www.wjpr.net Vol 3, Issue 3, 2014. 5041
EVALUATION OF THE DPPH FREE RADICAL SCAVENGING
ACTIVITY OF WRIGHTIA TINCTORIA R. Br. LEAF, BARK AND
SEED EXTRACTS
Beena Jose1*, Jesy E.J.1, Ritty J. Nedumpara2
1
Department of Chemistry, Vimala College, Thrissur, Kerala, 680009, India. 2
Department of Physics, Vimala College, Thrissur, Kerala, 680009, India.
ABSTRACT
The DPPH free radical scavenging activity of the leaf, bark and seed
extracts of Wrightia tinctoria (Roxb.) R. Br. (Apocynaceae) was
evaluated. Among these, DPPH radical could be scavenged most
effectively by Wrightia tinctoria bark ethyl acetate extract with IC50 value 67.30±2.4µg/ml and was quite comparable with the standard
antioxidant L-ascorbic acid (IC50 value 62.5±2.5µg/ml). The antioxidant activity of W. tinctoria leaf methanol extract
(IC50:72.9±3.5µg/ml) was not significantly different from that of L- ascorbic acid (IC50: 62.5 ±2.5µg/ml). The remarkable antioxidant activity exhibited by the plant extracts can be attributed to the synergic
effect of the active compounds present in it. The results obtained showed that the bark ethyl
acetate and leaf methanol extracts of Wrightia tinctoria can be considered as good sources of
natural antioxidants and can be incorporated into the drug formulations.
Key words: Wrightia tinctoria, Apocynaceae, DPPH radical scavenging activity, natural antioxidants, drug formulations.
INTRODUCTION
In the recent years, the antioxidant actions have received much attention. It is well known
that reactive oxygen species (ROS) formed in vivo, such as superoxide anion, hydroxyl
radical and hydrogen peroxide, are highly reactive and potentially damaging transient
chemical species [1]. ROS, causing damage to DNA, proteins and lipids, have been associated with carcinogenesis, coronary heart disease, and many other health problems [2]. Free radicals are known to play a definite role in a wide variety of pathological manifestations of pain,
Volume 3, Issue 3, 5041-5048. Research Article ISSN 2277 – 7105
Article Received on 12 March 2014,
Revised on 05 April 2014, Accepted on 28 April 2014
*Correspondence for Author
Dr. Beena Jose
www.wjpr.net Vol 3, Issue 3, 2014. 5042 inflammation, cancer, diabetes, alzheimer; hepatic damage etc. antioxidants fight free radicals
and protect us from various diseases. They exert their action either by scavenging the reactive
oxygen species or protecting the antioxidant defense mechanisms [3]. Minimizing oxidative damage may well be one of the most important approaches to the primary prevention of these
oxidative stress-related diseases and health problems, since antioxidants terminate direct ROS
attacks and radical-mediated oxidative reactions [4]. Plants are the primary sources of naturally occurring antioxidants for humans [5].
W. tinctoria R.Br. Belongs to family Apocynaceae [6], is a small deciduous tree, generally up to 1.8 m tall and often under 60 cm girth, sometimes up to 7.5 m high, distributed all over
India. It is commonly known as “indrajav”, has been important in the traditional healing and
widely recognized medicinal plant [7]. The wrightial a new terpene and other phytoconstituents such as cycloartenone, cycloeucalenol were isolated identified by
fractionation of methanol extract of the immature seed pods [8]. The ursolic acid and isoricinolic acid has been also isolated from the seed pods [9]. The characterization of lingo-cellulosic seed fiber from W. tinctoria has been carried out [10].Almost every part of plant is useful - leaves pungent chewed for relief from tooth ache, bark and seeds are antidysenteric,
antidiarrhoel and antihaemorrhagic [11]. Oil emulsion of leaves and pods is used to treat psoriasis [12, 13]. The plant is also reported for its antimicrobial, wound healing and hepatoprotective activity [14, 15, 16, 17].
Earlier chemical investigations on Wrightia tinctoria reported alkaloids [18], triterpenoids [19], steroids, flavonol [20] etc. as major chemical constituents in the leaves of this plant. However, the antioxidant activity of the seed, bark and leaf extracts and leaf coconut oil extract of this
plant were not revealed completely. DPPH radical scavenging activity of the leaf methanol
extract was reported [21]. In the present study, the antioxidant activity of the leaf, bark and seed extracts of Wrightia tinctoria were evaluated by DPPH radical scavenging assay. The
results showed that the methanolic and ethyl acetate extracts of the leaf and bark of Wrightia
tinctoria is a good source of active compounds and antioxidants.
MATERIALS AND METHODS Plant Material
The leaf, bark and seeds of Wrightia tinctoria were collected from Thrissur district of Kerala,
South India and authenticated by Dr. Kochuthressia M.V., HOD, Department of Botany,
www.wjpr.net Vol 3, Issue 3, 2014. 5043 herbarium, Department of Botany, Vimala College, Thrissur.
Preparation of Plant Extracts
Fifty grams of the powered plant material were extracted successively with 150mL of
petroleum ether, chloroform, ethyl acetate and methanol as solvents for 24hours by Soxhlet
equipment. Leaf coconut oil extracts were prepared by soaking the fresh leaves of Wrightia
tinctoria (500g) in 1Kg coconut oil and kept for 1, 4, 7 and 15 days in the sunlight.
Preliminary Phytochemical analysis
The sample extracts were analysed for the presence of various phytoconstituents like
flavonoids, alkaloids, glycosides, steroids, phenols, saponins and tannins according to
standard methods [22].
DPPH free radical scavenging assay
The DPPH free radical is a stable free radical, which has been widely accepted as a tool for
estimating free radical-scavenging activities of antioxidants [23]. Hydrogen or electron donation abilities of the compounds were measured from the bleaching of the purple-colored
methanol solution of 1, 1-diphenyl-2-picrylhydrazyl (DPPH). This spectrophotometer assay
uses the stable radical DPPH as a reagent. The sample solution of material (50 µL) at four
concentrations (1.0, 0.5, 0.25 and 0.125 mg/mL) were mixed with freshly prepared
methanolic solution of DPPH (634 µM) and allowed to stand for 30 min at room temperature.
The absorbance was then measured at 515nm using a spectrophotometer and the inhibition of
free radical DPPH in percent (%) was calculated using the formula below:
The percent of inhibition of DPPH reduction (decolourization)
Asample
A0
A0 X 100
% of inhibition =
where (A0) is the absorbance of the control (blank) and (A sample) is the absorbance of the test compound. The compound concentration demonstrating 50% inhibition (IC50) was calculated from the plot of inhibition percentage against sample concentration. Tests were carried out in
triplicate. Samples and DPPH were dissolved in methanol. L-ascorbic acid was used as
www.wjpr.net Vol 3, Issue 3, 2014. 5044 RESULTS
Antioxidant activity
The antioxidant activity of Wrightia tinctoria leaf, bark and seed extracts in solvents of
varying polarity were measured in terms of hydrogen donating or radical scavenging ability,
using the stable radical, DPPH. The method is based on the reduction of alcoholic DPPH·
solutions in the presence of a hydrogen donating antioxidant. DPPH· solutions show a strong
absorption band at 515 nm appearing as a deep violet color. The absorption vanishes and the
resulting decolourization is stoichiometric with respect to degree of reduction. The remaining
DPPH·, measured after a certain time, corresponds inversely to the radical scavenging
activity of the antioxidant. The results of the free radical scavenging activity of the leaf
extracts of W.tinctoria assessed by DPPH assay were summarized in table 1. The DPPH
radical scavenging activity of the bark and seed extracts is given in table 2.
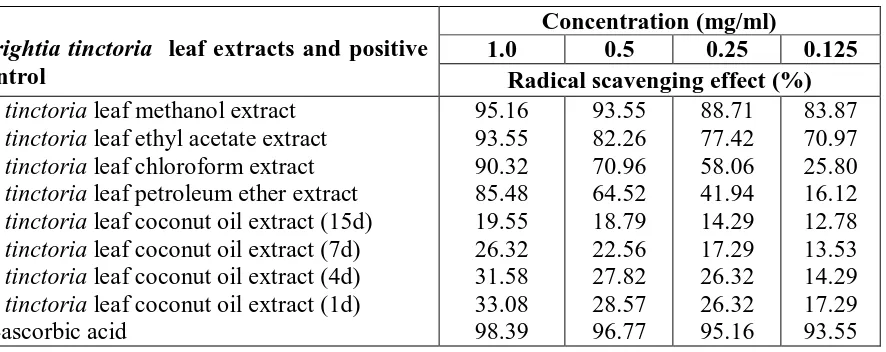
Table 1: The DPPH radical scavenging activity of the leaf extracts of Wrightia tinctoria
Wrightia tinctoria leaf extracts and positive
control
Concentration (mg/ml)
1.0 0.5 0.25 0.125
Radical scavenging effect (%) W. tinctoria leaf methanol extract
[image:4.595.91.534.352.528.2]W. tinctoria leaf ethyl acetate extract W. tinctoria leaf chloroform extract W. tinctoria leaf petroleum ether extract W. tinctoria leaf coconut oil extract (15d) W. tinctoria leaf coconut oil extract (7d) W. tinctoria leaf coconut oil extract (4d) W. tinctoria leaf coconut oil extract (1d) L-ascorbic acid 95.16 93.55 90.32 85.48 19.55 26.32 31.58 33.08 98.39 93.55 82.26 70.96 64.52 18.79 22.56 27.82 28.57 96.77 88.71 77.42 58.06 41.94 14.29 17.29 26.32 26.32 95.16 83.87 70.97 25.80 16.12 12.78 13.53 14.29 17.29 93.55
Table 2: The DPPH radical scavenging activity of the seed and bark extracts of Wrightia
tinctoria
Wrightia tinctoria
bark & seed extracts, positive control
Seed extracts Bark extracts
Concentration (mg/ml) Concentration (mg/ml) 1.0 0.5 0.25 0.125 1.0 0.5 0.25 0.125
Radical scavenging effect (%) Methanol extract
EA extract
www.wjpr.net Vol 3, Issue 3, 2014. 5045 The amount of the extract needed for 50% inhibition of free radical activity is expressed by
IC50. Lower IC50 value indicates higher antioxidant activity. IC50 value of Wrightia tinctoria leaf methanol, leaf ethyl acetate, seed methanol, bark ethyl acetate extracts and the authentic
antioxidant L-ascorbic acid are given in table 3.
Table 3: Antioxidant activities of the W. tinctoria leaf, bark and seed extracts and positive control using the (DPPH) free radical - scavenging assay
Samples
Antioxidant activity IC50 (µg/ml)
W. tinctoria leaf methanol extract W. tinctoria leaf ethyl acetate extract W. tinctoria seed methanol extract W. tinctoria bark ethyl acetate extract L-ascorbic acid
72.90±3.5 87.50±3.2 86.53±2.5 67.30±2.4 62.5±2.5
DISCUSSION
DPPH free radical scavenging activity assay
The DPPH free radical scavenging activity of the leaf extracts of W. tinctoria are sorted in
descending order: leaf methanol extract> Leaf ethyl acetate extract> Leaf chloroform
extract> Leaf petroleum ether extract. Out of the four samples tested, W. tinctoria leaf
methanol extract showed the highest scavenging activity (% inhibition 95.16, 93.55, 88.71
and 83.87 at 1.0, 0.5, 0.25 and 0.125 mg/ml respectively), followed by W. tinctoria leaf ethyl
acetate extract. Leaf petroleum ether extract exhibited least DPPH radical scavenging ability
with % inhibition 85.48, 64.52, 41.94 and 16.12 at 1.0, 0.5, 0.25 and 0.125mg/ml
respectively.
The DPPH free radical scavenging activity of the leaf coconut oil extracts of Wrightia
tinctoria are: leaf extract (1d)> leaf extract (4d)> leaf extract (7d)> leaf extract (15d). Leaf
coconut oil extract with one day exposure to sunlight was found to be more active than the
extract with 15days of exposure to sunlight. Leaf coconut oil extracts are comparatively less
active (maximum % inhibition 33.08) than petroleum ether, chloroform, ethyl acetate and
methanol extracts of the leaf, seed and bark of W. tinctoria.
The ability of the W. tinctoria seed extract to scavenge the DPPH free radical is: seed
methanol extract> seed petroleum ether extract> seed ethyl acetate extract> seed chloroform
extract and bark extract is: bark ethyl acetate extract> bark methanol extract> bark
www.wjpr.net Vol 3, Issue 3, 2014. 5046 Among the W. tinctoria extracts studied, seed methanol extract, bark ethyl acetate extract and
leaf methanol extract showed considerable radical scavenging activity. The radical
scavenging activity of these extracts is compared with the standard L-ascorbic acid. Wrightia
tinctoria bark ethyl acetate showed greater ability to quench DPPH radicals and its activity
was found to be same as that of L-ascorbic acid. W. tinctoria leaf methanol extract exhibited
more activity than seed methanol extract.
The bark ethyl acetate extract of W. tinctoria was found to be the significant one for
scavenging DPPH radicals with IC50 value of 67.30±2.4 µg/ml and was quite comparable with the standard antioxidant L-ascorbic acid 62.5±2.5 µg/ml. IC50 value of Wrightia tinctoria leaf ethyl acetate extract 87.5±3.2 µg/ml and seed methanol extract 86.53±2.5 were
almost same. By comparing the of the IC50 value of W. tinctoria leaf methanol extract with that of the authentic antioxidant L-ascorbic acid, it was found that the antioxidant activity of
W. tinctoria leaf methanol extract (IC50:72.9±3.5 µg/ml) was not significantly different from that of L- ascorbic acid (IC50: 62.5 ±2.5 µg/ml).
CONCLUSIONS
Among the leaf, bark and seed extracts of Wrightia tinctoria studied, bark ethyl acetate
extract showed potent scavenging activity on DPPH free radical and its activity was quite
comparable with the standard antioxidant L-ascorbic acid. Antioxidant activities of the
extracts from medicinal plants are mainly attributed to the active compounds present in them.
This can be due to the high percentage of main constituents, but also to the presents of other
constituents in small quantities or to synergy among them [24, 25].
Phytochemical screening of the bark ethyl acetate extract of W. tinctoria revealed the
presence of phenolics and tannins. The leaf methanol extract of W.tinctoria was rich in
phenolics, saponins, flavonoids, alkaloids, glycosides and steroids [26]. Appreciable amounts of phenolics, saponins, flavonoids and glycosides were present in the seed methanol extract.
Bark methanol extract showed appreciable amount of tannins, saponins, flavonoids and
phenolics. The antioxidant potential of leaf, bark and seed extracts can be attributed to the
presence of these phytochemicals. The results obtained shows that the methanolic extracts of
the seeds and leaf, ethyl acetate extracts of the bark and laves of W.tinctoria can be
considered good source of natural antioxidants. The study supports the use of this plant for
drug formulations and reinforces the ethnobotanical importance of plant as a potential source
www.wjpr.net Vol 3, Issue 3, 2014. 5047 ACKNOWLEDGEMENT
One of the authors (Beena Jose) sincerely acknowledges the University Grants Commission
(UGC), New Delhi for the financial support to carry out the Major Research Project.
REFERENCES
1. Patel DK, Kumar R, Laloo D, Hemalatha S. Evaluation of phytochemical and antioxidant
activities of the different fractions of Hybanthus enneaspermus (Linn.) F. Muell.
(Violaceae). Asian Pac J Trop Med, 2011; 4(5): 391-396.
2. Sasidharan I, Menon AN. A study of antioxidant properties of different extracts of curry
leaf (Murraya koenigii L.). Electron J Environ Agric Food Chem, 2010; 9(6): 1036-1046.
3. Beena Jose, Joji LR. Evaluation of Antibacterial and DPPH Radical Scavenging
Activities of the Leaf Extracts of Cassia Fistula Linn from South India. Open Access
Scientific Reports 2013; 2 (8): 773-777.
4. Sanz A, Stefanatos RK. The mitochondrial free radical theory of aging: a critical view.
Curr Aging Sci, 2008; 1: 10-21.
5. Ramalakshmi S, Edaydulla N, Ramesh P, Muthuchelian K. Investigation on cytotoxic,
antioxidant, antimicrobial and volatile profile of Wrightia tinctoria (Roxb.) R. Br. flower
used in Indian medicine. Asian Pacific J of Tropical Disease, 2012; 68-75.
6. Anonymous. The Wealth of India. Raw materials, publication and Information
Directorate, CSIR, New Delhi, India vol. X, 1976: 588.
7. Khyade MS, Vaikos NP. Comparative phytochemical and antibacterial studies on the
bark of Wrightia tinctoria and Wrightia arborea. Int J Pharm Bio Sci, 2011; 2: 176-181.
8. Ramchandra P, Basheermiya M, Krupadanam GLD, Srimannaryana G. Wrightial: A new
Terpene from Wrightia tinctoria. J Nat Prod, 1993; 56: 1811-1812.
9. Ahmad I, Lie Ken Jie, MSF: Oleochemicals from Wrightia tinctoria seed oil. Ind Eng
Chem Res, 2008; 47:2091-2095.
10. Jain PS, Bari SB. Isolation of lupeol, stigmasterol and campesterol from petroleum ether
extract of woody stem of Wrightia tinctoria. Asian J. Plant Sci, 2010; 9: 163-167.
11. Singh VP, Sharma SK .Pharmacognostical studies on Wrightia tinctoria Bark. Ind Drugs
1980; 17: 7-10.
12. Mitra SK, Seshadri SJ, Venkataranganna MV, Gopumadhvan S. Reversal of
parakeratosis, a feature of Psoriasis By Wrightia tinctiria (In Emulsion) Histological
www.wjpr.net Vol 3, Issue 3, 2014. 5048 13. Krishnamoorthy JR, Ranganathan S. Antipityrosporum ovale activity of a herbal drug
combination of W. tinctoria and Hibiscus rosa-sinensis. Ind J dermatol, 2000; 45:
125-126.
14. Dang R, Sabitha JS, Shivanand BG. Anti-microbial activity of herbs used in psoriasis.
The Pharm Rev, 2005; 9: 31-32.
15. Veerapur VP, Palkar MB, Srinivasa H, Kumar MS, Patra S, Rao PGM, Srinivasan KK.
The effect of ethanolic extract of Wrightia tinctoria bark on wound healing in rats. J Nat
Rem, 2004; 4(2):155-159.
16. Chandrashekar VM, Nagappa AN. Hepatoprotective activity of Wrightia tinctoria (Roxb)
in rats. Ind Drugs, 2004; 41(6): 366-370.
17. Pritam S, Sanjay B. Antibacterial and anti fungal activity of extracts of woody stem of
Wrightia tinctoria R.Br. Int J Pharm Recent Res 2009; 1(1):18-21.
18. George V, Koshy AS, Singh OV, Nayar MNS, Pushpangadan P. Tryptanthrin from
Wrightia tinctoria. Fitoterapia, 1996; 67 (6): 553-554.
19. Ramchandra P, Basheermiya M, Krupadanam GLD, Srimannarayana G. Wrightial, a new
terpene from Wrightia tinctoria. J Nat Prod, 1993; 56 (10): 1811-1812.
20. Daniel M, Sabnis SD. Chemotaxonomical studies on Apocynaceae. Ind J Exp Biol, 1978;
16 (4): 512-513.
21. Jyotiram A, Sawale CV, Panchal, Suhas Padmane, Poul BN, Patel JR. In vitro antioxidant
and anti-inflammatory activity of Wrightia tinctoria leaves. World J Pharm Pharmaceut
Sci, 2014; 3(4): 964-972.
22. Harborne JB. Pytochemical methods, A guide to Modern techniques of plant analysis,
London: Chapman and Hall Ltd; 1973.
23. Fenglin H, Ruili L, Bao H, Liang M. Free radical scavenging activity of extracts
prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia, 2004; 75:
14-23.
24. Abdalla, AE, Roozen, JP. Effect of plant extracts on the oxidative stability of sunflower
oil and emulsion. Food Chemistry 1999; 64: 323–329.
25. Hamdoon AM, Salmin KA, Awad GA. Antioxidant and Quantitative Estimation of
Phenolic and Flavonoids of Three Halophytic Plants Growing in Libya. J Pharm
Phytochem 2013; 2(3): 89-94.
26. Muruganandam AV, Bhattacharya SK, Ghosa S. Indole and flavonoid constituents of
Wrightia tinctoria, W. tomentosa and W. coccinea. Ind J Chem (B), 2000; 39 (2): 125 –