organic papers
Acta Cryst.(2006). E62, o2421–o2422 doi:10.1107/S1600536806018174 Tonget al. C
20H21BN2
o2421
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
Bis(2-methylphenylamino)phenylborane
Hong-Bo Tong, Dian-Sheng Liu* and Mei-Su Zhou
Institute of Modern Chemistry, Shanxi University, Shanxi, People’s Republic of China
Correspondence e-mail: tong@sxu.edu.cn
Key indicators
Single-crystal X-ray study T= 213 K
Mean(C–C) = 0.002 A˚ Rfactor = 0.040 wRfactor = 0.098
Data-to-parameter ratio = 14.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 5 May 2006 Accepted 16 May 2006
#2006 International Union of Crystallography All rights reserved
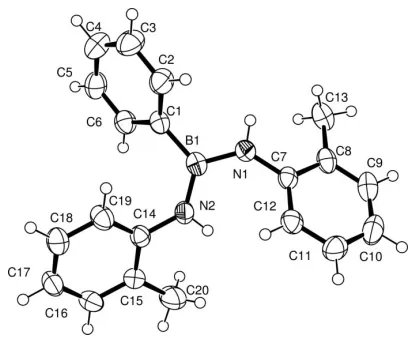
In the title compound, C20H21BN2, the B and N atoms show
trigonal planar geometry. The B—N bond lengths of 1.4177 (19) and 1.418 (2) A˚ indicate covalent partial double bonds.
Comment
Among the group 3 elements B, Al, Ga, In and Tl, the orga-nometallic chemistry of boron and aluminium clearly predo-minates. Organoboron chemistry is of interest from many perspectives, including electronic and structural, as well as its heterocyclic chemistry (Elschenbroich & Salzer, 1992). Recently, we have reported the synthesis and structure of two five-membered heterocyclic compounds incorporating boron in which the coordination around the B atom was distorted tetrahedral with B—N bond lengths in the range 1.685 (4)– 1.734 (4) A˚ (Tonget al., 2002, 2004).
In the title molecule, bis(2-methylphenylamino)phenyl-borane, (I), in which the B and N atoms show trigonal planar geometry (Fig. 1), the two B—N bonds of 1.4177 (19) and 1.418 (2) A˚ are significantly shorter than in the two above-mentioned compounds, indicating their covalent partial double-bond character. The observed B–N bond lengths in (I) compare well with the values reported for similar compounds (Chiverset al., 2004). Atom B1 lies 0.032 (2) A˚ out of the plane defined by the C and two N atoms bonded to it.
Experimental
All manipulations were carried out under argon using standard Schlenk techniques. Diethyl ether was dried by distillation over sodium, and CH2Cl2 was distilled from CaH2. n-Butyllithium was
added at 273 K, and the mixture was warmed to room temperature and stirred for an additional 12 h to give a white precipitate (LiCl). The mixture was filtered, the solvent evaporated with a vacuum pump and the residue extracted with CH2Cl2. The CH2Cl2solution of (I)
was concentrated carefully under vacuum, yielding colourless crystals of the title compound.
Crystal data
C20H21BN2
Mr= 300.20
Monoclinic,P21=c
a= 9.607 (3) A˚
b= 17.454 (5) A˚
c= 10.307 (3) A˚
= 93.272 (5)
V= 1725.6 (8) A˚3
Z= 4
Dx= 1.156 Mg m 3
MoKradiation
= 0.07 mm 1
T= 213 (2) K Block, colourless 0.300.200.20 mm
Data collection
Siemens SMART CCD area-detector diffractometer
!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin= 0.980,Tmax= 0.987
7056 measured reflections 3030 independent reflections 2112 reflections withI> 2(I)
Rint= 0.038
max= 25.0
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.040
wR(F2) = 0.099
S= 0.92 3030 reflections 210 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0499P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.14 e A˚ 3
min= 0.15 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
B1—N1 1.4177 (19) B1—N2 1.418 (2) B1—C1 1.560 (2)
N1—C7 1.4090 (17) N2—C14 1.4059 (17)
N1—B1—N2 119.45 (14) N1—B1—C1 116.59 (14) N2—B1—C1 123.81 (13)
C7—N1—B1 132.18 (13) C14—N2—B1 130.97 (12)
All H atoms were positioned geometrically and allowed to ride on their parent atoms, withUiso(H) = 1.2Ueq(C,N) or 1.5Ueq(methyl C).
A torsion parameter was refined for the methyl group.
Data collection:SMART(Siemens, 1996); cell refinement:SAINT
(Siemens, 1996); data reduction: SAINT; program(s) used to solve structure: SHELXS97(Sheldrick, 1990); program(s) used to refine
structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3(Farrugia, 1997); software used to prepare material for publication:SHELXL97.
We thank the Natural Science Foundation of China (grant No. 29872024 to LDS), the Natural Science Foundation of Shanxi Province (grant No. 20011008, to LDS), the Youth Foundation of Shanxi University (grant No. 2006026, China) and the Youth Foundation of Shanxi Province (grant No. 20031015, China).
References
Chivers, T., Fedorchuk, C. & Parvez, M. (2004).Inorg. Chem.43, 2643–2653. Elschenbroich, Ch. & Salzer, A. (1992).Organometallics, 2nd ed., revised, pp.
57–75. Weinheim, New York: VCH. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565. Sheldrick, G. M. (1990).Acta Cryst.A46, 467–473.
Sheldrick, G. M. (1996).SADABS. University of Go¨ttingen, Germany. Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany. Siemens (1996).SMARTandSAINT. Siemens Analytical X-ray Instruments
Inc., Madison, Wisconsin, USA.
Tong, H.-B., Wei, X.-H., Huang, S.-P., Li, J.-F. & Liu, D.-S. (2002).Acta Cryst.
E58, o1389–o1391.
[image:2.610.336.542.71.240.2]Tong, H.-B., Wei, X.-H., Liu, D.-S. & Huang, S.-P. (2004).Acta Cryst.E60, o2270–o2272.
Figure 1
supporting information
sup-1 Acta Cryst. (2006). E62, o2421–o2422
supporting information
Acta Cryst. (2006). E62, o2421–o2422 [https://doi.org/10.1107/S1600536806018174]
Bis(2-methylphenylamino)phenylborane
Hong-Bo Tong, Dian-Sheng Liu and Mei-Su Zhou
Bis(2-methylphenylamino)phenylborane
Crystal data C20H21BN2
Mr = 300.20 Monoclinic, P21/c
a = 9.607 (3) Å b = 17.454 (5) Å c = 10.307 (3) Å β = 93.272 (5)° V = 1725.6 (8) Å3
Z = 4
F(000) = 640 Dx = 1.156 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 2200 reflections θ = 2.3–24.8°
µ = 0.07 mm−1
T = 213 K Block, colourless 0.30 × 0.20 × 0.20 mm
Data collection
Siemens SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996) Tmin = 0.980, Tmax = 0.987
7056 measured reflections 3030 independent reflections 2112 reflections with I > 2σ(I) Rint = 0.038
θmax = 25.0°, θmin = 2.1°
h = −10→11 k = −20→20 l = −11→12
Refinement Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.040
wR(F2) = 0.099
S = 0.92 3030 reflections 210 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0499P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.14 e Å−3
Δρmin = −0.15 e Å−3
Special details
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
B1 0.96932 (19) 0.08993 (9) 0.63674 (15) 0.0363 (4)
N1 0.97828 (13) 0.04594 (6) 0.75254 (10) 0.0389 (3)
H1 1.0603 0.0449 0.7906 0.047*
N2 0.84148 (12) 0.09276 (6) 0.56094 (10) 0.0399 (3)
H2 0.7817 0.0586 0.5806 0.048*
C1 1.10692 (16) 0.12811 (7) 0.59577 (13) 0.0373 (4)
C2 1.19769 (17) 0.16555 (9) 0.68393 (14) 0.0498 (4)
H2A 1.1729 0.1712 0.7693 0.060*
C3 1.32325 (19) 0.19465 (9) 0.64894 (17) 0.0601 (5)
H3 1.3818 0.2196 0.7103 0.072*
C4 1.36214 (19) 0.18693 (9) 0.52348 (17) 0.0590 (5)
H4 1.4469 0.2066 0.4996 0.071*
C5 1.27534 (18) 0.15014 (9) 0.43388 (15) 0.0524 (4)
H5 1.3016 0.1443 0.3490 0.063*
C6 1.14967 (17) 0.12191 (8) 0.46919 (14) 0.0435 (4)
H6 1.0912 0.0979 0.4067 0.052*
C7 0.87828 (16) 0.00356 (8) 0.81707 (12) 0.0354 (4)
C8 0.91883 (17) −0.06505 (8) 0.87853 (12) 0.0405 (4)
C9 0.8203 (2) −0.10517 (9) 0.94328 (14) 0.0518 (4)
H9 0.8459 −0.1510 0.9839 0.062*
C10 0.6861 (2) −0.07963 (10) 0.94966 (15) 0.0590 (5)
H10 0.6221 −0.1078 0.9943 0.071*
C11 0.64681 (18) −0.01225 (10) 0.88981 (14) 0.0524 (4)
H11 0.5558 0.0054 0.8935 0.063*
C12 0.74276 (16) 0.02927 (9) 0.82404 (13) 0.0427 (4)
H12 0.7159 0.0751 0.7839 0.051*
C13 1.06398 (17) −0.09445 (9) 0.87273 (14) 0.0523 (4)
H13A 1.0722 −0.1426 0.9176 0.078*
H13B 1.1280 −0.0583 0.9132 0.078*
H13C 1.0853 −0.1014 0.7836 0.078*
C14 0.79586 (15) 0.14192 (8) 0.45916 (13) 0.0362 (4)
C15 0.68811 (15) 0.11913 (8) 0.37228 (14) 0.0421 (4)
C16 0.64406 (16) 0.16933 (9) 0.27430 (15) 0.0502 (4)
H16 0.5709 0.1549 0.2164 0.060*
C17 0.70503 (18) 0.23959 (9) 0.26004 (16) 0.0522 (4)
H17 0.6747 0.2720 0.1926 0.063*
C18 0.81084 (18) 0.26130 (9) 0.34603 (16) 0.0539 (5)
H18 0.8534 0.3087 0.3370 0.065*
C19 0.85485 (17) 0.21345 (8) 0.44596 (14) 0.0465 (4)
supporting information
sup-3 Acta Cryst. (2006). E62, o2421–o2422
C20 0.6214 (2) 0.04237 (10) 0.38408 (19) 0.0748 (6)
H20A 0.5462 0.0374 0.3192 0.112*
H20B 0.5859 0.0373 0.4688 0.112*
H20C 0.6892 0.0030 0.3718 0.112*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
B1 0.0472 (11) 0.0312 (9) 0.0303 (9) 0.0024 (8) 0.0015 (8) −0.0034 (7)
N1 0.0397 (8) 0.0431 (7) 0.0337 (7) −0.0031 (6) −0.0016 (6) 0.0040 (5)
N2 0.0443 (8) 0.0391 (7) 0.0362 (7) −0.0051 (6) 0.0005 (6) 0.0075 (5)
C1 0.0464 (10) 0.0322 (8) 0.0330 (8) 0.0008 (7) 0.0002 (7) 0.0012 (6)
C2 0.0556 (11) 0.0555 (10) 0.0386 (9) −0.0092 (9) 0.0046 (8) −0.0036 (8)
C3 0.0572 (12) 0.0645 (12) 0.0583 (11) −0.0187 (9) 0.0014 (9) −0.0088 (9)
C4 0.0526 (12) 0.0603 (11) 0.0655 (12) −0.0127 (9) 0.0139 (10) 0.0022 (9)
C5 0.0596 (12) 0.0546 (10) 0.0443 (10) 0.0014 (9) 0.0151 (9) 0.0046 (8)
C6 0.0503 (11) 0.0427 (9) 0.0373 (9) 0.0001 (8) 0.0012 (8) −0.0006 (7)
C7 0.0428 (10) 0.0378 (8) 0.0257 (7) −0.0039 (7) 0.0022 (6) −0.0022 (6)
C8 0.0580 (11) 0.0384 (9) 0.0251 (7) −0.0007 (8) 0.0019 (7) −0.0026 (6)
C9 0.0766 (14) 0.0436 (10) 0.0357 (9) −0.0050 (9) 0.0074 (9) 0.0044 (7)
C10 0.0720 (14) 0.0642 (12) 0.0417 (10) −0.0217 (10) 0.0120 (9) 0.0043 (9)
C11 0.0463 (11) 0.0706 (12) 0.0408 (9) −0.0057 (9) 0.0079 (8) −0.0044 (8)
C12 0.0479 (11) 0.0475 (10) 0.0325 (8) −0.0005 (8) 0.0012 (7) −0.0009 (7)
C13 0.0689 (12) 0.0481 (10) 0.0398 (9) 0.0147 (9) 0.0031 (8) 0.0048 (7)
C14 0.0385 (9) 0.0381 (9) 0.0323 (8) 0.0045 (7) 0.0046 (7) 0.0021 (6)
C15 0.0356 (9) 0.0457 (9) 0.0448 (9) 0.0004 (7) 0.0005 (7) 0.0056 (7)
C16 0.0403 (10) 0.0602 (11) 0.0489 (10) 0.0053 (8) −0.0078 (8) 0.0066 (8)
C17 0.0518 (11) 0.0556 (11) 0.0492 (10) 0.0113 (9) 0.0019 (9) 0.0189 (8)
C18 0.0604 (12) 0.0384 (9) 0.0624 (11) 0.0013 (8) 0.0000 (9) 0.0126 (8)
C19 0.0549 (11) 0.0365 (9) 0.0469 (9) 0.0011 (8) −0.0075 (8) 0.0015 (7)
C20 0.0595 (13) 0.0701 (13) 0.0910 (14) −0.0192 (10) −0.0285 (11) 0.0213 (11)
Geometric parameters (Å, º)
B1—N1 1.4177 (19) C9—H9 0.9300
B1—N2 1.418 (2) C10—C11 1.371 (2)
B1—C1 1.560 (2) C10—H10 0.9300
N1—C7 1.4090 (17) C11—C12 1.380 (2)
N1—H1 0.8600 C11—H11 0.9300
N2—C14 1.4059 (17) C12—H12 0.9300
N2—H2 0.8600 C13—H13A 0.9600
C1—C2 1.3867 (19) C13—H13B 0.9600
C1—C6 1.3942 (19) C13—H13C 0.9600
C2—C3 1.376 (2) C14—C19 1.3811 (19)
C2—H2A 0.9300 C14—C15 1.3877 (19)
C3—C4 1.373 (2) C15—C16 1.385 (2)
C3—H3 0.9300 C15—C20 1.493 (2)
C4—H4 0.9300 C16—H16 0.9300
C5—C6 1.372 (2) C17—C18 1.364 (2)
C5—H5 0.9300 C17—H17 0.9300
C6—H6 0.9300 C18—C19 1.374 (2)
C7—C12 1.383 (2) C18—H18 0.9300
C7—C8 1.3996 (19) C19—H19 0.9300
C8—C9 1.380 (2) C20—H20A 0.9600
C8—C13 1.490 (2) C20—H20B 0.9600
C9—C10 1.369 (2) C20—H20C 0.9600
N1—B1—N2 119.45 (14) C11—C10—H10 120.2
N1—B1—C1 116.59 (14) C10—C11—C12 119.78 (16)
N2—B1—C1 123.81 (13) C10—C11—H11 120.1
C7—N1—B1 132.18 (13) C12—C11—H11 120.1
C7—N1—H1 113.9 C11—C12—C7 120.81 (15)
B1—N1—H1 113.9 C11—C12—H12 119.6
C14—N2—B1 130.97 (12) C7—C12—H12 119.6
C14—N2—H2 114.5 C8—C13—H13A 109.5
B1—N2—H2 114.5 C8—C13—H13B 109.5
C2—C1—C6 116.23 (14) H13A—C13—H13B 109.5
C2—C1—B1 122.34 (13) C8—C13—H13C 109.5
C6—C1—B1 121.30 (13) H13A—C13—H13C 109.5
C3—C2—C1 122.02 (15) H13B—C13—H13C 109.5
C3—C2—H2A 119.0 C19—C14—C15 119.39 (13)
C1—C2—H2A 119.0 C19—C14—N2 120.87 (13)
C4—C3—C2 120.03 (16) C15—C14—N2 119.72 (13)
C4—C3—H3 120.0 C16—C15—C14 118.35 (14)
C2—C3—H3 120.0 C16—C15—C20 120.82 (14)
C5—C4—C3 119.58 (17) C14—C15—C20 120.83 (13)
C5—C4—H4 120.2 C17—C16—C15 121.98 (15)
C3—C4—H4 120.2 C17—C16—H16 119.0
C4—C5—C6 120.06 (15) C15—C16—H16 119.0
C4—C5—H5 120.0 C18—C17—C16 119.09 (14)
C6—C5—H5 120.0 C18—C17—H17 120.5
C5—C6—C1 122.07 (15) C16—C17—H17 120.5
C5—C6—H6 119.0 C17—C18—C19 120.30 (15)
C1—C6—H6 119.0 C17—C18—H18 119.9
C12—C7—C8 119.52 (14) C19—C18—H18 119.8
C12—C7—N1 121.56 (13) C18—C19—C14 120.86 (15)
C8—C7—N1 118.89 (14) C18—C19—H19 119.6
C9—C8—C7 118.18 (15) C14—C19—H19 119.6
C9—C8—C13 120.92 (14) C15—C20—H20A 109.5
C7—C8—C13 120.89 (14) C15—C20—H20B 109.5
C10—C9—C8 122.12 (16) H20A—C20—H20B 109.5
C10—C9—H9 118.9 C15—C20—H20C 109.5
C8—C9—H9 118.9 H20A—C20—H20C 109.5
C9—C10—C11 119.59 (16) H20B—C20—H20C 109.5