metal-organic papers
m562
Andrea Berenbaumet al. [Fe(C5H4)2(CH3Si)]2O DOI: 10.1107/S1600536802016690 Acta Cryst.(2002). E58, m562±m563 Acta Crystallographica Section EStructure Reports
Online ISSN 1600-5368
An oxygen-bridged bis(sila[1]ferrocenophane),
[Fe(
g
-
C
5H
4)
2Si(Me)]
2O
Andrea Berenbaum, Alan J. Lough* and Ian Manners
Department of Chemistry, University of Toronto, Toronto, Ontario, Canada M5S 3H6
Correspondence e-mail: alough@chem.utoronto.ca
Key indicators Single-crystal X-ray study
T= 100 K
Mean(C±C) = 0.004 AÊ
Rfactor = 0.029
wRfactor = 0.069
Data-to-parameter ratio = 17.8
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
In the title compound, [Fe(C5H4)2(CH3Si)]2O, the
cyclopenta-dienyl rings in both ferrocenophane moieties are tilted towards the bridging Si atoms, with tilt angles of 19.7 (2) and 20.7 (2); the central SiÐOÐSi angle is 143.25 (12).
Comment
The title compound was isolated in trace amounts as an impurity from aged samples of the silicon-bridged [1]ferrocenophane, (II), (Zechelet al., 1996), which possesses a hydrolytically sensitive SiÐCl moiety. The bis(sila[1]-ferrocenophane), (I), is of potential utility as a crosslinking agent in the polymerization of other sila[1]ferrocenophanes (MacLachlanet al., 1996, 2000; Kulbabaet al., 2001; Kulbaba & Manners, 2001); however, attempts to produce it on a large scale have been unsuccessful to date.
In (I), the cyclopentadienyl (Cp) rings of both of the ferrocenophane groups are tilted towards the bridging Si atoms. The angle between the least-squares planes of the Cp rings bonded to Fe1 is 19.7 (2), and the angle between the Cp
rings bonded to Fe2 is 20.7 (2). The tilt of the Cp rings is also
re¯ected in the variation of the FeÐC distances in each of the ferrocene groups. The FeÐC distances in the ferrocene group containing Fe1 range from 2.010 (2) to 2.082 (3) AÊ, and in the ferrocene group containing Fe2, the FeÐC distances range from 2.009 (3) to 2.087 (3) AÊ. The shortest FeÐC distances are for the C atoms which are also bonded to the bridging Si atoms, namely C1, C6, C11 and C16 (see Table 1). The overall conformation of the molecule is twisted, such that the ferro-cene groups are rotated about the SiÐO bonds so that they are approximately perpendicular to each other (see Fig. 1). The degree of rotation can be described by a torsion angle calculated using the non-bonded atoms Fe1 Si1 Si2 Fe2, which gives a value of 75.07 (5). The Si1ÐO1ÐSi2 angle is
143.25 (12).
Experimental
Crystals of (I) were obtained at 243 K in trace amounts from the hexanes-soluble fraction of a reaction mixture which initially contained (II). No other hexanes-soluble products were formed in
this reaction, and an investigation of the sample of (II) utilized in the synthesis revealed the presence of trace (< 5%) amounts of (I). For (I):1H NMR (400 MHz, C
6D6, 298 K): = 4.46±4.44 (m, 4H, Cp),
4.42±4.39 (m, 8H, Cp), 3.87±3.86 (m, 4H, Cp), 0.58 (s, 6H, Me);
13C{1H} NMR (100.4 MHz, C
6D6, 298 K):= 78.4, 77.8, 75.9, 74.5
(Cp), 38.7 (ipso-Cp), ÿ0.6 (Me); 29Si{1H} NMR (79.3 MHz, C 6D6,
298 K):=ÿ13.4.
Crystal data [Fe(C5H4)2(CH3Si)]2O Mr= 470.28
Orthorhombic,P212121 a= 7.5203 (1) AÊ b= 11.4213 (3) AÊ c= 22.2986 (6) AÊ V= 1915.26 (8) AÊ3 Z= 4
Dx= 1.631 Mg mÿ3
MoKradiation
Cell parameters from 19591 re¯ections
= 2.9±27.5
= 1.65 mmÿ1 T= 100 (1) K Plate, orange 0.350.320.10 mm
Data collection
Nonius Kappa±CCD diffractometer 'scans and!scans withoffsets Absorption correction: multi-scan (DENZO-SMN; Otwinowski & Minor, 1997)
Tmin= 0.596,Tmax= 0.853
19591 measured re¯ections
4381 independent re¯ections 3810 re¯ections withI> 2(I) Rint= 0.075
max= 27.5 h=ÿ9!9 k=ÿ14!14 l=ÿ28!28 Re®nement
Re®nement onF2 R[F2> 2(F2)] = 0.029 wR(F2) = 0.069 S= 1.00 4381 re¯ections 246 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0359P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.31 e AÊÿ3
min=ÿ0.48 e AÊÿ3
Absolute structure: (Flack, 1983), 1871 Friedel pairs
Flack parameter =ÿ0.009 (13)
Table 1
Selected geometric parameters (AÊ,).
Fe1ÐC1 2.010 (2)
Fe1ÐC6 2.016 (2)
Fe1ÐC2 2.025 (3)
Fe1ÐC7 2.032 (3)
Fe1ÐC5 2.032 (3)
Fe1ÐC10 2.037 (3)
Fe1ÐC4 2.068 (3)
Fe1ÐC9 2.076 (3)
Fe1ÐC8 2.076 (3)
Fe1ÐC3 2.082 (3)
Fe2ÐC11 2.009 (3) Fe2ÐC16 2.020 (3) Fe2ÐC20 2.025 (3) Fe2ÐC12 2.027 (3)
Fe2ÐC15 2.028 (3) Fe2ÐC17 2.030 (2) Fe2ÐC14 2.079 (3) Fe2ÐC13 2.080 (3) Fe2ÐC18 2.080 (3) Fe2ÐC19 2.087 (3)
Si1ÐO1 1.6308 (18)
Si1ÐC21 1.851 (3)
Si1ÐC6 1.882 (3)
Si1ÐC1 1.885 (3)
Si2ÐO1 1.6377 (19)
Si2ÐC22 1.847 (3) Si2ÐC16 1.871 (3) Si2ÐC11 1.879 (3)
O1ÐSi1ÐC6 111.18 (11) O1ÐSi1ÐC1 111.21 (11) C6ÐSi1ÐC1 96.82 (11) O1ÐSi2ÐC16 110.51 (11)
O1ÐSi2ÐC11 110.11 (11) C16ÐSi2ÐC11 96.72 (11) Si1ÐO1ÐSi2 143.25 (12)
Fe1ÐSi1ÐSi2ÐFe2 75.07 (5)
All H atoms were included in calculated positions, with distances of 1.00 AÊ (for Cp CÐH) and 0.98 AÊ (for methyl CÐH). In the re®nement H atoms were included in riding-motion approximation, withUiso= 1.2Ueq(1.5eqfor methyl) of the carrier atom.
Data collection:COLLECT(Nonius 1997±2001); cell re®nement:
DENZO-SMN (Otwinowski & Minor, 1997); data reduction:
DENZO-SMN; program(s) used to solve structure: SHELXTL
(Sheldrick, 1999); program(s) used to re®ne structure:SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication:SHELXTL.
The authors acknowledge NSERC Canada and the University of Toronto for ®nancial support.
References
Flack, H. D. (1983).Acta Cryst.A39, 876±881.
Kulbaba, K. & Manners, I. (2001).Macromol. Rapid Commun.22, 711±724. Kulbaba, K., Resendes, R., Cheng, A., Bartole, A., Safa-Sefat, A., Coombs, N.,
Stoever, H. D. H., Greedan, J. E., Ozin, G. A. & Manners, I. (2001).Adv. Mater.13, 732±736.
MacLachlan, M. J., Ginzburg, M., Coombs, N., Coyle, T. W., Raju, N. P., Greedan, J. E., Ozin, G. A. & Manners, I. (2000).Science,287, 1460±1463. MacLachlan, M. J., Lough, A. J. & Manners, I. (1996).Macromolecules, 29,
8562±8564.
Nonius (1997±2001).COLLECT. Nonius BV, Delft, The Netherlands. Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
Macromolecular Crystallography, Part A, edited by C. W. Carter Jr and R. M. Sweet, pp. 307±326. New York: Academic Press.
Sheldrick, G. M. (1999).SHELXTL/PC. Version 5.1 for Windows NT. Bruker AXS Inc., Madison, USA.
Zechel, D. L., Hultzsch, K. C., Rulkens, R., Balaishis, D., Ni, Y., Pudelski, J. K., Lough, A. J., Manners, I. & Foucher, D. A. (1996).Organometallics,15, 1972±1978.
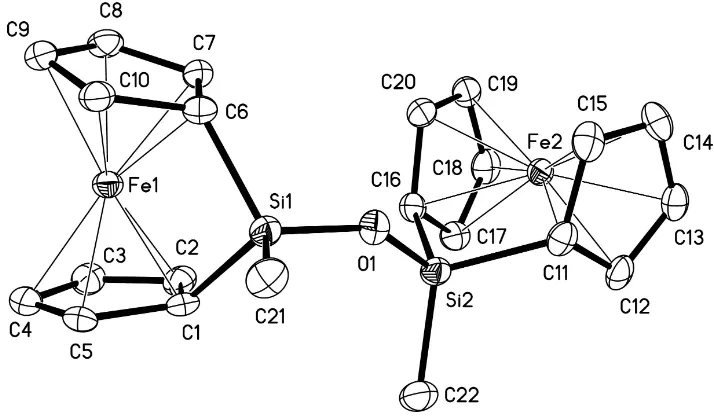
Figure 1
supporting information
sup-1
Acta Cryst. (2002). E58, m562–m563supporting information
Acta Cryst. (2002). E58, m562–m563 [doi:10.1107/S1600536802016690]
An oxygen-bridged bis(sila[1]ferrocenophane), [Fe(
η
-
C
5H
4)
2Si(Me)]
2O
Andrea Berenbaum, Alan J. Lough and Ian Manners
S1. Comment
The title compound was isolated in trace amounts as an impurity from aged samples of the silicon-bridged
[1]ferrocenophane, (II), (Zechel et al., 1996) which possesses a hydrolytically sensitive Si—Cl moiety. The bis-(sila[1]ferrocenophane), (I), is of potential utility as a crosslinking agent in the polymerization of other
sila[1]ferrocenophanes (MacLachlan et al., 1996, 2000; Kulbaba et al., 2001; Kulbaba & Manners, 2001); however, attempts to produce it on a large scale have been unsuccessful to date.
In (I), the cyclopentadienyl (Cp) rings of both of the ferrocenophane groups are tilted towards the bridging Si atoms.
The angle between the least-squares planes of the Cp rings bonded to Fe1 is 19.7 (2)°, and the angle between the Cp rings
bonded to Fe2 is 20.7 (2)°. The tilt of the Cp rings is also reflected in the variation of the Fe—C distances in each of the
ferrocene groups. The Fe—C distances in the ferrocene group containing Fe1 range from 2.010 (2) to 2.082 (3) Å and in
the ferrocene group containing Fe2, the Fe—C distances range from 2.009 (3) to 2.087 (3) Å. The shortest Fe—C
distances are for the C atoms which are also bonded to the bridging Si atoms, namely C1, C6, C11 and C16 (see Table 1).
The overall conformation of the molecule is twisted such that the ferrocene groups are rotated about the Si—O bonds so
that the they are approximately perpendicular to each other (sse Fig. 1). The degree of rotation can be described by a
torsion angle calculated using the non-bonded atoms Fe1—Si1—Si2—Fe2, which gives a value of 75.07 (5)°. The Si1—
O1—Si2 angle is 143.25 (12)°.
S2. Experimental
Crystals of (I) were obtained at 243 K in trace amounts from the hexanes-soluble fraction of a reaction mixture which
initially contained (II). No other hexanes-soluble products were formed in this reaction, and an investigation of the
sample of (II) utilized in the synthesis revealed the presence of trace (< 5%) amounts of (I). For 1: 1H NMR (400 MHz,
C6D6, 298 K): δ = 4.46–4.44 (m, 4 H, Cp), 4.42–4.39 (m, 8 H, Cp), 3.87–3.86 (m, 4 H, Cp), 0.58 (s, 6 H, Me); 13C{1H}
NMR (100.4 MHz, C6D6, 298 K): δ = 78.4, 77.8, 75.9, 74.5 (Cp), 38.7 (ipso-Cp), −0.6 (Me); 29Si{1H} NMR (79.3 MHz,
C6D6, 298 K): δ = −13.4.
S3. Refinement
All hydrogen atoms were included in calculated positions, with distances of 1.00 Å (for Cp C—H) and 0.98 Å (for
methyl C—H). In the refinement hydrogen atoms were included in riding-motion approximation, with Uiso = 1.2Ueq (1.5eq
Figure 1
View of molecule (I), with displacement ellipsoids drawn at the 50% probability level.
(I)
Crystal data
[Fe(C5H4)2(CH3Si)]2O
Mr = 470.28
Orthorhombic, P212121
Hall symbol: P 2ac 2ab
a = 7.5203 (1) Å
b = 11.4213 (3) Å
c = 22.2986 (6) Å
V = 1915.26 (8) Å3
Z = 4
F(000) = 968
Dx = 1.631 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 19591 reflections
θ = 2.9–27.5°
µ = 1.65 mm−1
T = 100 K Plate, orange
0.35 × 0.32 × 0.10 mm
Data collection
Nonius Kappa-CCD diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 9 pixels mm-1
φ scans and ω scans with κ offsets Absorption correction: multi-scan
(DENZO-SMN; Otwinowski & Minor, 1997)
Tmin = 0.596, Tmax = 0.853
19591 measured reflections 4381 independent reflections 3810 reflections with I > 2σ(I)
Rint = 0.075
θmax = 27.5°, θmin = 2.9°
h = −9→9
k = −14→14
l = −28→28
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.029
wR(F2) = 0.069
S = 1.00 4381 reflections 246 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
supporting information
sup-3
Acta Cryst. (2002). E58, m562–m563(Δ/σ)max = 0.001
Δρmax = 0.31 e Å−3
Δρmin = −0.48 e Å−3
Absolute structure: (Flack, 1983), 1871 Friedel pairs
Absolute structure parameter: −0.009 (13)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Fe1 0.48696 (5) 0.40794 (3) 0.070092 (16) 0.01818 (9) Fe2 0.91547 (5) 0.44410 (3) 0.332136 (17) 0.01771 (9) Si1 0.73986 (9) 0.55859 (7) 0.10626 (3) 0.01936 (16) Si2 0.77709 (10) 0.57814 (6) 0.24443 (3) 0.01971 (16) O1 0.8254 (2) 0.55946 (16) 0.17346 (8) 0.0220 (4) C1 0.4897 (4) 0.5665 (2) 0.10918 (11) 0.0211 (5) C2 0.3935 (4) 0.4853 (2) 0.14549 (13) 0.0240 (6)
H2A 0.4265 0.4623 0.1873 0.029*
C3 0.2454 (3) 0.4395 (3) 0.11270 (13) 0.0251 (6)
H3A 0.1593 0.3790 0.1271 0.030*
C4 0.2476 (4) 0.4922 (2) 0.05511 (13) 0.0266 (7)
H4A 0.1628 0.4747 0.0217 0.032*
C5 0.3937 (4) 0.5711 (2) 0.05198 (13) 0.0233 (6)
H5A 0.4267 0.6202 0.0165 0.028*
C6 0.7550 (3) 0.4089 (2) 0.07139 (12) 0.0211 (5) C7 0.6860 (4) 0.3082 (2) 0.10450 (12) 0.0212 (6)
H7A 0.7060 0.2923 0.1481 0.025*
C8 0.5825 (4) 0.2377 (2) 0.06505 (13) 0.0238 (6)
H8A 0.5154 0.1655 0.0765 0.029*
C9 0.5844 (4) 0.2900 (2) 0.00733 (12) 0.0223 (6)
H9A 0.5193 0.2609 −0.0288 0.027*
C10 0.6909 (3) 0.3940 (2) 0.01038 (12) 0.0219 (6)
H10A 0.7142 0.4487 −0.0237 0.026*
C11 0.9868 (4) 0.5920 (2) 0.28970 (12) 0.0213 (5) C12 0.9703 (4) 0.6127 (2) 0.35374 (12) 0.0241 (6)
H12A 0.8856 0.6687 0.3730 0.029*
C13 1.0925 (3) 0.5392 (2) 0.38468 (13) 0.0243 (6)
H13A 1.1057 0.5331 0.4292 0.029*
C14 1.1873 (4) 0.4722 (3) 0.34135 (13) 0.0274 (7)
H14A 1.2790 0.4111 0.3500 0.033*
C15 1.1240 (3) 0.5050 (3) 0.28363 (13) 0.0261 (6)
C16 0.6991 (3) 0.4375 (2) 0.27854 (12) 0.0205 (5) C17 0.6473 (3) 0.4380 (2) 0.34176 (12) 0.0220 (6)
H17A 0.5732 0.4996 0.3615 0.026*
C18 0.7214 (4) 0.3389 (2) 0.37108 (14) 0.0260 (6)
H18A 0.7108 0.3204 0.4148 0.031*
C19 0.8180 (4) 0.2732 (2) 0.32837 (13) 0.0242 (6)
H19A 0.8875 0.2003 0.3366 0.029*
C20 0.8060 (4) 0.3321 (2) 0.27218 (13) 0.0223 (6)
H20A 0.8638 0.3056 0.2342 0.027*
C21 0.8374 (4) 0.6748 (2) 0.05862 (13) 0.0263 (6)
H21A 0.8372 0.7491 0.0806 0.040*
H21B 0.7668 0.6830 0.0219 0.040*
H21C 0.9598 0.6537 0.0481 0.040*
C22 0.6212 (4) 0.7006 (3) 0.25725 (14) 0.0319 (7)
H22A 0.6698 0.7722 0.2395 0.048*
H22B 0.6048 0.7120 0.3004 0.048*
H22C 0.5064 0.6824 0.2386 0.048*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-5
Acta Cryst. (2002). E58, m562–m563Geometric parameters (Å, º)
Fe1—C1 2.010 (2) C4—C5 1.423 (4)
Fe1—C6 2.016 (2) C4—H4A 1.0000
Fe1—C2 2.025 (3) C5—H5A 1.0000
Fe1—C7 2.032 (3) C6—C10 1.454 (4)
Fe1—C5 2.032 (3) C6—C7 1.462 (4)
Fe1—C10 2.037 (3) C7—C8 1.424 (4)
Fe1—C4 2.068 (3) C7—H7A 1.0000
Fe1—C9 2.076 (3) C8—C9 1.419 (4)
Fe1—C8 2.076 (3) C8—H8A 1.0000
Fe1—C3 2.082 (3) C9—C10 1.434 (4)
Fe1—Si1 2.6885 (8) C9—H9A 1.0000
Fe2—C11 2.009 (3) C10—H10A 1.0000
Fe2—C16 2.020 (3) C11—C15 1.439 (4)
Fe2—C20 2.025 (3) C11—C12 1.453 (4)
Fe2—C12 2.027 (3) C12—C13 1.423 (4)
Fe2—C15 2.028 (3) C12—H12A 1.0000
Fe2—C17 2.030 (2) C13—C14 1.424 (4)
Fe2—C14 2.079 (3) C13—H13A 1.0000
Fe2—C13 2.080 (3) C14—C15 1.422 (4)
Fe2—C18 2.080 (3) C14—H14A 1.0000
Fe2—C19 2.087 (3) C15—H15A 1.0000
Fe2—Si2 2.6929 (8) C16—C20 1.454 (4)
Si1—O1 1.6308 (18) C16—C17 1.463 (4)
Si1—C21 1.851 (3) C17—C18 1.421 (4)
Si1—C6 1.882 (3) C17—H17A 1.0000
Si1—C1 1.885 (3) C18—C19 1.413 (4)
Si2—O1 1.6377 (19) C18—H18A 1.0000
Si2—C22 1.847 (3) C19—C20 1.425 (4)
Si2—C16 1.871 (3) C19—H19A 1.0000
Si2—C11 1.879 (3) C20—H20A 1.0000
C1—C2 1.429 (4) C21—H21A 0.9800
C1—C5 1.466 (4) C21—H21B 0.9800
C2—C3 1.431 (4) C21—H21C 0.9800
C2—H2A 1.0000 C22—H22A 0.9800
C3—C4 1.418 (4) C22—H22B 0.9800
C3—H3A 1.0000 C22—H22C 0.9800
C1—Fe1—C6 88.78 (11) C4—C3—H3A 126.3
C1—Fe1—C2 41.46 (11) C2—C3—H3A 126.3
C6—Fe1—C2 109.43 (11) Fe1—C3—H3A 126.3
C1—Fe1—C7 109.52 (11) C3—C4—C5 108.8 (2)
C6—Fe1—C7 42.35 (10) C3—C4—Fe1 70.52 (15)
C2—Fe1—C7 100.76 (11) C5—C4—Fe1 68.31 (15)
C1—Fe1—C5 42.54 (10) C3—C4—H4A 125.6
C6—Fe1—C5 110.06 (11) C5—C4—H4A 125.6
C7—Fe1—C5 147.55 (11) C4—C5—C1 108.4 (2) C1—Fe1—C10 110.26 (11) C4—C5—Fe1 71.09 (15)
C6—Fe1—C10 42.03 (10) C1—C5—Fe1 67.95 (14)
C2—Fe1—C10 146.94 (11) C4—C5—H5A 125.8
C7—Fe1—C10 69.41 (11) C1—C5—H5A 125.8
C5—Fe1—C10 101.64 (11) Fe1—C5—H5A 125.8
C1—Fe1—C4 70.10 (11) C10—C6—C7 105.2 (2)
C6—Fe1—C4 150.46 (11) C10—C6—Si1 118.24 (19)
C2—Fe1—C4 68.23 (11) C7—C6—Si1 119.00 (19)
C7—Fe1—C4 164.39 (12) C10—C6—Fe1 69.75 (14)
C5—Fe1—C4 40.60 (11) C7—C6—Fe1 69.38 (14)
C10—Fe1—C4 125.88 (11) Si1—C6—Fe1 87.14 (10)
C1—Fe1—C9 150.85 (11) C8—C7—C6 109.1 (2)
C6—Fe1—C9 70.15 (11) C8—C7—Fe1 71.41 (15)
C2—Fe1—C9 164.83 (11) C6—C7—Fe1 68.27 (14)
C7—Fe1—C9 68.32 (11) C8—C7—H7A 125.4
C5—Fe1—C9 125.66 (11) C6—C7—H7A 125.4
C10—Fe1—C9 40.79 (11) Fe1—C7—H7A 125.4
C4—Fe1—C9 120.01 (11) C9—C8—C7 108.5 (2)
C1—Fe1—C8 149.76 (11) C9—C8—Fe1 70.01 (15)
C6—Fe1—C8 70.12 (11) C7—C8—Fe1 68.04 (14)
C2—Fe1—C8 124.99 (12) C9—C8—H8A 125.7
C7—Fe1—C8 40.55 (11) C7—C8—H8A 125.7
C5—Fe1—C8 165.43 (12) Fe1—C8—H8A 125.7
C10—Fe1—C8 68.33 (11) C8—C9—C10 108.1 (2)
C4—Fe1—C8 136.73 (11) C8—C9—Fe1 70.02 (15)
C9—Fe1—C8 39.98 (10) C10—C9—Fe1 68.15 (15)
C1—Fe1—C3 69.81 (11) C8—C9—H9A 125.9
C6—Fe1—C3 149.89 (11) C10—C9—H9A 125.9
C2—Fe1—C3 40.76 (10) Fe1—C9—H9A 125.9
C7—Fe1—C3 124.60 (11) C9—C10—C6 109.1 (2)
C5—Fe1—C3 68.31 (12) C9—C10—Fe1 71.06 (15)
C10—Fe1—C3 165.66 (11) C6—C10—Fe1 68.22 (14)
C4—Fe1—C3 39.96 (11) C9—C10—H10A 125.4
C9—Fe1—C3 136.73 (11) C6—C10—H10A 125.4
C8—Fe1—C3 119.28 (12) Fe1—C10—H10A 125.4
supporting information
sup-7
Acta Cryst. (2002). E58, m562–m563C20—Fe2—C17 69.14 (11) C12—C13—C14 108.1 (2) C12—Fe2—C17 102.09 (12) C12—C13—Fe2 67.75 (15) C15—Fe2—C17 146.79 (11) C14—C13—Fe2 69.98 (15) C11—Fe2—C14 69.77 (11) C12—C13—H13A 125.9 C16—Fe2—C14 148.86 (11) C14—C13—H13A 125.9 C20—Fe2—C14 124.23 (12) Fe2—C13—H13A 125.9 C12—Fe2—C14 68.28 (12) C15—C14—C13 107.8 (3) C15—Fe2—C14 40.49 (11) C15—C14—Fe2 67.82 (15) C17—Fe2—C14 166.36 (11) C13—C14—Fe2 69.99 (15) C11—Fe2—C13 69.84 (11) C15—C14—H14A 126.1 C16—Fe2—C13 150.27 (11) C13—C14—H14A 126.1 C20—Fe2—C13 164.17 (11) Fe2—C14—H14A 126.1 C12—Fe2—C13 40.52 (11) C14—C15—C11 109.7 (2) C15—Fe2—C13 68.05 (11) C14—C15—Fe2 71.69 (16) C17—Fe2—C13 126.48 (11) C11—C15—Fe2 68.42 (14) C14—Fe2—C13 40.04 (11) C14—C15—H15A 125.2 C11—Fe2—C18 150.40 (12) C11—C15—H15A 125.2 C16—Fe2—C18 70.15 (11) Fe2—C15—H15A 125.2 C20—Fe2—C18 68.02 (12) C20—C16—C17 104.2 (2) C12—Fe2—C18 126.29 (11) C20—C16—Si2 119.84 (19) C15—Fe2—C18 164.42 (12) C17—C16—Si2 118.2 (2) C17—Fe2—C18 40.43 (11) C20—C16—Fe2 69.11 (15) C14—Fe2—C18 137.53 (12) C17—C16—Fe2 69.17 (14) C13—Fe2—C18 121.03 (11) Si2—C16—Fe2 87.48 (10) C11—Fe2—C19 149.46 (11) C18—C17—C16 109.6 (2) C16—Fe2—C19 70.02 (11) C18—C17—Fe2 71.69 (15) C20—Fe2—C19 40.52 (11) C16—C17—Fe2 68.48 (13) C12—Fe2—C19 165.80 (11) C18—C17—H17A 125.2 C15—Fe2—C19 124.84 (12) C16—C17—H17A 125.2 C17—Fe2—C19 67.84 (11) Fe2—C17—H17A 125.2 C14—Fe2—C19 119.60 (12) C19—C18—C17 108.3 (2) C13—Fe2—C19 137.40 (11) C19—C18—Fe2 70.43 (16) C18—Fe2—C19 39.65 (11) C17—C18—Fe2 67.88 (14) O1—Si1—C21 111.50 (12) C19—C18—H18A 125.8
O1—Si1—C6 111.18 (11) C17—C18—H18A 125.8
C21—Si1—C6 112.97 (13) Fe2—C18—H18A 125.8 O1—Si1—C1 111.21 (11) C18—C19—C20 108.0 (2) C21—Si1—C1 112.39 (13) C18—C19—Fe2 69.92 (15) C6—Si1—C1 96.82 (11) C20—C19—Fe2 67.41 (15) O1—Si2—C22 112.89 (12) C18—C19—H19A 126.0 O1—Si2—C16 110.51 (11) C20—C19—H19A 126.0 C22—Si2—C16 112.84 (13) Fe2—C19—H19A 126.0 O1—Si2—C11 110.11 (11) C19—C20—C16 109.9 (2) C22—Si2—C11 112.69 (13) C19—C20—Fe2 72.06 (16) C16—Si2—C11 96.72 (11) C16—C20—Fe2 68.76 (15) Si1—O1—Si2 143.25 (12) C19—C20—H20A 125.1
C2—C1—C5 105.5 (2) C16—C20—H20A 125.1
C5—C1—Si1 117.58 (19) Si1—C21—H21A 109.5
C2—C1—Fe1 69.82 (15) Si1—C21—H21B 109.5
C5—C1—Fe1 69.51 (14) H21A—C21—H21B 109.5
Si1—C1—Fe1 87.24 (11) Si1—C21—H21C 109.5
C1—C2—C3 110.0 (2) H21A—C21—H21C 109.5
C1—C2—Fe1 68.72 (15) H21B—C21—H21C 109.5
C3—C2—Fe1 71.74 (15) Si2—C22—H22A 109.5
C1—C2—H2A 125.0 Si2—C22—H22B 109.5
C3—C2—H2A 125.0 H22A—C22—H22B 109.5
Fe1—C2—H2A 125.0 Si2—C22—H22C 109.5
C4—C3—C2 107.4 (2) H22A—C22—H22C 109.5
C4—C3—Fe1 69.52 (15) H22B—C22—H22C 109.5
C2—C3—Fe1 67.50 (15)