plant in Indian System of Medicine. All parts of the
tree can be applied externally to treat parasitic infections of the skin like scabies, ringworm,
antifungal tape worm eye-disease, cancer, diabetes, diarrhoea, jaundice, malaria,
urino-genital infection, swellings of joints from acute rheumatism and testes from suppressed
gonorrhoea, etc.[14] Pharmacological studies have revealed purgative, anthelmintic,[15] antioxidant,[16] insecticidal, anti-microbial,[17-19] anti-lithic [20], antifertility[21], antitumour,[22] heptoprotective [23] activities. On account of its wound healing and antimicrobial properties, M. phillippinensis dye could be used in production of skin friendly protective fabrics. The
plant has been investigated for different groups of phytochemicals including amino acids,
carbohydrates, flavonoids, gum, oil and resins, proteins, phenolic groups, saponins, steroids,
tannins and terpenoids.[24] The chemical constituents such as betulin, friedelin, kamaladiol‐3‐acetate,[25] lupeol, tannic acid, 3α‐hydroxy‐D‐A-friedoolean‐3‐en‐2‐one, 2β‐hydroxy‐D‐A‐friedooleanan‐3‐one and 3α‐hydroxy‐D‐A ‐friedooleanan‐2‐one, were
reported from the stem bark.[26-36]
The colouring matter is present in the red glandular pubescence covering of the ripe capsules
and is usually collected in February-March when the fruits ripen. The granules which cover
the ripe fruit are used in India as a dye (Kamala powder) for textile dyeing. The red granules
are usually separated by beating and shaking the ripe fruits, or by stirring the fruits vigorously
in water when the dye settles down as sediment. The sediment is then collected, dried and
pieces of pericarp and other refuse separated by sifting.[37] The dyeing material, known in trade as Kamala, Kamala Powder or Kamala Dye, is used in the dyeing of silk and wool that
produces a bright orange or flame colour.[38] Kamala dye is insoluble in cold water, slightly soluble in boiling water and freely soluble in alkalies, alcohol and ether forming deep red
solutions. The principal colouring constituents, salmon-coloured rottlerin (C30H28O8) and its
[image:3.595.99.501.333.546.2]yellow isomer, isorottlerin together constitute c. 11 % of the powder. In addition, it also
contains a small amount of homo-rottlerin, a low-melting yellow resin (5%), wax (c. 2%),
traces of a volatile oil, tannins, gum and citric and oxalic acids.[36,39]
The yield of kamala powder is only 1.4-3.7 % of the fruits.[39] Low yield of dye by traditional extraction method makes the product very expensive and commercially unfeasible. Present
research work was designed to address this issue through optimization of extraction process
so as to get maximum possible yield of dye from M. philippinensis fruits.
MATERIALS AND METHODS Plant Materials and Fabrics
Fruits of M. philippinensis were collected from the premises of Forest Research Institute,
Dehradun, India. The collected fruits were properly cleaned and then allowed for air drying in
shade. Pure silk, wool and cotton textile fabrics were purchased from Gandhi Ashram, an
authorized outlet of KVIC Dehradun, India. The fabrics were washed with nonionic detergent
(1% owf) for 30 min, rinsed and dried at room temperature. The scoured material was wetted
in water for 30 min prior to dyeing or mordanting.
Chemical and Analytical
All the chemicals viz., acetic acid, alum, copper sulphate, ferrous sulphate, potassium
dichromate and stannous chloride used as mordants were of L.R. grade and were procured
from local dealers. The optical density (OD) of dye extracts were recorded on a UV-Vis
spectrophotometer [Chemito make (Model 2700)], IR spectra were recorded on a FT-IR
(Shimadzu) spectrophotometer. Colour fastness of the extracted dye on different types of
dyed fabric against washing, light, perspiration and rubbing were measured by using
Laundrometer (Make: Metrex Scientific Instrument (P) Ltd.), Digital Light Fastness Tester
(Make-Labin Scientific Instruments and Calibration Pvt Ltd.), Perspirometer and Crockmeter
(Make-Globe–Tex Industries) respectively. The CIEL*a*b*, hue, chroma and K/S values
were determined on a Reference Bench-top Spectrophotometer (Make-Gretag Mac Beth;
Colour-Eye 7000A).
Optimization of Dye Extraction Parameters
Optimization of the conditions such as combination of plant material and extractant (material
to liquor ratio i.e. MLR), alkali content of the extraction medium and extraction time was
extraction experiment was done in 500 mL beaker containing 100 mL of distilled water with
(0.1g-0.7g) of sodium carbonate (Na2CO3) and different amounts of dried fruits of M.
philippensis ranging from 2-12 g and then heated at 95-980 C for varying time ranging from 15 to 80 minutes. The dye extracts obtained from each of the experiments were cooled,
filtered and made to 100 mL. 1 mL of aliquot from each of the extracts were diluted to 100
mL and subjected to optical density (OD) measurement. Each of the extraction experiments
were replicated thrice. Based on the highest value of OD, optimum values of MLR, alkali
content and time for dye extraction were determined. Natural dye from fruits of M.
philippinensis was extracted using calculated values of MLR, alkali content and time.
Optimization of Dyeing Parameters for Different Fabrics
Natural dye extracted under optimum extraction condition was used for dying silk, wool and
cotton fabrics by using fabric to dye solution ratio 1:20 - 1: 50 for 30-75 min. Dyeing of silk,
wool and cotton samples was done by post mordanting method to produce improved shades
in terms of hue and darkness. The dye bath was set with optimum fabric to dye solution ratio
and was heated maintaining the solution level for optimum time calculated for different
fabrics. The dyeing temperature was maintained at 80-850 C for silk and wool and 95-980 C for cotton. The dye bath was cooled to room temperature and the dyed fabrics were directly
transferred to mordant bath for mordanting.
Mordanting of Dyed Fabrics
Most of the natural dyes are mordant dyes and require a mordant for fixing it to the fabric.
Post mordnating method was adopted for mordantoing of dyed fabrics. Common mordants
like alum, copper sulphate, potassium dichromate, iron salts and tin salts have the affinity for
both dye and fabrics and form an insoluble precipitate in combination with dye on the fibre.
For mordanting of dyed fabrics, optimization of mordant concentration, fabric to mordant
solution ratio and mordanting time were done through series of experimental trials. Varying
concentration (0.25, 0.5, 0.75 and 1.0 %) of different mordants, fabric to mordant solution
ratio (1:20-1: 50) and mordanting time 30, 45 and 60 min for silk and wool and 45, 60 and
75 min for cotton were trialled so as to achieve best possible mordating.
After standardizing the mordanting conditions, the dyed samples were directly immersed in
the mordant bath. Mordanting of dyed fabrics was done as per standardized condition of
fabric to dye solution ratio and time. Mordants used for study were metallic salts like: alum,
like Terminalia chebula fruit pericarp powder and Phyllanthus emblica fruit powder was also
employed for mordanting of dyed textile fabrics. The dyed textile samples mordanted
separately with different mordants were codified as MPD 1 (without mordant), MPD 2
(Alum), MPD 3 (Copper Sulphate), MPD 4 (Ferrous Sulphate), MPD 5 Potassium
dichromate), MPD 6 (Stannous chloride), MPD 7 (Phyllanthus emblica) and MPD 8
(Terminalia chebula). Numeric values are indicative of mordant treatments for the three
types of fabrics i.e. silk, wool and cotton for further studies.
Determination of Colourfastness of Dyed Fabrics
Colourfastness of dyed textile fabrics signifies their resistance by which the fabrics opposes
to changing or losing its shade when subjected to the action of various agents such as light,
washing, rubbing, perspiration, staining of the other textiles, etc. Usually, a textile fabric
faces many external conditions which can affect the colour of the textiles. Therefore the
samples of dyed fabrics were tested for their colour fastness properties against washing, light,
crocking/rubbing and perspiration. Colour fastness of dyed fabrics to light, wash, crocking
and perspiration was carried out by standard methods; IS: 2454: 1985, IS-687:1979 IS-766:
1988 and IS-971: 1983 respectively.[40] The tested samples were assessed against the standard grey scales for change of colour and staining on adjacent and rubbing fabrics, while for light
fastness, comparison up to Blue wool standard 5 was done.
Determination of Colour Coordinates
All the dyed and mordanted silk, wool and cotton samples were tested for their L*, a*, b*,
C*, h0 and K/S values using Reference Bench Top Spectrophotometer. CIE stands for Commission Internationale de l’Eclairage system of colour analysis, an international clearing
house for colour research. The terms, L*, a*, and b* signify respectively the luminosity or
lightness, redness or greenness and yellowness and blueness of a sample. Positive a* stands
for red and negative a* for green where as positive b* stands for yellow and negative b* for
blue. Other aspects, like hue, chroma and K/S ratio of the dyed fabrics were also studied so as
to get a more precise result of the colour coordinates.
Hue
Hue is the attribute by which a sample differs from achromatic (i.e. hueless) colour of same
Chroma
Chroma is the colourfulness of an area judged in proportion to the brightness of a similarly
illuminated area that appears to be white or highly transmitting.
K/S Values
Kubelka-Monk equation defines a relationship between spectral reflectance (R in %) of the
sample and its absorption (K) and scattering (S) characteristics, represented as follows:
[1 – 0.01 R2] K/S =
2[0.01 R]
K/S grows to infinity as reflectance decreases to zero
RESULTS AND DISCUSSIONS
Optimization of Dye Extraction Parameters
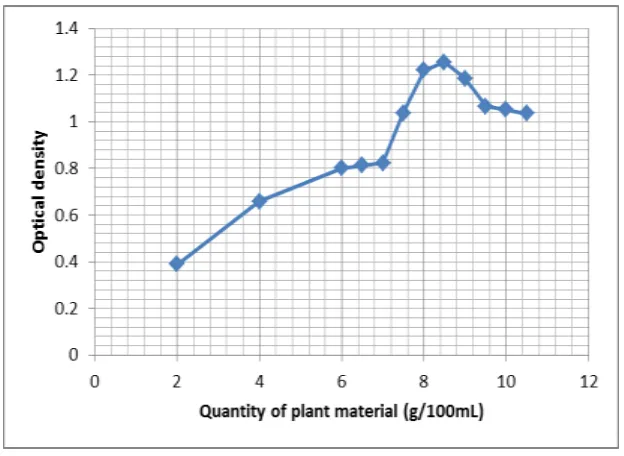
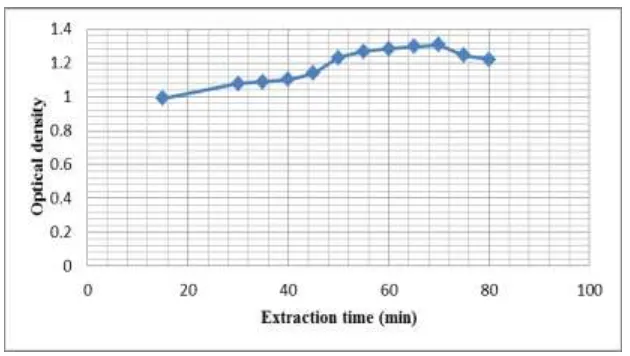
Extraction parameters viz., MLR, alkali content and time were optimized based on optical
density (OD) of extracted dye with different levels of the three variables. The highest optical
density 1.256, 2.471 and 1.306 was recorded for MLR of 8.5 g/100 mL (Fig. 3), alkali
[image:7.595.142.454.428.656.2]content of 0.3 g/100 mL sodium carbonate (Fig. 4) and extraction time of 70 min (Fig. 5).
Fig. 4: Standardization of alkali content (pH)
Figure 5: Standardization of extraction time
Based on the highest optical density the optimum conditions MLR (8.5 g/100 mL), alkali
content (sodium carbonate 0.3 g/100 mL) and extraction time (70 min) for extraction of dye
from fruits of M. philippinensis was determined.
Dye Yield with Optimum Extraction Condition
Natural dye was extracted from M. philippinensis with optimized condition of MLR,
alkalinity of extractant and extraction time. The yield of the dye was recorded to be 12.8 %
on moisture free basis. It is, therefore obvious that the yield of natural dye obtained under
optimized conditions is considerably high as compared that obtained by traditional extraction
method.
Dye Absorptivity and Dyeing of Fabrics
Experimental trials were carried out to determine optimal quantity/concentration of dye
[image:8.595.141.456.281.459.2]0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 g/100 mL of dye solution was recorded to be 38.35, 39.83,
41.67, 43.63, 43.86, 34.20 and 32.26 % for silk; 31.32, 32.86, 34.63, 36.86, 43.20, 35.14 and
30.34% for wool and 24.07, 27.57, 22.79, 31.60, 32.92, 29.56 and 27.94% for cotton
respectively. It is evident from absorption percentage data; maximum absorption took place
in case of 1.0g/100mL of dye solution for all the test fabrics i.e. silk (43.86 %), wool
(43.20%) and cotton (32.92%).
Dyeing and Mordanting of Fabrics
Dyeing of silk and wool with M. philippinensis dye was carried out in 1.0 % dye solution
with material to liquor ratio of 1:40 for 45 minutes at a temperature of 80-850 C, while cotton fabric was dyed with the same MLR at 90-950C for 60 minutes. Mordant concentration of 0.5 g/100 mL and mordanting time 30 minutes for silk and wool and 45 minutes for cotton were
found to be optimum by visual observations of dyed and mordanted samples. The criteria for
evaluation were the depth of colour, evenness of dye and brightness of shade. The dyed
samples were directly immersed in the mordant bath by keeping the fabrics to liquor ratio of
1:40. The bath was heated to a temperature of 80-850C for 30 minutes for fixing the dye on silk and wool samples. For cotton samples the mordanting was continued for 60 minute at a
temperature of 95-980C. The samples were taken out from the cooled bath, washed with water and dried in shade. Mordants including metallic salts viz., alum, copper sulphate,
ferrous sulphate, potassium dichromate, stannous chloride and two natural mordants,
Phyllanthus emblica and Terminalia chebula were used for mordanting experiments.
Determination of Colourfastness of Dyed Fabrics
Different types of fabrics i.e. silk, wool and cotton, dyed using M. philippinensis dye were
subjected to colour fastness studies against washing, perspiration, rubbing and light and
determination of colour coordinates including CIEL*a*b* and K/S values as per Indian
Standards (IS).
Colourfastness to Washing
The data regarding colourfastness of dyed and mordanted fabrics to washing are given in
Table 1. The data showed that change in colour on grey scale in silk fabrics in sample
MPD1, MPD2, MPD3, MPD5, MPD6, MPD7 and MPD8 were 4-5 and in MPD4 it was 4/5.
In wool MPD1, MPD2, MPD4 and MPD6 were 4 and in MPD3, MPD5 and MPD7: 4-5
while MPD8 exhibited 3-4. In cotton the change in colour on grey scale in MPD1, MPD2,
respectively. Staining on adjacent fabrics 1 and 2 and in silk, wool and cotton was 4-5 in all
the samples.
Table 1: Colourfastness to washing (As per IS 687: 1979)
The data of colourfastness to washing properties indicate that the washing fastness of silk and
wool is very good to excellent in all the samples however due to weaker affinity of dye with
cotton fabrics the washing fastness is lower in cotton fabrics in comparison with silk and
wool fabrics.
Colourfastness to Light
Colour change of the dyed fabric samples were assessed against the grey scale and the blue
wool standard 5. Light fastness values of all dyed and mordanted samples of silk, wool and
[image:10.595.99.493.134.325.2]on grey scale as well as blue wool scale are summarized in Table 2.
Table 2: Colourfastness to light method (As per: IS: 2454: 1985) S. No Test
Fabric
Change in Colour of Test Fabric on Blue Wool Standard
Silk Wool Cotton
1. MPD1 4 4 3-4
2. MPD2 4 4 4
3. MPD3 4 4 4
4. MPD4 4 4 4
5. MPD5 4 4 4
6. MPD6 4 4 4
7. MPD7 4 4 3-4
8. MPD8 3-4 4 4
The data revealed that change in colour of test fabric on blue wool standard of silk fabrics for
fabrics exhibited change in colour of test fabric on blue wool standard: 4 for the test
specimens viz. MPD1, MPD2, MPD3, MPD4, MPD5, MPD6, MPD7 and MPD8. However
cotton fabrics exhibited lower rating of colour fastness to light in comparison with wool and
silk fabrics. Various tests of light fastness of natural plant based dyes have shown that the
majority have a maximum rating of four (4).[41-43] Results of this study therefore are in conformity with the minimum performance requirements for application of the dye in textile
industry.[44]
Colourfastness to Rubbing
Determination of colourfastness of silk, wool and cotton samples to rubbing and their staining
on adjacent fabrics 1 and 2 was carried out in dry and wet conditions. The staining of the
rubbing fabrics were assessed with grey scale for assessment of staining. The results of
rubbing fastness studies presented in Table-3 revealed that silk fabrics under dry and wet
rubbing test showed 4-5 and 4/5 for all the test samples viz. MPD1, MPD2, MPD3, MPD4,
[image:11.595.78.515.406.569.2]MPD5, MPD6, MPD7 and MPD8.
Table 3: Colourfastness to rubbing (as per IS: 766: 1988) S. No. Test
Fabric
Staining on rubbing cloth Dry rubbing
Staining on rubbing cloth Wet rubbing
Silk Wool Cotton Silk Wool Cotton
1. MPD1 4-5 4-5 4 4-5 4-5 4
2. MPD2 4/5 4-5 4-5 4/5 4/5 4-5
3. MPD3 4-5 4 4-5 4-5 4-5 4-5
4. MPD4 4-5 4-5 4 4/5 4-5 4
5. MPD5 4-5 4-5 4 4-5 4-5 4-5
6. MPD6 4/5 4-5 4-5 4/5 4/5 4-5
7. MPD7 4-5 4-5 4 4-5 4-5 4-5
8. MPD8 4-5 4-5 4-5 4-5 4-5 4-5
Dry rubbing of test fabrics of wool: MPD1, MPD2, MPD4, MPD5, MPD6, MPD7, MPD8
MPD3 exhibited 4-5 while MPD3 exhibited: 4. Rubbing in wet conditions wool fabrics
exhibited 4-5 and 4/5 for all the test samples. Dry rubbing of cotton revealed the rubbing
fastness of 4-5 for MPD2, MPD3, MPD6, MPD8; while dry rubbing fastness for MPD1,
MPD4, MPD5, MPD7, were 4. Colourfastness to rubbing under wet conditions, cotton
fabrics showed 4-5 for MPD2, MPD3, MPD5, MPD6, MPD7, MPD8; and 4 for MPD1 and
MPD4. Silk and wool fabrics exhibited better rubbing fastness properties over cotton. Good
rubbing fastness indicated no unfixed dyes left on the fibre surface after soaping and
fibres, associated with ionic interactions or hydrogen bonding or by complexation with
mordant or with functional groups of dyes.[45]
Colourfastness to Perspiration
Dyed samples were tested for their colourfastness against perspiration (Table-4).
Table 4: Colourfastness to perspiration (Silk) As per IS 971: 1983
Colour change on grey scale and staining on adjacent fabric 1 & 2 by acidic perspiration on
silk indicated that colour change for test sample of MPD2, MPD4, MPD5, MPD7 and MPD8
exhibited colourfastness of 4-5; MPD1 and MPD6: 4 and MPD3: 4/5. Staining on adjacent
fabrics-1 were 4-5 in the case of test sample MPD1, MPD2, MPD4, MPD5, MPD7, MPD8;
MPD3 exhibited 4/5 rating and MPD6 exhibited 4 on grey scale. Staining on adjacent
fabrics-2, the rating for all the samples were 4-5 except MPD3 in which it was 4/5. In
alkaline perspiration change of colour on grey scale were found to be 4-5 for test samples
MPD1, MPD2, MPD5, MPD6, MPD7, MPD8, and 4/5 for test samples MPD3 and MPD4.
The staining on adjacent fabrics 1 and 2 was 4-5 and 4/5 for the all test sample under
investigation.
For wool samples (Table-5) the change in colour on grey scale in acidic perspiration, the
rating for test samples: MPD2, MPD4, MPD5, MPD7, MPD8 were 4-5; MPD3: 4/5; MPD1,
Table 5: Colourfastness to perspiration (Wool) as per IS 971: 1983
Change in colour on grey scale in alkaline perspiration, the colourfastness were 4-5, 4-5, 4/5,
4/5, 4-5, 4, 4-5 and 4-5 for the test samples MPD1, MPD2, MPD3, MPD4, MPD5, MPD6,
MPD7 and MPD8 respectively. The staining on adjacent fabrics 1 and 2 was 4-5 and 4/5 for
the all test sample of alkaline perspiration.
Cotton fabrics exhibited change in colour on grey scale for test sample MPD1 and MPD6-4;
MPD3-4/5 and for rest of the samples: 4-5 (Table-6).
Table 6: Colourfastness to perspiration (Cotton) as per IS 971: 1983
Staining on adjacent fabrics 1 and 2 were 4, 4-5 and 4-5 for all the test samples. The data of
perspiration fastness specify the very good colour fastness to perspiration for silk, wool and
cotton fabrics which are acceptable for textile dyeing.
CIEL*a*b* and K/S Values of Dyed Fabrics
CIEL*a*b* and K/S values of dyed silk, wool and cotton fabrics are summarized in Table 7
The values of L*, a* and b* dyed silk fabrics was calculated as L*: 45.68, 44.89, 49.42,
29.18, 42.25, 49.60, 45.56 and 46.40; a*: 10.35, 9.08, 8.99, 2.74, 10.89, 9.32, 9.53 and 9.17;
b*: 26.78, 25.82, 28.59, 9.43, 28.12, 27.01, 25.47 and 26.09 for MPD1, MPD2, MPD3,
14.84, 16.02, 16.87, 15.52, 12.95, 15.20 and 14.62. The data for variation in ‘L’ values
indicated maximum dark shade (L=29.18) in MPD4 which is mordanted with ferrous
sulphate in silk. Highest value of redness (a*=10.89) in silk was obtained by mordnated with
potassium dichromate (MPD5) while highest value of yellowness (b*=28.59) in silk was
obtained by mordanting with copper sulphate (MPD3). K/S value (16.87) in silk fabrics was
highest in the sample which was mordanted with ferrous sulphate (MPD4). As per
Kubelka-Monk equation as the value of K/S grows, reflectance decreases thereby absorbance
increases, hence it indicates that MPD4 has maximum absorbance.
Table 7: CIEL*a*b* and K/S values of dyed and mordanted fabrics
S.No. Mordant L* a* b* C* h0 K/S
Silk
1. MPD1 45.68 10.35 26.78 28.71 68.86 15.41
2. MPD2 44.89 9.08 25.82 27.37 70.62 14.84
3. MPD3 49.42 8.99 28.59 29.97 22.54 16.02
4. MPD4 29.18 2.74 9.43 9.82 73.78 16.87
5. MPD5 42.25 10.89 28.12 30.15 68.82 15.52
6. MPD6 49.60 9.32 27.01 28.58 70.96 12.95
7. MPD7 45.56 9.53 25.47 27.20 89.50 15.20
8. MPD8 46.40 9.17 26.09 28.27 71.04 14.62
Wool
1. MPD1 49.74 9.26 27.58 29.09 71.44 18.02
2. MPD2 49.42 8.99 28.59 29.97 72.54 16.02
3. MPD3 38.13 5.60 22.72 23.40 76.16 23.16
4. MPD4 29.68 1.12 8.76 8.83 82.72 21.74
5. MPD5 32.91 8.68 25.43 26.87 71.14 29.68
6. MPD6 52.64 9.55 30.75 32.20 72.75 17.74
7. MPD7 44.24 10.26 27.35 29.21 69.43 23.16
8. MPD8 42.42 7.80 24.90 25.16 72.44 18.20
Cotton
1. MPD1 66.29 4.02 16.56 17.04 76.35 4.29
2. MPD2 66.73 3.00 20.41 20.63 81.65 4.00
3. MPD3 60.78 2.98 23.26 23.45 82.69 6.53
4. MPD4 47.91 1.68 7.79 7.97 77.81 6.17
5. MPD5 60.56 4.70 22.56 23.05 78.23 5.74
6. MPD6 71.26 3.55 16.14 16.53 77.58 2.40
7. MPD7 64.03 3.74 17.15 17.55 77.69 4.52
8. MPD8 65.56 3.07 18.77 19.07 80.30 3.98
L*, a* and b* for wool fabrics were L*: 49.74, 49.42, 38.13, 29.68, 32.91, 52.64, 44.24 and
42.42; a*: 9.26, 8.99, 5.60, 1.12, 8.68, 9.55, 10.26 and 7.80; b*: 27.58, 28.59, 22.72, 8.76,
25.43, 30.75, 27.35 and 24.90 for MPD1, MPD2, MPD3, MPD4, MPD5, MPD6, MPD7 and
17.74, 23.16 and18.20 respectively for MPD1, MPD2, MPD3, MPD4, MPD5, MPD6, MPD7
and MPD8. The values of L* indicate that dyed wool fabric, mordanted with ferrous sulphate
(MPD4) has maximum dark shade (L=29.68) which is close to silk fabric. Highest values of
redness (a*=10.26) and yellowness (b*=30.75) was in wool fabrics in MPD7 and MPD6
respectively. K/S values for wool fabrics were 18.02, 16.02, 23.16, 21.74, 29.68, 17.74, 23.16
and18.20 for MPD1, MPD2, MPD3, MPD4, MPD5, MPD6, MPD7 and MPD8 respectively.
It shows that MPD5 has maximum absorbance in wool fabrics.
Cotton fabrics appear less dark than silk and wool. The data of L*a*b* and K/S for cotton
fabrics are:- L*: 66.29, 66.73, 60.78, 47.91, 60.56, 71.26, 64.03, and 65.56; a*: 4.02, 3.00,
2.98, 1.68, 4.70, 3.55, 3.74 and 3.07; b*: 16.56, 20.41, 23.26, 7.79, 22.56, 16.14, 17.15 and
18.77; K/S: 4.29, 4.00, 6.53, 6.17, 5.74, 2.40, 4.52 and 3.98 for MPD1, MPD2, MPD3,
MPD4, MPD5, MPD6, MPD7 and MPD8 respectively. Analysis of the data indicate that
cotton fabric mordanted with ferrous sulphate (MPD4) has darkest shade (L=47.91). Highest
values of redness (a*=4.70) and yellowness (b*=23.26) in cotton fabrics was in MPD5 and
MPD3 respectively.
CONCLUSION
In the present study the extraction procedure for extraction of natural dyes from M.
philippinensis fruits was optimized leading to significantly high recovery of natural dye from
the plant as compared to conventional method. Different kind of fabrics including silk, wool
and cotton dyed with extracted dye exhibited excellent fastness properties against various
colour-waning factors, thus establishing commercial significance of the dye. A systematic
study of extraction and characterization of the M. philippinensis fruits dye is must to
minimize the cost investment, yield maximization and dye purity. The research outcome may
contribute to the scale-up of M. philippinensis fruits dye production useful for textile
industries. Further, the overall research effort is also a very good example of efficient
approach towards extraction operation in the area of natural dye production.
On account of their environment friendliness and non-toxicity to living biota, natural dyes are
gaining world-wide acceptance. However, dye manufacturing units and industries using
natural dyes are facing raw material crisis and availability of limited range of colours. The
outcome of the research work has led to the development of protocol for extraction of dye in
higher yield from M. philippinensis fruits exhibiting tremendous fastness traits on different
philippinensis fruit dye have high medicinal value in general and antimicrobial activity in
particular, thus can be used in production of textiles for medical application and protective
clothing.
ACKNOWLEDGEMENTS
Authors would like to express sincere gratitude to the Director, Forest Research Institute,
Dehradun, India for providing necessary facilities. Authors are also thankful the Indian
Council of Forestry Research and Education (ICFRE, an autonomous body under
MoEF&CC, Govt.of India), for funding support.
REFERENCES
1. Uddin MG, Ghosh NC, Reza MS. Study on the performance of ecoalkali in dyeing of
cotton fabric with reactive dyes. International Journal of Textile Sciences, 2014; 3:
51-58.
2. Patil PD, Rao CR, Wasif AI. Revival of natural dyes: Smart use of biodiversity.
Colourage., 2012; 10: 33-38
3. Teli MD, Paul R, Pardeshi PD. Natural Dyes: Classification, chemistry and extraction
methods, Part–I: Chemical classes, extraction methods and future prospects. Colourage.,
2000; 47(12): 43-48.
4. Prusty AK, Das T, Nayak A, Das NB. Colourimetric analysis and antimicrobial study of
natural dyes and dyed silk. J. Cleaner Prod., 2010; 18: 1750-1756.
5. Dayal R, Dobhal PC. Natural dye from Indian Plants. Colourage, 2001; 48(8): 33-38.
6. Harivaindaran KV, Rebecca OPS, Chandran S.. Study of optimal temperature, Ph and
stability of dragon fruit (Hylocereus polyrhizus) peel for use as potential natural
colorant. Pak. J. Biol. Sci., 2008; 11: 2259-2263.
7. Mansour HF, Gamal AM. Environmental assessment of osage orange extraction and its
dyeing properties on protein fabrics Part I: Standardization of extraction. J. Environ. Sci.
Technol., 2011; 4: 395-402.
8. Boyo AO, Shitta MBO, Oluwa T, Adeola S. Bitter leaf (Vernonia amygdalin) for dye
sensitized solar cell. Trends Applied Sci. Res., 2012; 7: 558-564.
9. Sinha K, Saha PD, Ramya V, Datta S. Improved extraction of natural blue dye from
butterfly pea using microwave assisted methodology to reduce the effect of synthetic
10. Sekar N. Application of natural colourants to textiles– principles and limitations.
Colourage, 1999; 46(7): 33-34.
11. Gulrajani ML. Present status of natural dyes. Indian Journal of Fibre and Textile
Research, 2001; 26(12):191-201.
12. Sharma J, Varma R. Historical review on Mallotus philippienensis (Lam.) M.Arg.
endangered plant. International Human Research Journal, 2014; 2(2): 1-9.
13. Gangwar M, Dalai A, Chaudhary A, Tryambak D, Singh SK, Goel RK, Nath G. Study
on activity of alcoholic extract of glands and hairs of fruits of M. philippinensis in
murine cestodal infection model. International Journal of Pharmacy and Pharmaceutical
Sciences, 2012; 4: 643-645.
14. Zafar R, Yadav K. Pharmacognostical and phytochemical investigations on the leaves of
M. philippinensis Muell. Arg., Hamdard Medicus, 1993; 36(3): 41-45.
15. Verma P,GuptaSS, Aggarwal SK. Purgative and anthelmintic activity in Mallotus
philippinenesis. Ind. J. Pharmacol., 1983; 13(1): 103.
16. Muhammad Arfan, Hazrat Amin, Karamać Magdalena, Kosińska Agnieszka,
Wiczkowski Wiesław and Amarowicz Ryszard. Antioxidant activity of phenolic
fractions of M. philippinensis bark extract. Czech Journal of Food Sciences., 2009; 27:
109-117.
17. Moorthy K, Srinivasan K, Subramanian C, Mohanasundari C, Palaniswamy M.
Phytochemical screening and antibacterial evaluation of stem bark of M. philippinensis
var. Tomentosus. African Journal of Biotechnology, 2007; 6: 1521-1523.
18. Sharma A, Chandraker S, Patel VK, Padmini R. Antibacterial activity of medicinal
plants against pathogens causing complicated urinary tract infections. Indian Journal of
Pharmaceutical Sciences, 2009; 71: 136-139.
19. Velanganni J, Kadamban D, Tangavelou AC. Phytochemical screening and antimicrobial
activity of the stem of Mallotus philippinensis (Lam.) Muell. Arg. Var. Philippensis
(Euphorbiaceae). International Journal of Pharmacy and Pharmaceutical Sciences, 2011;
3(2): 160-163.
20. Gupta SS, Verma P. Lithontriptic effect of M. philippinensis Muell.Arg. (Kamala). J .
Res. Ayur. Siddha, 1989; 10(3): 175-178.
21. Thakur SC, Thakur SS, Chaube SK, Singh SP. An ethereal extract of Kamala (M.
philippinensis (Moll.Arg) Lam.) seed induce adverse effects on reproductive parameters
22. Arya Vikrant. A Review on Anti-Tubercular Plants. International Journal of PharmTech
Research CODEN (USA), 2011; 3: 872-880.
23. Ramakrishna S, Geetha KM, Bhaskar Gopal PVVS, Ranjit Kumar P, Charan Madav P,
Umachandar L. Effect of Mallotus philippienensis Muell. Arg leaves against
hepatotoxicity of Carbon tetrachloride in rats. International Journal of Pharma Sciences
and Research, 2011; 2: 74-83.
24. Kadam VB, Ahire PP. Biochemical evaluation of three endangerd taxa of south Gujrat
forest. Plant Archives. 2007; 7: 897-898.
25. Nair SP, Rao JM. Kamaladiol‐3‐acetate from the stem bark of M. philippinensis.
Phytochemistry, 1993; 32: 407‐409.
26. Bandopadhyay M et al. Triterpenoid and other components of M. philippinensis,
Euphorbiaceae. Phytochemistry, 1972; 11: 1511.
27. Ahluwalia VK, Sharma ND, Mittal B, Gupta SR. Novel prenylated flavonoids from M.
philippinensis Muell. Arg., Indian J. Chem., 1988; 27B(3): 238-241.
28. Saijo, R. et al. Tanins and related compounds. LXXXVIII. Isolation and characterization
of hydrozable tannins from Mallotus japonicum (Thumb) Muller Arg., Mallotus
philippensis (Lam.) Muller Arg., Chem. Pharm. Bull., 1989; 37: 2940-2947.
29. Tanaka T, Ito T, Iinuma M, Takahashi Y, Naganawa H. Dimeric chalcone derivatives
from Mallotus philippienensis. J. Phytochemistry, 1998; 48(8): 1423-1427.
30. Pandey BP. People and Plants of India: Tannins and Dyes. New Delhi: S. Chand &
Company Ltd, 1981.
31. Cragg GM, Newman DJ. Biodiversity: A continuing source of novel drug leads. Pure
Appl Chem., 2005; 77: 7‐24.
32. Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. Germany:
Springer‐Verlag Berlin/Heidelberg, 2007.
33. Reiko T, Tomoko N, Chiharu Y, Shun‐Ichi W, Takeshi Y, Harukuni T. Potential
Anti‐Tumor‐Promoting activity of 3a‐Hydroxy‐D: A‐friedooleanan‐2‐one from the Stem
Bark of Mallotus philippensis. Planta Medica, 2008; 74: 413‐416.
34. Rivie`re Hoai N, Dejaegher B, Tistaert C, Nguyen Thi T, Kim J, Quetin-Leclercq.
Mallotus species from Vietnamese mountainous areas: Phytochemistry and
35. Likai Xia.,Young Rok Lee. Efficient Synthesis of Biologically Interesting Natural
Pyranochalcones from Mallotus philippienensis and Their Unnatural Derivatives.
Bull.Korean Chem. Soc., 2011; 32(8): 2921-2927.
36. Gangwar MR. Goel RK, Gopal N. M. philippinensis Muell. Arg (Euphorbiaceae):
Ethnopharmacology and Phytochemistry Review. Bio. Med. Research International,
2014; 1-13.
37. The Ayurvedic Pharmacopoeia of India, Part-I. Department of AYUSH, Ministry of
Health and Family Welfare, Govt. of India, New Delhi, 1989; 1: 55.
38. The Wealth of India, Raw Materials, Vol. VI. Publications and Information Directorate,
CSIR, New Delhi, 1992; 229-233.
39. The Wealth of India. A Dictionary of Indian Raw materials & Industrial Products. First
supplement series (Raw materials), (J-Q), National Institute of Science Communication
and Information Resources (CSIT-NISCAIR), New Delhi, 2003, 9-32.
40. Handbook of Textile Testing, 1989; 4(15-4): 127, 137,157 & 193.
41. Tim P, Sheila L. The lightfastness of the natural dyes. Stud. Conserv, 1966; 11(4):
181-198.
42. Kunio Y, Patricia CC. Characteristics of fading of wool cloth dyed with selected natural
dyestuffs on the basis of solar radiant energy. Dyes & Pigments, 2003; 58: 199-204.
43. Patricia CC The fading rates of some natural dyes. Stud. Conserv, 1987; 32(2): 65-72.
44. Kadolph JS. Identification of plant residue with commercial potential as natural
dyestuffs. Leopald Centre for Sustainable Agriculture Progress Report, Iowa State
University, 2005; 14: 56.
45. Anna H, Christian RV. The potential use of organically grown dye plants in the organic
textile industry. Experiences and Results on the Cultivation and Yields of Dyers
chemimile (Anhemis tinctoria L.), Dyers Knotweed (Polygonum tinctoria Ait) and Weld