metal-organic papers
m1298
Fan and Zhu [Mn(C7H4O6S)(C12H8N2)(H2O)2] doi:10.1107/S1600536805017861 Acta Cryst.(2005). E61, m1298–m1300 Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
catena
-Poly[[
cis
-diaqua(1,10-phenanthroline-
j
2N
,
N
000)-manganese(II)]-
l
2-5-sulfonatosalicylato-
j
2
O
:
O
000]
Sai-Rong Fan and Long-Guan Zhu*
Department of Chemistry, Zhejiang University, Hangzhou 310027, People’s Republic of China Correspondence e-mail: chezlg@zju.edu.cn
Key indicators
Single-crystal X-ray study
T= 295 K
Mean(C–C) = 0.003 A˚
Rfactor = 0.030
wRfactor = 0.084
Data-to-parameter ratio = 13.2
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
The title complex, [Mn(C7H4O6S)(C12H8N2)(H2O)2]n, comprises a one-dimensional chain in which each Mn atom displays a distorted octahedral geometry. Hydrogen-bonding interactions between chains generate a two-dimensional architecture.
Comment
Recently, much attention has been paid to metal complexes of 5-sulfosalicylic acid (H3ssal) because of the structural diversity and biological interest of this ligand (Fan, Caiet al., 2005; Gao et al., 2005; Fan, Zhuet al., 2005a). In our previous work on the manganese(II) 1,10-phenanthroline (phen) complex [Mn(phen)2(H2O)2](Hssal)4H2O, (II), the doubly deproto-nated 5-sulfosalicylate does not coordinate to the metal atom (Fan, Zhu et al., 2005b), partly because the phen ligand can strongly chelate to the metal atom. Therefore, a new synthetic procedure, a two-step reaction method, was introduced to the Mn2+/H3ssal/phen system and, as expected, the Hssal
2
coor-dinated complex, (I), was synthesized.
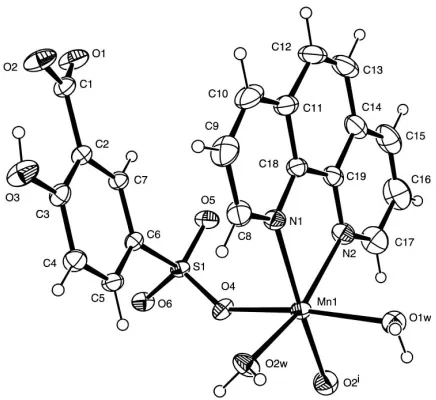
In the title complex, the MnII atom adopts a distorted octahedral geometry defined by two N donors from one phen ligand, two O atoms from twociswater molecules and two O atoms, one from a sulfonyl and one from a carboxyl group of two different Hssal2ligands (Fig. 1 and Table 1). The Mn—N and Mn—Ow distances are similar to those of (II). The 5-sulfonatosalicylate ligand acts as a linker using one sulfonyl and one carboxyl O atom to extend the structure into a one-dimensional chain (Fig. 2). Hydroxy and uncoordinated carboxyl O atoms form intrachain hydrogen bonds with a carboxyl group and a water molecule, respectively. Between chains, water molecules and sulfonyl O atoms form inter-molecular hydrogen bonds, generating a two-dimensional hydrogen-bonding network (Fig. 3 and Table 2).
Comparison of (II), [Mn(phen)2(H2O)2] 2+
, with (I), [Mn(phen)(H2O)2(Hssal)], shows that one phen ligand in II is replaced by an Hssal2ligand in (I). Two such diverse struc-tures were constructed by different synthetic procedures. For (II), a one-pot synthesis was used, while for (I), a two-step reaction method was used,i.e.phen was added after the metal salt had completely reacted with 5-sulfosalicylic acid in the presence of a weak base. Therefore, multi-step reactions are very useful in the construction of different architectures.
Experimental
A mixture of MnCl24H2O (0.199 g, 1.0 mmol), 5-sulfosalicylic acid
dihydrate (0.050 g, 0.20 mmol), and 4% pyridine (2 ml) in water (20 ml) was stirred for 17 h. 1,10-Phenanthroline (0.040 g, 0.20 mmol) was then added. The resulting solution was put aside and the solvent was allowed to evaporate at room temperature. After three weeks, pale-yellow block-shaped crystals were obtained.
Crystal data
[Mn(C7H4O6S)(C12H8N2)(H2O)2] Mr= 487.34
Monoclinic,P21=c a= 14.4166 (9) A˚
b= 7.7234 (5) A˚
c= 18.6868 (11) A˚ = 107.421 (1)
V= 1985.2 (2) A˚3 Z= 4
Dx= 1.631 Mg m
3
MoKradiation Cell parameters from 5871
reflections = 2.3–28.2
= 0.82 mm1 T= 295 (2) K Block, pale yellow 0.430.250.16 mm
Data collection
Bruker SMART APEX area-detector diffractometer ’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 2002)
Tmin= 0.719,Tmax= 0.880 10821 measured reflections
3888 independent reflections 3537 reflections withI> 2(I)
Rint= 0.019
max= 26.0
h=14!17
k=9!9
l=23!13
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.030 wR(F2) = 0.084
S= 1.07 3888 reflections 295 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0466P)2
+ 0.5664P] whereP= (Fo
2
+ 2Fc 2
)/3 (/)max= 0.001
max= 0.24 e A˚
3
min=0.36 e A˚
3
Table 1
Selected geometric parameters (A˚ ,).
Mn1—O1w 2.2316 (14) Mn1—O2w 2.1255 (14) Mn1—O2i
2.1174 (13) Mn1—O4 2.1835 (12) Mn1—N1 2.2659 (15)
Mn1—N2 2.2447 (15) S1—O4 1.4595 (12) S1—O5 1.4479 (13) S1—O6 1.4627 (12)
O2i
—Mn1—O1w 88.61 (5) O2i
—Mn1—O2w 97.59 (6) O2i—Mn1—O4 81.49 (5) O2i
—Mn1—N1 173.00 (6) O2i
—Mn1—N2 99.68 (6) O4—Mn1—O1w 169.86 (5) O4—Mn1—N1 96.03 (5) O4—Mn1—N2 91.36 (5) O1w—Mn1—N1 93.55 (5) O1w—Mn1—N2 88.13 (6) O2w—Mn1—O4 89.39 (5)
O2w—Mn1—O1w 94.12 (5) O2w—Mn1—N1 88.91 (6) O2w—Mn1—N2 162.63 (6) N2—Mn1—N1 73.76 (6) O5—S1—O4 113.94 (8) O5—S1—O6 112.67 (8) O4—S1—O6 109.13 (7) O5—S1—C6 107.69 (8) O4—S1—C6 105.45 (7) O6—S1—C6 107.50 (8)
Symmetry code: (i)x;yþ1 2;z12.
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O2w—H1B O6ii
0.84 (1) 1.89 (1) 2.7214 (18) 169 (2) O1w—H1A O5iii
0.84 (1) 2.01 (1) 2.8233 (18) 163 (2) O1w—H2A O1i
0.85 (1) 1.90 (1) 2.7076 (19) 158 (2) O2w—H2B O6iii
0.84 (1) 1.88 (1) 2.7127 (17) 173 (2) O3—H3A O2 0.84 (1) 1.80 (1) 2.578 (2) 152 (2) Symmetry codes: (i)x;yþ1
2;z 1
2; (ii)x;y 1 2;zþ
1
2; (iii)x;y1;z.
The aromatic H atoms were positioned geometrically, and were included in the refinement in the riding-model approximation [C—
metal-organic papers
Acta Cryst.(2005). E61, m1298–m1300 Fan and Zhu [Mn(C
7H4O6S)(C12H8N2)(H2O)2]
m1299
Figure 2
[image:2.610.333.551.70.270.2]A view of the one-dimensional chain in (I). Hydrogen bonds are shown as dashed lines and H atoms have been omitted for clarity.
Figure 3
A view of the two-dimensional hydrogen-bonding (dashed lines) network for (I). The phen ligand and H atoms have been omitted for clarity. Figure 1
AnORTEP-3(Farrugia, 1997) view of a segment of (I). Displacement ellipsoids are drawn at the 50% probability level. [Symmetry code: (i)x,
1 2y,z
[image:2.610.314.566.403.555.2]H = 0.93 A˚ andUiso(H) = 1.2Ueq(C)]. The water and hydroxy H atoms
were located in difference Fourier maps and were refined with a distance restraint of O—H = 0.85 (1) A˚ and fixed isotropic displacement parameters ofUiso(H) = 0.05 A˚2.
Data collection:SMART(Bruker, 2002); cell refinement:SAINT
(Bruker, 2002); data reduction: SAINT; program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3 for Windows (Farrugia, 1997); software used to prepare material for publication:WinGX(Farrugia, 1999).
We thank the National Natural Science Foundation of China (50073019).
References
Bruker (2002).SADABS,SAINTandSMART. Bruker AXS Inc., Madison, Wisconsin, USA.
Fan, S. R., Cai, G. Q., Zhu, L. G. & Xiao, H. P. (2005).Acta Cryst.C61, m177– m179.
Fan, S. R., Zhu, L. G., Xiao, H. P. & Ng, S. W. (2005a).Acta Cryst.E61, m377– m378.
Fan, S. R., Zhu, L. G., Xiao, H. P. & Ng, S. W. (2005b).Acta Cryst.E61, m563– m565.
Farrugia, L. J. (1997).J. Appl. Cryst.30, 565. Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Gao, S., Zhu, Z. B., Huo, L. H. & Ng, S. W. (2005).Acta Cryst.E61, m279– m281.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
metal-organic papers
m1300
Fan and Zhu [Mn(Csupporting information
sup-1 Acta Cryst. (2005). E61, m1298–m1300
supporting information
Acta Cryst. (2005). E61, m1298–m1300 [https://doi.org/10.1107/S1600536805017861]
catena
-Poly[[
cis
-diaqua(1,10-phenanthroline-
κ
2N
,
N
′
)manganese(II)]-
µ2
-5-sulfonatosalicylato-
κ
2O
:
O
′
]
Sai-Rong Fan and Long-Guan Zhu
(I)
Crystal data
[Mn(C12H8N2)(C7H4SO6)(H2O)2]
Mr = 487.34 Monoclinic, P21/c Hall symbol: -P 2ybc
a = 14.4166 (9) Å
b = 7.7234 (5) Å
c = 18.6868 (11) Å
β = 107.421 (1)°
V = 1985.2 (2) Å3
Z = 4
F(000) = 996
Dx = 1.631 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5871 reflections
θ = 2.3–28.2°
µ = 0.82 mm−1
T = 295 K
Block, pale yellow 0.43 × 0.25 × 0.16 mm
Data collection
Bruker SMART APEX area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 2002)
Tmin = 0.719, Tmax = 0.880
10821 measured reflections 3888 independent reflections 3537 reflections with I > 2σ(I)
Rint = 0.019
θmax = 26.0°, θmin = 2.3°
h = −14→17
k = −9→9
l = −23→13
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.030
wR(F2) = 0.084
S = 1.07 3888 reflections 295 parameters 5 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0466P)2 + 0.5664P] where P = (Fo2 + 2Fc2)/3
supporting information
sup-2 Acta Cryst. (2005). E61, m1298–m1300
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-3 Acta Cryst. (2005). E61, m1298–m1300
H15 0.6081 0.4119 0.4035 0.084* C16 0.50256 (19) 0.3512 (4) 0.31150 (18) 0.0745 (8) H16 0.5324 0.3957 0.2777 0.089* C17 0.41093 (17) 0.2743 (3) 0.28572 (14) 0.0598 (6) H17 0.3804 0.2696 0.2343 0.072* C18 0.36159 (13) 0.1394 (2) 0.45521 (10) 0.0367 (4) C19 0.41045 (13) 0.2154 (2) 0.40632 (11) 0.0382 (4) H1B 0.0361 (10) −0.003 (3) 0.2752 (11) 0.050* H1A 0.2766 (16) −0.2578 (19) 0.2977 (12) 0.050* H2A 0.2758 (15) −0.174 (3) 0.2338 (6) 0.050* H2B 0.0833 (16) −0.1544 (13) 0.3015 (12) 0.050* H3A 0.0776 (13) 0.249 (3) 0.6318 (8) 0.050*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-4 Acta Cryst. (2005). E61, m1298–m1300
Geometric parameters (Å, º)
Mn1—O1W 2.2316 (14) C4—C5 1.374 (2) Mn1—O2W 2.1255 (14) C4—H4 0.9300 Mn1—O2i 2.1174 (13) C5—C6 1.391 (2) Mn1—O4 2.1835 (12) C5—H5 0.9300 Mn1—N1 2.2659 (15) C6—C7 1.379 (2) Mn1—N2 2.2447 (15) C7—H7 0.9300 S1—O4 1.4595 (12) C8—C9 1.394 (3) S1—O5 1.4479 (13) C8—H8 0.9300 S1—O6 1.4627 (12) C9—C10 1.364 (4) S1—C6 1.7620 (16) C9—H9 0.9300 N1—C8 1.328 (3) C10—C11 1.395 (4) N1—C18 1.358 (2) C10—H10 0.9300 N2—C17 1.321 (3) C11—C18 1.408 (3) N2—C19 1.357 (2) C11—C12 1.442 (3) O1—C1 1.236 (2) C12—C13 1.333 (4) O2—C1 1.277 (2) C12—H12 0.9300 O2—Mn1ii 2.1174 (13) C13—C14 1.433 (4) O3—C3 1.349 (2) C13—H13 0.9300 O3—H3A 0.842 (10) C14—C15 1.403 (4) O1W—H1A 0.838 (10) C14—C19 1.406 (3) O1W—H2A 0.851 (9) C15—C16 1.347 (4) O2W—H1B 0.841 (9) C15—H15 0.9300 O2W—H2B 0.837 (10) C16—C17 1.396 (3) C1—C2 1.497 (2) C16—H16 0.9300 C2—C7 1.397 (2) C17—H17 0.9300 C2—C3 1.402 (2) C18—C19 1.435 (3) C3—C4 1.393 (3)
supporting information
sup-5 Acta Cryst. (2005). E61, m1298–m1300
O5—S1—C6 107.69 (8) C11—C10—H10 119.9 O4—S1—C6 105.45 (7) C10—C11—C18 117.4 (2) O6—S1—C6 107.50 (8) C10—C11—C12 124.3 (2) C8—N1—C18 118.61 (16) C18—C11—C12 118.3 (2) C8—N1—Mn1 126.57 (13) C13—C12—C11 121.7 (2) C18—N1—Mn1 114.80 (12) C13—C12—H12 119.2 C17—N2—C19 118.43 (18) C11—C12—H12 119.2 C17—N2—Mn1 126.00 (15) C12—C13—C14 121.5 (2) C19—N2—Mn1 115.57 (12) C12—C13—H13 119.3 C1—O2—Mn1ii 133.93 (12) C14—C13—H13 119.3 C3—O3—H3A 103.6 (16) C15—C14—C19 117.1 (2) S1—O4—Mn1 140.48 (8) C15—C14—C13 124.1 (2) Mn1—O1W—H1A 118.3 (16) C19—C14—C13 118.8 (2) Mn1—O1W—H2A 101.4 (15) C16—C15—C14 120.2 (2) H1A—O1W—H2A 106 (2) C16—C15—H15 119.9 Mn1—O2W—H1B 118.2 (16) C14—C15—H15 119.9 Mn1—O2W—H2B 124.4 (15) C15—C16—C17 119.3 (2) H1B—O2W—H2B 109 (2) C15—C16—H16 120.3 O1—C1—O2 124.24 (16) C17—C16—H16 120.3 O1—C1—C2 120.23 (16) N2—C17—C16 122.7 (2) O2—C1—C2 115.53 (16) N2—C17—H17 118.6 C7—C2—C3 118.69 (15) C16—C17—H17 118.6 C7—C2—C1 119.43 (15) N1—C18—C11 122.15 (19) C3—C2—C1 121.88 (15) N1—C18—C19 117.94 (15) O3—C3—C4 116.66 (16) C11—C18—C19 119.87 (18) O3—C3—C2 123.21 (16) N2—C19—C14 122.2 (2) C4—C3—C2 120.13 (16) N2—C19—C18 117.90 (16) C5—C4—C3 120.28 (16) C14—C19—C18 119.88 (18) C5—C4—H4 119.9
supporting information
sup-6 Acta Cryst. (2005). E61, m1298–m1300
O5—S1—O4—Mn1 54.18 (14) C19—C14—C15—C16 −0.2 (4) O6—S1—O4—Mn1 −178.92 (11) C13—C14—C15—C16 177.9 (2) C6—S1—O4—Mn1 −63.70 (14) C14—C15—C16—C17 0.5 (4) O2i—Mn1—O4—S1 −151.32 (13) C19—N2—C17—C16 0.2 (3) O2W—Mn1—O4—S1 110.91 (13) Mn1—N2—C17—C16 −179.48 (19) O1W—Mn1—O4—S1 −138.7 (2) C15—C16—C17—N2 −0.5 (4) N2—Mn1—O4—S1 −51.73 (13) C8—N1—C18—C11 0.1 (3) N1—Mn1—O4—S1 22.08 (13) Mn1—N1—C18—C11 −178.88 (14) Mn1ii—O2—C1—O1 9.3 (3) C8—N1—C18—C19 178.01 (17) Mn1ii—O2—C1—C2 −170.49 (13) Mn1—N1—C18—C19 −1.0 (2) O1—C1—C2—C7 13.9 (3) C10—C11—C18—N1 0.2 (3) O2—C1—C2—C7 −166.31 (17) C12—C11—C18—N1 178.59 (17) O1—C1—C2—C3 −166.39 (18) C10—C11—C18—C19 −177.61 (18) O2—C1—C2—C3 13.4 (3) C12—C11—C18—C19 0.7 (3) C7—C2—C3—O3 177.04 (17) C17—N2—C19—C14 0.2 (3) C1—C2—C3—O3 −2.7 (3) Mn1—N2—C19—C14 179.87 (14) C7—C2—C3—C4 −3.3 (3) C17—N2—C19—C18 −178.64 (18) C1—C2—C3—C4 177.00 (17) Mn1—N2—C19—C18 1.0 (2) O3—C3—C4—C5 −176.80 (17) C15—C14—C19—N2 −0.2 (3) C2—C3—C4—C5 3.5 (3) C13—C14—C19—N2 −178.38 (18) C3—C4—C5—C6 −1.0 (3) C15—C14—C19—C18 178.64 (19) C4—C5—C6—C7 −1.8 (3) C13—C14—C19—C18 0.4 (3) C4—C5—C6—S1 176.53 (14) N1—C18—C19—N2 0.0 (2) O5—S1—C6—C7 14.68 (16) C11—C18—C19—N2 177.92 (16) O4—S1—C6—C7 136.70 (14) N1—C18—C19—C14 −178.88 (16) O6—S1—C6—C7 −106.96 (14) C11—C18—C19—C14 −0.9 (3)
Symmetry codes: (i) x, −y+1/2, z−1/2; (ii) x, −y+1/2, z+1/2.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O2W—H1B···O6iii 0.84 (1) 1.89 (1) 2.7214 (18) 169 (2) O1W—H1A···O5iv 0.84 (1) 2.01 (1) 2.8233 (18) 163 (2) O1W—H2A···O1i 0.85 (1) 1.90 (1) 2.7076 (19) 158 (2) O2W—H2B···O6iv 0.84 (1) 1.88 (1) 2.7127 (17) 173 (2) O3—H3A···O2 0.84 (1) 1.80 (1) 2.578 (2) 152 (2)