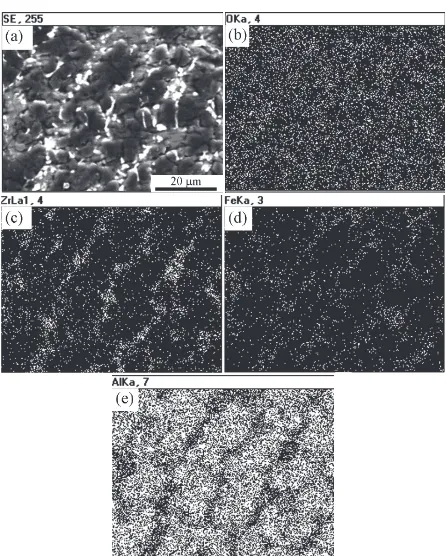
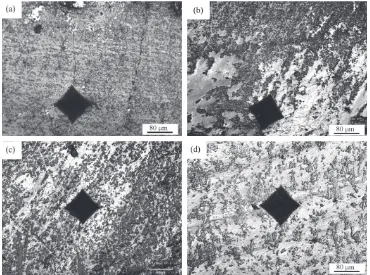
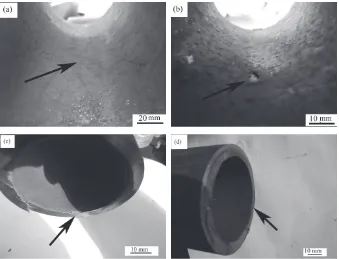
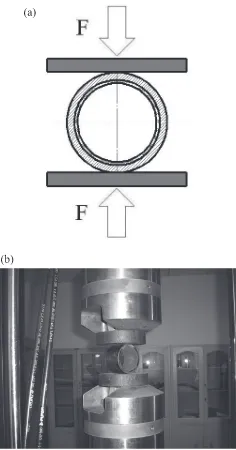
High Performance Ceramic Lined Composite Pipes with ZrO2 Additive Prepared by Centrifugal SHS Process
Full text
Figure

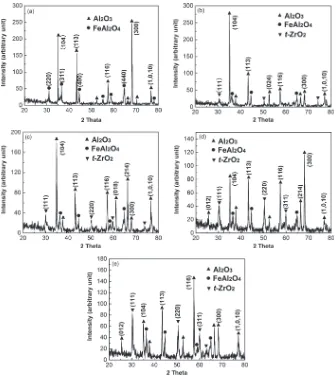
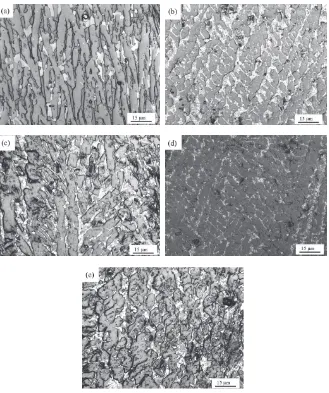
Related documents
ing whether or not during this period any time was spent in child health conferences, school health services, out-patient clinics, hospital ward rounds, teaching, and other
Phylogenetic, antigenic and biological characterization of pigeon paramyxovirus type 1 circulating in China RESEARCH Open Access Phylogenetic, antigenic and biological characterization
implementation of the Double-Multiple Streamtube model was modified so that the blade pitch angle was determined at each azimuth angle location based on the angle of attack
albicans in FLC is eliminated by cyclosporine experiments 1 and 2 are fully dominant with respect to ( Marchetti et al. 2000) and may be mitigated by alter- MIC or fitness and
A three-step procedure for the flexibility matrix calculation is presented in this paper: (i) a complete geomechanical model of the site with geological
Figure 6 shows a comparison between the simulated and measured normalized radiation pattern results of the proposed dual band-notched UWB antenna at selected frequencies (3, 5,
Objective: To estimate the cost-effectiveness of three alternative dietetic strategies for cow’s milk allergy in Brazil: 1) using an extensively hydrolyzed casein formula (eHCF;
In this research, classical data augmentation techniques along with CGAN based on a deep transfer learning model for COVID-19 detection in chest CT scan images will be