Immersion-Induced Changes in Structure
and Properties of a Calcium Sulfate Cement
Wei-Luen Chen
1,+1, Nai-Yen Fan
1,+1, Jing-Wei Lee
2, Ruey-Mo Lin
3,
Chien-Ping Ju
1and Jiin-Huey Chern Lin
1,+21Department of Materials Science and Engineering, National Cheng Kung University,
No. 1 University Road, 70101, Tainan City, Taiwan, R. O. China
2Division of Plastic Surgery, Department of Surgery, National Cheng Kung University Hospital,
70403, Tainan City, Taiwan, R. O. China
3Department of Orthopedics, National Cheng Kung University College of Medicine and Hospital,
Dou-Liou Branch, Yunlin City, Taiwan, R. O. China
This research was devoted to the investigation of changes in structure and properties of a calcium sulfate hemihydrates (CSH) cement immersed in Hanks’solution for different periods of time. XRD patterns indicated a quick phase change (a hydration process) of CSH into calcium sulfate dihydrate (CSD) after the cement powder was mixed with the setting solution. After the hardened cement was immersed in Hanks’solution for 1 d, CSD became the dominant phase. The long-term (up to 30 d) pH value of the Hanks’solution wherein the cement was immersed remained in the range of 67. The hardened cement gradually lost its weight and increased its porosity level with immersion time. When immersed for 1 d, the average compressive strength (CS) value of the cement reached its maximum value. After 30 d, the compressive strength (CS) value of the cement still remained>25 MPa. SEM showed that, after being immersed for 30 d, numerous large, faceted CSD crystals were observed throughout the sample. Cytotoxicity test indicated that the present cement is a biocompatible implant material.
[doi:10.2320/matertrans.M2011362]
(Received November 24, 2011; Accepted March 26, 2012; Published May 25, 2012)
Keywords: biomaterials, microstructure, mechanical properties, calcium sulfate hemihydrates (CSH)
1. Introduction
Bioresorbable bioceramic has become one of the most promising bone substitute materials today.14) Its resorption rate often depends on such material parameters as chemical composition, crystallinity, microstructure, etc.59) When its resorption rate is adjusted to be similar to the growth rate of natural bone, the implanted material can be gradually replaced by new bone.10) Calcium sulfates as well as some of calcium phosphates, are typical such bioresorbable materials.11,12) Furthermore, due to its unique injectable feature, calcium sulfate cement can be used in bonding, filling, and repairing damaged natural bone in orthopedic, dental, maxillofacial and other medical applications via minimally invasive procedures.1315)
Despite its many advantages, such as excellent biocompat-ibility, osteoconductivity, and being X-ray detectable, most currently-used calcium phosphate and calcium sulfate materials have their respective disadvantages. For example, as being used as a bone substitute material, most calcium phosphates exhibit relatively low bioresorption rates,13)while most calcium sulfates demonstrate relatively low mechanical strengths and high rates of dissolution.12) For many applications, insufficient mechanical strength may cause premature disintegration of the implant. This problem becomes especially serious when such bone substitute material has to bear high loading after being implanted. On the other hand, dissolution rates being too high may not allow new bone cells to effectively grow into bone cavity.16,2123) The mismatch in resorption rate between implant material
and natural bone further weakens the implantation site, even causing local fractures.2528)
It is known that, as soon as CSH powder contacts water to form a cement paste, hydration takes place which involves supersaturation, nucleation and crystal growth of CSD phase.1720) This phase transformation (hardening of the paste) usually proceeds very fast, only about 23 min. The rapid hardening process not only can affect the mechanical strength, but also can increase the difficulty of surgery-especially when a minimally invasive procedure is pursued. In a preliminary study of the present authors’ laboratory, use of 0.0375 M (NH4)2HPO4as setting solution was found effective in prolonging the setting process (slowing down the CSH-CSD phase transformation process) of CSH cement. To further learn the behavior of this CSH cement in physiological solution, the present study is devoted to the investigation of the changes in structure and properties of the CSH cement immersed in Hanks’solution for different periods of time.
2. Materials and Methods
To evaluate the physiological solution-immersion effects on various properties of hardened CSH cement, CSH cement paste was first prepared by mixing CSH powder (Showa, Toyko, Japan) and 0.0375 M (NH4)2HPO4 setting solution at a liquid/powder (L/P) ratio of 0.35 mL/g for 1 min. The setting solution was prepared by mixing an appropriate amount of (NH4)2HPO4powder (Showa, Tokyo, Japan) with de-ionized water. Thus-formed cement paste was packed into a 6 mm dia., 12 mm deep cylindrical stainless steel mold under a pressure of 1.4 MPa for 30 min. After being removed from the mold, the hardened cement samples were immersed +1Graduate Student, Cheng Kung University
in Hanks’ physiological solution (Table 1)24) which was maintained at 37°C and agitated daily to help maintain uniform ion concentrations.
After immersion, samples were removed from the solution for compressive strength (CS) testing while they were still wet. The CS testing was conducted according to ASTM 451-99a method using a desk-top mechanical tester (Shimadzu AGS-500D, Tokyo, Japan) at a crosshead speed of 1.0 mm/min.
The working time of cement paste was determined by the time after that the cement paste was no longer workable. The setting time of cement paste was measured according to the standard method set forth in ISO 1566 for dental zinc phosphate cements. The cement is considered set when a 400 g weight loaded onto a Vicat needle with a 1 mm dia. tip fails to make a perceptible circular indentation on the surface of the cement.
The early stage (during hardening process) variation in pH value was determined using a pH meter (Suntex Instruments SP2000, Taipei, Taiwan) that was buried in the cement paste immediately after the powder and setting liquid were mixed. The pH value of the Hanks’ solution wherein the cement paste sample was immersed was monitored using the same pH meter. After powder and setting solution were mixed for 5 min, 2 g cement paste was taken and immersed in 20 mL Hanks’solution with a pH value of 7.05. The solution was maintained at 37°C throughout testing and continually stirred to help maintain uniform ion concentrations of the solution. The porosity of cement samples was measured according to ASTM C830-00 (2006) method.
The various phases of the cement under different conditions were analyzed using a Rigaku D-MAX B X-ray diffractometer (XRD) (Tokyo, Japan) with Ni-filtered CuK¡
radiation operated at 30 kV and 20 mA at a scanning speed of 1°/min. Each phase was identified by comparing its characteristic peaks with data compiled in the JCPDSfiles.
Scanning electron microscopy (SEM) was conducted using
a field-emission scanning electron microscope (FE-SEM)
(XL-40, Philips, Holland) operated at 10 kV. The fracture surface being examined was coated with a thin layer of gold using an ion sputtering system (JFC-1100, JEOL, Japan) to facilitate conducting the sample.
The cytotoxicity test was performed according to ISO 10993-5, wherein an extraction method was used.
NIH/3T3 fibroblasts with a seeding density of 5000 per
well were pre-cultured for 24 h in Dulbecco’s modified essential medium (DMEM) supplemented with bovine serum (10%) and PSF (1%). An extract was prepared by immersing the hardened cement sample in the culture medium at a ratio of 0.1 g/mL (a typical ratio for porous materials) at 37°C for 24 h, followed by collection of the liquid by centrifugation. The extract was placed into a 96-well microplate (100 µL per 96-well) incubated in a 5% CO2 -humidified atmosphere at 37°C. After 24 h, the extract was sucked out, leaving cells at the bottom of the wells. A mixture of the culture medium (100 µL) and WST-1 (10 µL) was subsequently added to the wells and incubated for 1 h at 37°C. Cell viability was measured by using a WST-1 assay, which is a colorimetric assay of mitochondrial dehydrogen-ase activity wherein the absorbance at 450 nm is proportional to the amount of dehydrogenase activity in the cell. After incubation for 1 h, the mixture of medium and WST-1 was transferred to a 96-well microplate and the absorbance at 450 nm was measured with an ELISA reader. Al2O3powder was also assayed as a control. Four bars were tested for each sample (n=4).
3. Results and Discussion
3.1 Working time, setting time and mechanical stability in Hanks’solution
As mentioned in“Materials and Methods,”to prepare the calcium sulfate cement for the study, an appropriate amount of the CSH cement powder was mixed with 0.0375 M (NH4)2HPO4setting solution with a L/P ratio of 0.35 mL/g. The resultant cement paste exhibited an average working time and setting time of 8.2 and 10.2 min, respectively, which are generally considered appropriate for most orthopedic and dental surgeries.
To evaluate the mechanical stability of the cement paste in Hanks’solution, after mixing powder and setting solution for 1 min, the cement paste was directly injected into Hanks’ solution at 37°C using a 5 mL syringe with needle removed. After 1 h, the cement hardened in Hanks’ solution still remained in its original shape, and was not dispersed even after shaking of the container. This non-dispersive nature, that may reduce the possibilities of complications such as cement embolism, is a good indication for its safety for clinical use.
3.2 Immersion-induced changes in pH value
The early-stage (up to 15 min post-mixing) variation in pH value of the cement is shown in Fig. 1. As shown in the figure, the pH value of the cement paste slightly increased from its 3-min value of 6.3 to 15-min value of 6.6 during the hardening process, after that a plateau was reached.
[image:2.595.47.291.84.208.2]Figure 2 shows the long-term variation in pH of Hanks’ solution wherein the cement was immersed for different periods of time. As shown in thefigure, the pH value of the Hanks’solution gradually increased from its 1-d value of 6.1 to 14-d value of 6.9, followed by slowly reducing to 6.8 after 30 d. The long-term decrease in pH value of the Hanks’ solution is probably due to the hydration of CSH and the dissolution of CSD,12)which could cause the pH value of the solution to decrease.
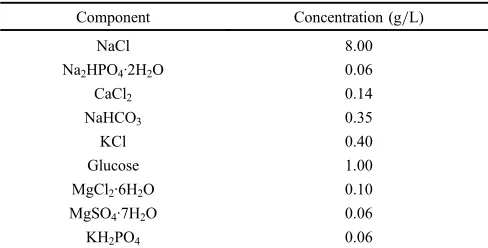
Table 1 Composition of Hanks’solution used for the study.24)
Component Concentration (g/L)
NaCl 8.00
Na2HPO4·2H2O 0.06
CaCl2 0.14
NaHCO3 0.35
KCl 0.40
Glucose 1.00
MgCl2·6H2O 0.10
MgSO4·7H2O 0.06
3.3 Immersion-induced changes in weight
As shown in Fig. 3, the hardened cement had 31.9% in porosity and lost 14.6% in weight after being immersed in Hanks’ solution for 1 d. After 1 d, the cement weight loss gradually increased with time. For example, after 30 d, the cement increased its porosity to 56% and weight loss to 25.2%. The dissolution of CSH and CSD (formed by a hydration process of CSH during immersion) is considered to be the major reason for the immersion-induced porosity and weight loss due to their high dissolution rates in the solution.12)
3.4 Immersion-induced changes in phases and micro-structure
Shown in Fig. 4 are typical XRD patterns of the various starting powders and the hardened cement immersed in Hanks’solution for different periods of time. The character-istic peak locations of CSD and CSH phases are given as a reference. As indicated in the figure, after the hardened cement was immersed in Hanks’solution for 1 d or longer, CSD became the dominant phase of the cement.
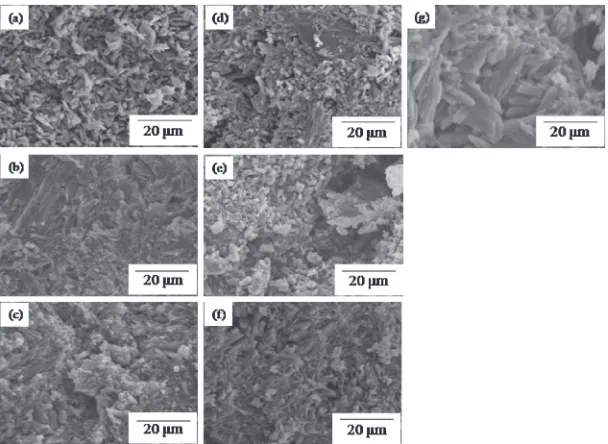
Figure 5 represents typical scanning electron micrographs of the cement immersed in Hanks’ solution for different periods of time. As shown in the micrographs, the hardened cement immersed for 20 min or 1 d had a relatively dense
morphology. After being immersed for 3 d or longer, small pores and relatively large crystals appeared. In the 30-d sample, numerous large crystals were observed throughout the sample. The XRD data (Fig. 4) and morphological information clearly indicate that these large (up to about 20 microns long and about 4 microns thick), faceted crystals are CSD crystals.12)
3.5 Immersion-induced changes in compressive strength
Figure 6 demonstrates the variation in CS of the hardened cement immersed in Hanks’solution for different periods of time. As shown in the figure, after immersion for 1 d, the average CS value of the cement reached 33.6 MPa. After that, the CS value decreased, but not in a drastic way. For example, even after being immersed for 30 d, the CS value of the cement still remained >25 MPa. It seems reasonable to attribute the long-term decrease in CS to the observed dissolution-induced increase in porosity level (Fig. 3). From a practical point of view, the maintaining of a relatively high strength of the cement for a reasonably long time can help avoiding the premature disintegration of the implant in patient’s body.
Fig. 2 Variation in pH value of the Hanks’solution wherein the cement is immersed for different periods of time.
Fig. 3 Weight loss and porosity values of the cement immersed in Hanks’ solution for different periods of time.
Fig. 4 XRD patterns of starting powders and the cement immersed in Hanks’solution for different periods of time.
[image:3.595.61.279.63.226.2] [image:3.595.318.534.67.234.2] [image:3.595.64.278.265.436.2] [image:3.595.311.541.284.467.2]3.6 Viability of cultured cells
Figure 7 demonstrates the viability values of cells incubated for 24 h in conditioned mediums adulterated with 24-h cement extraction, blank medium and Al2O3 powder control groups. As shown in the figure, the viability values (averages between 0.80 and 0.82 with standard deviations overlapping one another) of all three groups are statistically indistinguishable, indicating that the present cement is potentially a biocompatible implant material.
4. Conclusions
(1) The pH value of the present CSH cement paste slightly increased from its 3-min value of 6.3 to 15-min value of 6.6 during the hardening process. The long-term (up to 30 d) pH value of the Hanks’ solution wherein the cement was immersed remained in the range of 67. (2) The hardened cement gradually lost its weight and
increased porosity level with immersion time. It lost
weight by 14.6%after 1 d and 25.2%after 30 d, while increased porosity level from its 1-d value of 31.9 vol% to 30-d value of 56.0 vol%.
(3) XRD patterns indicated a quick hydration process of CSH into CSD phase in the solution. After the cement was immersed for 1 d, the CSD phase became the dominant phase.
(4) SEM showed that the hardened cement immersed in Hanks’solution for less than 3d had a relatively dense morphology. After being immersed for longer time, small pores and relatively large crystals appeared. In the 30-d sample, numerous large, faceted CSD crystals were observed throughout the sample.
(5) When immersed for 1 d, the average CS value of the cement reached 33.6 MPa. After that, the CS value gardually decreased. After 30 d, the CS value of the cement still remained>25 MPa.
Fig. 5 Scanning electron micrographs of the hardened cement immersed in Hanks’solution for different periods of time. (a) 5 min (b) 20 min (c) 1 d (d) 3 d (e) 7 d (f ) 14 d (g) 30 d.
Fig. 6 Variation in CS of the cement immersed in Hanks’solution for
different periods of time. Fig. 7 Viability values of cells incubated for 24 h in conditioned mediums adulterated with composite cement extraction, blank medium and Al2O3
[image:4.595.147.451.69.291.2] [image:4.595.62.279.346.510.2] [image:4.595.316.536.348.517.2](6) Cytotoxicity test showed that the viability values of all three groups (conditioned mediums adulterated with 24-h cement extraction, blank medium and Al2O3 powder control) are statistically indistinguishable, indicating that the present cement is a biocompatible implant material.
Acknowledgment
The authors would like to acknowledge the support for this research by the Southern Taiwan Science Park (Kaohsiung Science Park), Taiwan, ROC under the Research Grant# BZ-07-18-43-98.
REFERENCES
1) S. V. Dorozhkin:Glass. Ceram.64(2007) 442447.
2) V. A. Dubok:Powder Metall. Met. Ceram.39(2000) 381394.
3) H. Schliephake, R. Gruber, M. Dard, R. Wenz and S. Scholz:
J. Biomed. Mater. Res. A69(2004) 382390.
4) P. F. Heini and U. Berlemann:Eur. Spine. J.10(2001) S205213.
5) K. Ohura, M. Bohner, P. Hardouin, J. Lemaitre, G. Pasquier and B. Flautre:J. Biomed. Mater. Res.30(1996) 193200.
6) E. P. Frankenburg, S. A. Goldstein, T. W. Bauer, S. A. Harris and R. D. Poser: J. Bone Joint. Surg. Am.80(1998) 11121114.
7) D. Apelt, F. Theiss, A. O. El-Warrak, K. Zlinszky, R. Bettschart-Wolfisberger, M. Bohner, S. Matter, J. A. Auer and B. von Rechenberg:
Biomaterials25(2004) 14391451.
8) D. Knaack, M. E. P. Goad, M. Aiolova, C. Rey, A. Tofighi, P. Chakravarthy and D. D. Lee:J. Biomed. Mater. Res.43(1998) 399 409.
9) S. Yamada, D. Heymann, J. M. Bouler and G. Daculsi:Biomaterials18
(1997) 10371041.
10) W. C. Chen, C. P. Ju, Y. C. Tien and J. H. Chern Lin:Acta Biomater.5 (2009) 17671774.
11) M. Bohner:Injury31(2000) S-D 3747.
12) M. V. Thomas and D. A. Puleo: J. Biomed. Mater. Res. B88(2009) 597610.
13) H. Oda, K. Nakamura, T. Matsushita, S. Yamamoto, H. Ishibashi, T. Yamazaki and S. Morimoto:J. Orthop. Sci.11(2006) 167174.
14) G. D. Brown, B. L. Mealey, P. V. Nummikoski, S. I. Bifano and T. C. Waldrop:J. Periodontol.69(1998) 146157.
15) L. Comuzzi, E. Ooms and J. A. Jansen:Clin. Oral. Impl. Res.13(2002) 304311.
16) K. A. Hing, L. E. Wilson and T. Buckland:Spine J.7(2007) 475490.
17) A. S. Myerson:Handbook of Industrial Crystallization Butterworth-Heinemann Series in Chemical Engineering, (Butterworth-Heinemann Ltd, USA, 1993).
18) M. P. C. Weijnen and G. M. van Rosmalen: Industrial Crystallization84
(1984) 6166.
19) G. M. van Rosmalen, P. J. Daudey and W. G. J. Marchee: Industrial Crystallization81(1982) 147154.
20) J. R. Parsons, J. L. Ricci, H. Alexander and P. K. Bajpai:Ann. N. Y. Acad. Sci.523(1988) 190207.
21) M. Nilsson, E. Fernández, S. Sarda, L. Lidgren and J. A. Planell:
J. Biomed. Mater. Res.61(2002) 600607.
22) M. Nilsson, L. Wielanek, J. S. Wang, K. E. Tanner and L. Lidgren:
J. Mater. Sci. Mater. Med.14(2003) 399404.
23) M. Bohner:Biomaterials25(2004) 741749.
24) D. C. Mears: Int. Metals Rev.June(1997) 119153.
25) L. Xie and E. A. Monroe: Mater. Res. Soc. Symp. Proc.179(2002; 1977) 2539.
26) W. C. Chen, J. H. Chern Lin and C. P. Ju:J. Biomed. Mater. Res. A64 (2003) 664671.
27) W. C. Chen, C. P. Ju and J. H. Chern Lin:J. Oral. Rehabil.34(2007) 541551.