organic papers
Acta Cryst.(2006). E62, o3249–o3250 doi:10.1107/S1600536806025219 Duanet al. C
16H18N2O5S
o3249
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
1-Oxo-6-(2-phenylacetylamino)-1-penicillanic acid
Er-Hong Duan,aDi-Shun Zhao,b* Juan Wangaand Mei-Ling Lia
a
School of Chemical Engineering and Technology of Tianjin University, Tianjin 300072, People’s Republic of China, and bSchool of Chemical and Pharmaceutical
Engineering, Hebei University of Science and Technology, Shijiazhuang Hebei 050018, People’s Republic of China
Correspondence e-mail: dandinlion@yahoo.com.cn
Key indicators
Single-crystal X-ray study
T= 294 K
Mean(C–C) = 0.006 A˚
Rfactor = 0.038
wRfactor = 0.082
Data-to-parameter ratio = 12.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 8 June 2006 Accepted 29 June 2006
#2006 International Union of Crystallography
All rights reserved
The crystal structure of the title compound (CAS: 4052–54-4), C16H18N2O5S, contains O—H O and N—H O hydrogen
bonds. The four-membered ring is folded and the five-membered ring has an envelope conformation.
Comment
The title compound, (I), is an early derivative of penicillin which was prepared by Rogers & Folkers (1946). Penicillin G sulfoxide, which is the common name of 1-oxo-6-(2-phenyl-acetylamino)-1-penicillanic acid, decomposes at higher temperatures (>333 K), because it has an unsaturated S O bond. It is an important intermediate in the synthesis of 7-aminodeacetoxy-cefalosporanic acid (7-ADCA) (Pan et al.
2001).
Selected bond lengths and angles are listed in Table 1. The four-membered ring (C9–C11,N2) is folded and the five-membered ring (C10,N2,C12,S1,C14) has an envelope conformation. The crystal structure contains intermolecular O—H O and N—H O hydrogen bonds (Table 2), giving an infinite three-dimensional network. An intramolecular N— H O interaction forms a six-membered ring, which also has an envelope conformation, with atom N2 as the flap.
Experimental
1-Oxo-6-(2-phenylacetylamino)-1-penicillanic acid was synthesized according to the method described by Chowet al.(1962). Colorless single crystals of (I) were grown by slow evaporation of a methanol solution.
Crystal data
C16H18N2O5S
Mr= 350.38 Trigonal,P32
a= 11.6285 (12) A˚
c= 10.894 (2) A˚
V= 1275.7 (3) A˚3
Z= 3
Dx= 1.368 Mg m
3
MoKradiation
= 0.22 mm1
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 1997)
Tmin= 0.943,Tmax= 0.974
6825 measured reflections 2703 independent reflections 1994 reflections withI> 2(I)
Rint= 0.043
max= 25.5
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.039
wR(F2) = 0.082
S= 1.01 2703 reflections 220 parameters
H-atom parameters constrained
w= 1/[2
(Fo2) + (0.0359P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.003
max= 0.18 e A˚
3
min=0.16 e A˚
3
Absolute structure: Flack (1983), 1116 Freidel pairs
Flack parameter:0.04 (8)
Table 1
Selected geometric parameters (A˚ ,).
O1—C8 1.223 (4) O4—C13 1.182 (4) C1—C2 1.371 (5)
C7—C8 1.504 (4) C12—C13 1.512 (4) C14—C16 1.520 (5)
O3—S1—C10 103.47 (13) O3—S1—C14 105.21 (15) C10—S1—C14 89.03 (14)
C11—N2—C12 124.2 (2) C10—N2—C12 116.6 (2) C3—C2—C1 119.1 (5)
C4—C5—C6—C7 179.3 (4) C1—C6—C7—C8 99.7 (4) C9—N1—C8—O1 2.7 (4)
C6—C7—C8—O1 36.7 (5) C8—N1—C9—C10 105.6 (3) C10—N2—C12—C14 3.1 (3)
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O5—H5 O1i
0.82 1.79 2.597 (3) 166 N1—H1 O2ii
0.86 2.33 3.142 (3) 157 N1—H1 O3 0.86 2.40 2.844 (3) 113
Symmetry codes: (i)xþyþ1;xþ1;zþ1
3; (ii)yþ1;xyþ1;z 1 3.
The H atom of the OH group was initially located in a difference Fourier map, but subsequently the O—H distance was constrained to 0.82 A˚ and theUiso(H) value set equal to 1.2Ueq(O). All other H atoms were positioned geometrically (N—H = 0.86, C—H = 0.93– 0.98 A˚ ) and refined as riding, withUiso(H) = 1.2 or 1.5 times
Ueq(parent atom).
Data collection:SMART(Bruker, 1997); cell refinement:SAINT
(Bruker, 1997); data reduction: SAINT; program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
SHELXTL (Bruker, 1997); software used to prepare material for publication:SHELXTL.
References
Bruker (1997).SADABS,SMART,SAINTandSHELXTL. Bruker AXS Inc., Madison, Wisconsin, USA.
Chow, A. W., Hall, N. M. & Hoover, J. R. E. (1962).J. Org. Chem.27, 1381– 1383.
Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Pan, X. J., Liu, H. Z., An, Z. T., Wang, J. & Niu, G. G. (2001).Int. J. Pharm.220, 33–41.
Rogers, E. F. & Folkers, K. (1946). US Patent No. 2 483 235.
[image:2.610.319.566.73.169.2]Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
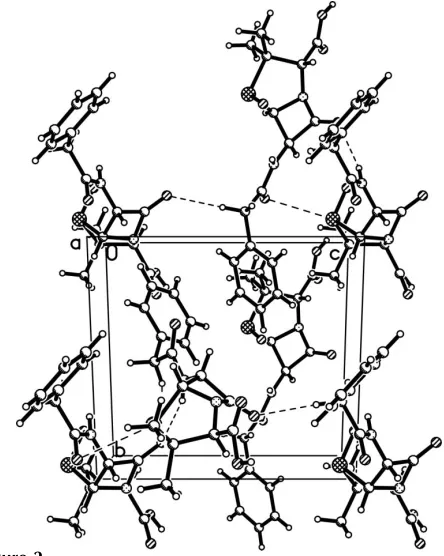
Figure 2
Packing diagram for (I), with hydrogen bonds shown as dashed lines.
Figure 1
[image:2.610.325.547.227.505.2]supporting information
sup-1
Acta Cryst. (2006). E62, o3249–o3250
supporting information
Acta Cryst. (2006). E62, o3249–o3250 [https://doi.org/10.1107/S1600536806025219]
1-Oxo-6-(2-phenylacetylamino)-1-penicillanic acid
Er-Hong Duan, Di-Shun Zhao, Juan Wang and Mei-Ling Li
1-oxo-6-(2-phenylacetylamino)-1-penicillanic acid
Crystal data
C16H18N2O5S
Mr = 350.38 Trigonal, P32
a = 11.6285 (12) Å c = 10.894 (2) Å V = 1275.7 (3) Å3
Z = 3 F(000) = 552
Dx = 1.368 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 1894 reflections θ = 2.8–21.2°
µ = 0.22 mm−1
T = 294 K Block, colourless 0.22 × 0.16 × 0.12 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 1997) Tmin = 0.943, Tmax = 0.974
6825 measured reflections 2703 independent reflections 1994 reflections with I > 2σ(I) Rint = 0.043
θmax = 25.5°, θmin = 2.0°
h = −14→12 k = −11→14 l = −8→13
Refinement
Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.082
S = 1.01 2703 reflections 220 parameters 1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0359P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.003
Δρmax = 0.18 e Å−3
Δρmin = −0.16 e Å−3
Absolute structure: Flack (1983), 1116 Freidel pairs
Absolute structure parameter: −0.04 (8)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-3
Acta Cryst. (2006). E62, o3249–o3250
H16B 0.7965 0.6057 0.2869 0.092* H16C 0.9360 0.7352 0.2889 0.092*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
S1 0.0469 (5) 0.0523 (5) 0.0350 (4) 0.0250 (5) −0.0026 (4) 0.0060 (4) O1 0.0548 (15) 0.0416 (14) 0.0703 (18) 0.0253 (12) −0.0005 (13) −0.0168 (12) O2 0.0510 (14) 0.0750 (16) 0.0383 (15) 0.0354 (13) 0.0005 (11) −0.0030 (13) O3 0.0521 (14) 0.0490 (13) 0.0605 (16) 0.0317 (12) −0.0049 (11) 0.0078 (12) O4 0.0548 (16) 0.0560 (16) 0.078 (2) 0.0307 (13) −0.0184 (13) 0.0052 (13) O5 0.0534 (16) 0.0490 (16) 0.135 (3) 0.0265 (14) −0.0441 (17) −0.0151 (15) N1 0.0350 (15) 0.0381 (15) 0.0475 (18) 0.0186 (13) −0.0077 (13) 0.0012 (12) N2 0.0360 (14) 0.0446 (16) 0.0361 (15) 0.0228 (13) −0.0043 (12) 0.0020 (12) C1 0.060 (3) 0.070 (3) 0.060 (3) 0.037 (2) −0.007 (2) 0.004 (2) C2 0.063 (3) 0.127 (5) 0.096 (4) 0.058 (3) 0.015 (3) 0.040 (3) C3 0.049 (3) 0.116 (5) 0.164 (7) 0.021 (3) −0.009 (4) 0.052 (5) C4 0.066 (4) 0.075 (3) 0.185 (7) 0.010 (3) −0.062 (4) −0.032 (4) C5 0.063 (3) 0.091 (3) 0.090 (3) 0.027 (3) −0.034 (2) −0.042 (3) C6 0.0394 (19) 0.052 (2) 0.046 (2) 0.0203 (17) −0.0138 (17) 0.0018 (18) C7 0.0450 (19) 0.062 (2) 0.047 (2) 0.0190 (18) −0.0099 (16) 0.0089 (18) C8 0.0387 (19) 0.048 (2) 0.0274 (18) 0.0207 (17) 0.0033 (15) −0.0062 (15) C9 0.0353 (17) 0.0349 (18) 0.046 (2) 0.0173 (15) −0.0011 (15) 0.0069 (15) C10 0.0394 (18) 0.0365 (18) 0.043 (2) 0.0211 (15) −0.0001 (14) −0.0009 (14) C11 0.0367 (19) 0.050 (2) 0.0343 (19) 0.0232 (16) 0.0050 (15) 0.0121 (16) C12 0.0356 (18) 0.0380 (18) 0.047 (2) 0.0209 (15) −0.0081 (15) −0.0051 (15) C13 0.045 (2) 0.050 (2) 0.046 (2) 0.0289 (19) −0.0107 (16) −0.0092 (18) C14 0.0395 (19) 0.049 (2) 0.046 (2) 0.0196 (17) 0.0027 (16) 0.0055 (16) C15 0.054 (2) 0.059 (2) 0.086 (3) 0.016 (2) 0.001 (2) 0.016 (2) C16 0.049 (2) 0.086 (3) 0.054 (2) 0.038 (2) 0.0113 (19) 0.001 (2)
Geometric parameters (Å, º)
C1—C6 1.372 (5) C14—C16 1.520 (5) C1—H1A 0.9300 C15—H15A 0.9600 C2—C3 1.364 (9) C15—H15B 0.9600 C2—H2 0.9300 C15—H15C 0.9600 C3—C4 1.352 (8) C16—H16A 0.9600 C3—H3 0.9300 C16—H16B 0.9600 C4—C5 1.373 (7) C16—H16C 0.9600
supporting information
sup-5
Acta Cryst. (2006). E62, o3249–o3250
C6—C1—C2—C3 0.0 (7) C10—N2—C11—O2 −164.2 (4) C1—C2—C3—C4 1.2 (8) C12—N2—C11—O2 −39.3 (5) C2—C3—C4—C5 −2.7 (9) C10—N2—C11—C9 15.4 (2) C3—C4—C5—C6 2.8 (9) C12—N2—C11—C9 140.3 (3) C4—C5—C6—C1 −1.5 (6) N1—C9—C11—O2 41.8 (5) C4—C5—C6—C7 179.3 (4) C10—C9—C11—O2 165.1 (4) C2—C1—C6—C5 0.1 (6) N1—C9—C11—N2 −137.7 (3) C2—C1—C6—C7 179.4 (3) C10—C9—C11—N2 −14.4 (2) C5—C6—C7—C8 −81.1 (4) C11—N2—C12—C13 125.7 (3) C1—C6—C7—C8 99.7 (4) C10—N2—C12—C13 −120.6 (3) C9—N1—C8—O1 2.7 (4) C11—N2—C12—C14 −110.6 (3) C9—N1—C8—C7 −178.2 (3) C10—N2—C12—C14 3.1 (3) C6—C7—C8—O1 36.7 (5) N2—C12—C13—O4 16.7 (5) C6—C7—C8—N1 −142.3 (3) C14—C12—C13—O4 −104.1 (4) C8—N1—C9—C11 −151.6 (3) N2—C12—C13—O5 −165.3 (3) C8—N1—C9—C10 105.6 (3) C14—C12—C13—O5 73.8 (3) C11—N2—C10—C9 −15.0 (2) N2—C12—C14—C15 143.0 (3) C12—N2—C10—C9 −145.8 (2) C13—C12—C14—C15 −92.7 (3) C11—N2—C10—S1 98.3 (2) N2—C12—C14—C16 −88.3 (3) C12—N2—C10—S1 −32.4 (3) C13—C12—C14—C16 36.1 (4) N1—C9—C10—N2 136.6 (3) N2—C12—C14—S1 27.5 (3) C11—C9—C10—N2 13.8 (2) C13—C12—C14—S1 151.9 (2) N1—C9—C10—S1 33.9 (4) O3—S1—C14—C15 −55.2 (3) C11—C9—C10—S1 −89.0 (2) C10—S1—C14—C15 −158.9 (3) O3—S1—C10—N2 −64.5 (2) O3—S1—C14—C16 −176.1 (2) C14—S1—C10—N2 40.9 (2) C10—S1—C14—C16 80.2 (2) O3—S1—C10—C9 28.8 (2) O3—S1—C14—C12 63.8 (2) C14—S1—C10—C9 134.2 (2) C10—S1—C14—C12 −39.9 (2)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O5—H5···O1i 0.82 1.79 2.597 (3) 166
N1—H1···O2ii 0.86 2.33 3.142 (3) 157
N1—H1···O3 0.86 2.40 2.844 (3) 113