1 THE ALANINE, VALINE, AND LEUCINE GROUP 1.1 Alanine
L-Alanine and the essential amino acids L-valine and L-leucine have a common precursor, i.e. pyruvic acid from the glycolytic pathway (Figure 1).
Biosynthesis of Food Constituents: Amino Acids:
2. The Alanine-Valine-Leucine, Serine-Cysteine-Glycine, and
Aromatic and Heterocyclic Amino Acids Groups – a Review
JAN VELÍŠEK and KAREL CEJPEK
Department of Food Chemistry and Analysis, Faculty of Food and Biochemical
Technology, Institute of Chemical Technology Prague, Prague, Czech Republic
Abstract
VELÍŠEK J., CEJPEK K. (2006): Biosynthesis of food constituents: Amino acids: 2. The alanine-valine-leucine, serine-cysteine-glycine, and aromatic and heterocyclic amino acids groups – a review.Czech J. Food Sci., 24: 45–58.
This review article gives a survey of principal pathways that lead to the biosynthesis of the proteinogenic amino acids of the alanine-valine-leucine group starting with pyruvic acid from the glycolytic pathway and serine-cysteine-glycine group starting with 3-phospho-D-glyceric acid from the glycolytic pathway. A survey is further given to the aromatic and heterocyclic amino acids (phenylalanine, tyrosine, tryptophan, histidine) starting with 3-phosphoenolpyruvic acid from the glycolytic pathway and D-erythrose 4-phosphate, an intermediate in the pentose phosphate cycle and Calvin cycle.
Keywords: biosynthesis; amino acids; alanine; valine; leucine; serine; glycine; cysteine; phenylalanine; tyrosine; tryp-tophan; histidine
Partly supported by the Ministry of Education, Youth and Sports of the Czech Republic, Project MSM 6046137305.
Alanine is formed from pyruvic acid in a simple transamination reaction (Figure 2) catalysed by alanine transaminase (EC 2.6.1.2) (SENGBUSCH). The transamination reactions are catalysed by transaminases dependent on the coenzyme pyri-doxal 5'-phosphate (PLP).
H3C COOH
O
L-glutamic acid
EC 2.6.1.2
CH3 COOH
NH2
2-oxoglutaric acid
[image:1.595.67.515.619.735.2]pyruvic acid L-alanine
Figure 1 Figure 2
pyruvic acid Glycolysis
L-valine
46
1.2 Valine
Valine is synthesised in a set of four reactions, and an extension of the valine pathway results in leucine synthesis (Figure 3) (IUBMB 2003). The first three enzymes involved in the biosynthesis of valine and leucine, i.e. acetolactate synthase (EC 2.2.1.6), ketol-acid reductoisomerase (EC 1.1.1.86), and di-hydroxyacid-dehydratase (EC 4.2.1.9), also catalyse analogous reactions leading to the biosynthesis of isoleucine. 3-Methyl-2-oxobutanoic acid is the common precursor of both amino acids, valine and leucine. This carboxylic acid then yields valine by the action of the branched-chain-amino-acid transaminase (EC 2.6.1.42).
1.3 Leucine
Leucine is synthesised from 3-methyl-2-oxobu-tanoic acid in six steps (Figure 3) (IUBMB 2003). The first step, catalysed by 2-isopropylmalate
[image:2.595.64.539.402.721.2]synthase (EC 2.3.3.13, requires K+ ions), is the condensation of 3-methyl-2-oxobutanoic acid with acetyl-CoA to (S)-2-isopropylmalic acid. The next two steps are catalysed by 3-isopropylmalate dehydratase (EC 4.2.1.33). 2-Isopropylmaleic acid arises from 2-isopropylmalic acid by elimination of water and addition of water to 2-isopropyl-maleic acid yields (2R,3S)-3-isopropylmalic acid. The enzyme also hydrates the product back to (S)-2-isopropylmalic acid, thus bringing about the interconversion between the two isomers. In the next step, 3-isopropylmalic acid is oxi-dised to (S)-2-isopropyl-3-oxosuccinic acid by the NAD+-dependent 3-isopropylmalate dehydroge-nase (EC 1.1.1.85). The product decarboxylates spontaneously to 4-methyl-2-oxopentanoic acid (4-oxoisocapronic acid), which is, in the last step, transformed to leucine by either branched-chain-amino-acid transaminase (EC 2.6.1.42) or more specific leucine transaminase (EC 2.6.1.6) that does not act on valine or isoleucine.
Figure 3
EC 2.6.1.6
OH
L-glutamic acid
H3C COOH
CH3 COOH
H3C COOH
CH3 COOH
OH
H3C COOH
CH3 COOH
O
H3C COOH
CH3 O
H3C COOH
CH3 NH2
EC 2.2.1.6
EC 4.2.1.9
H3C
O
COOH CH3
OH CO2
H2O
H3C COOH
CH3
O
H3C COOH
CH3
OH
(S)-2-hydroxy-2-methyl-3-oxobutanoic acid (R)-2,3-dihydroxy-3-methylbutanoic acid
3-methyl-2-oxobutanoic acid pyruvic acid
H3C COOH
O 2
EC 1.1.1.86
NADPH + H NADP
H3C COOH
CH3 COOH
OH
H3C S
O
CoA
HS CoA + H2O
H2O
H2O
NAD NADH + H CO2 2-oxoglutaric acid
(S)-2-isopropylmalic acid 2-isopropylmaleic acid
(2R,3S)-3-isopropylmalic acid (S)-2-isopropyl-3-oxosuccinic acid 4-methyl-2-oxopentanoic acid L-leucine
H3C COOH
CH3
NH2
L-valine
EC 2.3.3.13 EC 4.2.1.33
EC 4.2.1.33
EC 1.1.1.85 EC 2.6.1.42
EC 2.6.1.42
2 THE SERINE, GLYCINE, AND CYSTEINE GROUP
2.1 Serine
L-Serine is generated in a three-step reaction from 3-phospho-D-glyceric acid, formed in the glycolytic pathway, and becomes the precursor of glycine and L-cysteine (Figure 4).
2.2 Glycine
The major pathway of glycine biosynthesis is the direct transformation of L-serine, which is catalysed by PLP protein, i.e. serine hydroxymethyl trans-ferase (EC 2.1.2.1). The hydroxymethyl group of the serine side chain is split off and this C1 unit is ac-cepted by tetrahydrofolic acid (H4PteGlu, R = ben- zoyl glutamic acid residue) under the formation of 5,10-methylene-tetrahydrofolic acid (5,10-meth-ylene-H4PteGlu) (Figure 6) (SENGBUSCH).
2.3 Cysteine
Cysteine directly or indirectly provides sulfide for structural, regulatory, or catalytic purposes in peptides, proteins, and numerous low molecular weight compounds such as methionine, gluta- thione, biotin, and thiamine. Biosynthesis of the cysteine -SH group needs sulfur and thus provides an exclusive entry of reduced sulfur into cellular metabolism. Plants take up sulfur as inorganic sulfate ions. After its uptake by roots, sulfate is dis-tributed into different organs and, to be assimilated, it has to be reduced in a process called assimila-tory sulfate reduction performed in chloroplasts. The first step in the sulfate assimilation pathway is catalysed by ATP sulfurylase (EC 2.7.7.4). This enzyme activates sulfate via an ATP-dependent reaction that leads to the formation of adenylyl-The first step of serine biosynthesis is the
oxida-tion of 3-phospho-D-glyceric acid at C-2 to 3-phos-phopyruvic acid catalysed by 3-phosphoglycerate dehydrogenase (EC 1.1.1.95). This phosphorylated oxo-analogue of serine is transaminated by PLP-de-pendent phosphoserine transaminase (EC 2.6.1.52) in the second step yielding 3-phospho-L-serine. Finally, in the third step, phosphoserine is hydro-lysed by phosphoserine phosfatase (EC 3.1.3.3) to serine (Figure 5) (SENGBUSCH).
Figure 4
Figure 5
Figure 6
3-phospho-D-glyceric acid
L-serine L-cysteine
glycine Glycolysis
L-glutamic acid
PO COOH OH
PO COOH O
PO COOH
NH2
HO COOH
NH2
2-oxoglutaric acid NAD NADH + H
EC 1.1.1.95 EC 2.6.1.52 EC 3.1.3.3
3-phospho-D-glyceric acid 3-phosphopyruvic acid 3-phospho-L-serine L-serine
P H2O
NH2 COOH
HO COOH + H2O
NH2 EC 2.1.2.1
L-serine H4PteGlu glycine 5,10-methylene-H4PteGlu H
N
N N
N OH
H2N
N R
H H
5 10
N
N N
N OH
H2N
N R H
48
sulfate (adenosine-5'-phosphosulfuric acid, APS) and inorganic diphosforic acid (PP).
To accomplish the incorporation of sulphur into biomolecules, specifically amino acids, sulphate in APS is transformed into sulphite and this into sulphide. This process may occur through two different pathways, depending on the organism. One of them involves phosforylation of APS by adenosine 5'-phosphosulfate kinase (EC 2.7.1.25) using ATP to produce 3'-phosfoadenosine-5'-phos- phosulfate (PAPS) and ADP. In the following re-action, PAPS reductase (EC 1.8.4.8) firstly reacts with reduced thioredoxin and then with PAPS to generate free sulphite. The other pathway involves the direct reduction of APS by 5'-adenylylsulfate reductase (EC 1.8.4.9), which uses GSH as an electron source to produce sulphite. In yeasts and many bacteria, sulphite is synthesised via adeno-sine 5'-phosphosulfate kinase, whereas in plants, green algae, and phototrophic bacteria, sulfate is transformed into sulphite via 5'-adenylylsulfate reductase (Figure 7).
Once sulfate has been reduced to sulphite, the subsequent step is identical in bacteria, fungi,
and plants. Sulfite is reduced to sulphide at the expense of oxidising three molecules of NADPH, by sulphite reductase (EC 1.8.7.1)1.
Depending on the organism, there are two dif-ferent ways by which sulfide is incorporated into a carbon backbone to produce L-cysteine (Figure 8). First, the physiological O-ester substrate O -ace-tylserine is synthesised from L-serine by serine acetyltransferase (EC 2.3.1.30). Sulfide is condensed with O-acetylserine by the PLP-dependent enzyme O-acetylserine (thiol)-lyase (also called O -acetyl-serine sulfhydrylase, EC 2.5.1.47) to form cysteine directly. Because the substrate O-acetylserine is derived from the carbon and nitrogen assimilatory pathways, the O-acetylserine (thiol)-lyase links the sulfur and nitrogen assimilatory pathways together.
O-acetylserine sulphhydrylase (EC 2.5.1.47) also catalyses the condensation of sulfide with O -acetyl-homoserine to form L-homocysteine. O -Acetyl-homoserine is synthesised from L--Acetyl-homoserine by homoserine O-acetyltransferase (EC 2.3.1.31). Then homocysteine is transformed into cysteine by trans-sulfuration, i.e. L-homocysteine
asso-1Sulphite reductase contains a special acidic heme group called siroheme and catalyses the reduction of sulfite using
electrons donated by ferredoxin.
Figure 7
N N N
N NH2
CH2
OH OH
O
adenosine-5´-phosphate
N N N
N NH2
CH2
OH OH
O O P O
O OH
S OH
O O
adenosine-5´-phosphosulfate
HO SH HS
EC 1.8.4.8
thioredoxin (oxidized) thioredoxin (reduced)
O S OH OH
sulfurous acid adenosine-5´-phosphosulfate-3´-phosphate
N N N
N NH2
CH2
OH O O P O
O OH
S OH
O O
O P OH
OH O
N N N
N NH2
CH2
OH O P O O OH
O P OH
OH O
adenosine-3´,5´-diphosphate
N S R
O
S O
R H
N R
O H
R O
EC 2.7.1.25
ATP
ADP
O S OH OH
sulfurous acid
P O O OH
HO
+
EC 1.8.4.9 2 GSH G-S-S-G
ciates with serine to form L,L-cystathionine by action of cystathionine β-synthase (EC 4.3.1.22). Cystathionine in turn dissociates into cysteine, 2-oxobutyric acid, and ammonia by cystathionine γ-lyase (EC 4.4.1.1) (Figure 9) (JOST et al. 2000). Cysteine may also be transformed into homo-cysteine by reverse trans-sulphuration catalysed by cystathionine γ-synthase (EC 2.5.1.48) and cystathionine β-lyase (EC 4.4.1.8).
The serine acetyltransferase pathway is used by plants and by enteric bacteria. Fungi use different cysteine biosynthetic pathway depending on the species. In plants, cysteine can also be produced by the degradation of methionine.
3 THE AROMATIC AND HETEROCYCLIC AMINO ACIDS GROUP
3.1 Phenylalanine and tyrosine
Biosynthetic pathways of the aromatic and het-erocyclic amino acids family is schematically shown in Figure 10. The shikimic acid pathway provides a
route to the aromatic amino acids L-phenylalanine and L-tyrosine and to the heterocyclic amino acid L-tryptophan (IUBMB 2001). Shikimic acid2, a central intermediate in this pathway, is formed by a sequence of reactions from 3-phospho- enolpyruvic acid (from glycolysis) and D-eryth-rose 4-phosphate (from the pentose phosphate cycle and the Calvin cycle). Both shikimic acid and the subsequent chorismic acid are important intermediates. The latter is the starting compound for three different pathways that lead to the end products phenylalanine, tyrosine, and tryptophan. An activated form of D-ribose 5-phosphate (5-phospho-α-D-ribose 1-diphosphate), is an-other intermediate of tryptophan biosynthesis. It has also a key position in the biosynthesis of L-histidine. The biosynthetic pathway of histidine is unusual in that histidine is produced from a purine. Animals employ none of these pathways and, accordingly, these amino acids feature among those essential amino acids for man that have to be obtained in the diet.
2Shikimic acid has been isolated from plants of Illicium anisatum L., syn. I. religiosum Sieb. et Zucc., Japanese shikimi.
Figure 9 Figure 8
HO COOH
NH2
L-serine
O COOH H3C
O
NH2
HS COOH
NH2
acetyl-CoA HS-CoA H
2S CH3 COOH
O-acetyl-L-serine L-cysteine
EC 2.3.1.30 EC 2.5.1.47
COOH HO
NH2
L-homoserine
COOH
NH2
COOH
S HOOC
NH2
NH2
acetyl-CoA HS-CoA
L-cysteine L,L-cystathionine
EC 2..3.1.31
H3C O COOH
O NH2
H2S CH3 COOH
L-serine
H2O
H3C
COOH O + NH3
O-acetyl-L-homoserine
2-oxobutyric acid
EC 2.5.1.47
EC 4.2.1.22
EC 4.4.1.1
COOH HS
NH2
L-homocysteine
50
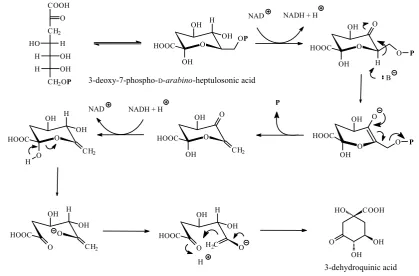
The shikimic acid pathway begins with an aldol-type condensation of phosphoenolpyruvic acid with D-erythrose 4-phosphate to give the seven-carbon 3-deoxy-D-arabino-heptulosonic acid 7-phosphate (3-deoxy-7-phospho-D-arabino-heptulosonic acid, Figure 11). This reaction catalysed by 3-deoxy-7-phosphoheptulonate synthase (EC 2.5.1.54) is mechanistically a complex reaction (Figure 12) (IUBMB 2002). Elimination of phosphoric acid from 3-deoxy-7-phospho-D-arabino-heptulosonic acid followed by intramolecular aldol reaction generates the first alicyclic intermediate 3-dehyd-roquinic acid. The elimination of phosphoric acid by 3-dehydroquinate synthase (EC 4.2.3.4) actually follows a NAD+-dependent oxidation (NAD+ is bound as a prosthetic group) of the central sec-ondary hydroxyl group, which is then re-formed in a NADH-dependent reduction reaction on the intermediate carbonyl compound prior to the aldol reaction occurring (Figure 13) (BENDER et al. 1989; GOURLEY et al. 1999; IUBMB 2001).
Reduction of 3-dehydroquinic acid with either quinate dehydrogenase (EC 1.1.1.24) or quinate/shi-
kimate dehydrogenase (EC 1.1.1.282) yields quinic acid, a fairly common acid frequently occurring in foods in the free and esterified form3. 3-Dehy-droshikimic acid is formed from 3-dehydroquinic acid by dehydration catalysed by 3-dehydroquin-ate dehydratase (EC 4.2.1.10) (Figure 14) (IUBMB 2001). Reduction of 3-dehydroshikimic acid by NADP+-dependent shikimate 3-dehydrogenase (EC 1.1.1.25) or quinate/shikimate dehydrogenase (EC 1.1.1.282) yields shikimic acid. This second shikimate dehydrogenase enzyme differs from shikimate 3-dehydrogenase (EC 1.1.1.25) in that it can use both quinate and shikimate as substrate and either NAD+ or NADP+ as acceptor.
An important branch point compound in the shikimate pathway is chorismic acid. Chorismic acid forms from shikimic acid, which is first phos-phorylated by shikimate kinase (EC 2.7.1.71) and yields 3-phosphoshikimic acid. 3-Phosphoshikimic acid reacts, under catalysis of 3-phosphoshikimate 1-carboxyvinyltransferase (EC 2.5.1.19), with fur-ther molecule of phosphoenolpyruvic acid, which is incorporated as an enol ether side-chain in 3Reduction of 3-dehydroquinic acid in bacteria can be achieved also by a quinoprotein, NAD(P)-independent
[image:6.595.64.534.83.404.2]quinate-dehydrogenase (pyrroloquinoline-quinone) (EC 1.1.99.25).
Figure 10
10
Glycolysis
Photosynthesis Pentose phosphate cycle
3-phosphoenolpyruvic acid
shikimic acid
chorismic acid
prephenic acid
L-phenylalanine L-tyrosine
L-tryptophan
L-histidine D-ribose 5-phosphate D-erythrose 4-phosphate
EC 2.5.1.54
EC 4.2.1.10
EC 1.1.1.25 EC 1.1.1.282 EC 2.7.1.71
EC 2.5.1.19
EC 4.2.3.5
EC 4.2.3.4 EC 1.1.1.24 EC 1.1.1.282
5-(1-carboxyvinyl)-3-phosphoshikimic acid chorismic acid
CH=O
H OH
H OH
CH2OP
H2C COOH
OP H2O
P
COOH
CH2
CH2OP
HO H
H OH
H OH
O P
OH
HO COOH
OH O
OH
HO COOH
OH HO
OH OH COOH
O OH
OH COOH
HO OH
OH COOH
PO
OH O COOH
PO COOH
CH2 H2C COOH
OP
OH O COOH
COOH CH2
phosphoenolpyruvic acid
D-erythrose 4-phosphate +
3-deoxy-7-phospho-D-arabino
-heptulosonic acid 3-dehydroquinic acid quinic acid
3-dehydroshikimic acid shikimic acid
H2O
NADPH + H NADP
NADP NADPH + H ATP
ADP
3-phosphoshikimic acid
phosphoenolpyruvic acid
P P
CH=O H OH H OH CH2OP
H2C COOH
OP
COOH CH2
CH2OP
HO H H OH H OH O
phosphoenolpyruvic acid
D-erythrose 4-phosphate
3-deoxy-7-phospho-D-arabino-heptulosonic acid
enzyme O
O H
H
P H
2O
enzyme O
O
CH2 COOH
O P
H
O O
COOH H2C
[image:7.595.64.531.87.726.2]enzyme
Figure 11
52
5-(1-carboxyvinyl)-3-phosphoshikimic acid (Fig-ure 15). The transformation of 5-(1-carboxyvinyl)-3-phosphoshikimic acid to chorismic acid involves a 1,4-elimination of phosphoric acid by chorismate synthase (EC 4.2.3.5) (Figure 16) (FLOSS et al. 1972; BORNEMANN et al. 1996, 2000; JAKEMAN et al. 1998; LEWIS et al. 1999; OSBORNE et al. 2000; IUBMB 2001).
The biologically unique conversion of choris-mic acid to prephenic acid by chorismate mutase (EC 5.4.99.5) is formally a Claisen rearrangement reaction (Figures 17 and 18). Decarboxylation and loss of hydroxyl group from prephenic acid (prephenate dehydratase, EC 4.2.1.51) yields phe-nylpyruvic acid, and the PLP-dependent transami-nation catalysed by aromatic aminotransferase
COOH CH2
CH2OP
HO H H OH H OH O
3-deoxy-7-phospho-D-arabino-heptulosonic acid
O O P
O OH HOOC
OH O
OH OH H
OP
OH
HOOC O
OH
OH HOOC
O
O OH
OH HOOC
O CH2
O OH
O HOOC
CH2
H OH H
O OH HOOC
CH2
H OH
O O
OH HOOC
H2C
H OH O
HO
OH COOH
OH O
NAD NADH + H
P
NAD NADH + H
3-dehydroquinic acid
O P
H B
H
3-dehydroquinic acid
HO
OH COOH
OH O
H2O
enzyme
B H
enzyme
N H
OH COOH
OH HO
N OH
OH COOH
enzyme
OH OH COOH
O
H2O
HO
OH COOH
OH N
H
H B
H2O
3-dehydroshikimic acid enzyme NH2
[image:8.595.89.509.86.362.2]Figure 13
OH OH COOH
PO
H2C COOH
OP
H
O OH COOH
PO
CH2
H
COOH O P
B
O OH COOH
PO
CH2
COOH
3-phosphoshikimic acid
phosphoenolpyruvic acid
5-(1-carboxyvinyl)-3-phosphoshikimic acid
P
COOH
PO OH
O COOH
CH2
COOH
OH O
COOH CH2
FMNH2 FMNH + P FMNH + P FMNH2+ H
5-(1-carboxyvinyl)-3-phosphoshikimic acid chorismic acid
COOH
OH O
COOH CH2
(EC 2.6.1.57) forms phenylalanine. In the presence of a NAD+-dependent dehydrogenase (prephen-ate dehydrogenase, EC 1.3.1.12), or a NADP+ -dependent prephenate dehydrogenase (NADP+)
[image:9.595.80.506.84.311.2](EC 1.3.1.13) decarboxylation occurs with reten-tion of the hydroxyl funcreten-tion. Transaminareten-tion of the resultant 4-hydroxyphenylpyruvic acid by aromatic-amino-acid transaminase (EC 2.6.1.57)
Figure 16 Figure 15
Figure 17
EC 1.3.1.13 EC 1.3.1.43
COOH O
phenylpyruvic acid
COOH
NH2
L-phenylalanine
COOH NH2
HO COOH
O HO
chorismic acid prephenic acid L-arogenic acid
O COOH
OH
COOH CH2
COOH O
O
H
O
OH
HOOC
OH
COOH NH2
4-hydroxyphenylpyruvic acid L-tyrosine
EC 5.4.99.5
EC 1.3.1.12
EC 4.2.1.51 EC 4.2.1.91
EC 2.6.1.57
EC 2.6.1.57 EC 2.6.1.57
H2O + CO2
H H
NAD
NADH + H + CO2
NAD(P)
NAD(P)H + H + CO2
CO2
H2O +
L-glutamic acid 2-oxoglutaric acid
L-glutamic acid 2-oxoglutaric acid
L-glutamic acid 2-oxoglutaric acid
54
OH HOOC
O COOH
pseudo-diequatorial pseudo-diaxial
prephenic acid chorismic acid conformers
OH
COOH O
HOOC
O O
H HOOC CH2 COOH COOH
O H
O CH2
O O H
or tyrosine transaminase (EC 2.6.1.5) subsequently gives tyrosine (CLARK et al. 1990; IUBMB 2001; MARTÍ et al. 2003).
L-Arogenic acid is the result of transamination of prephenic acid occurring prior to the decar-boxylation, and can be transformed into both phenylalanine and tyrosine depending on the absence or presence of a suitable enzyme activity, e.g. activity of arogenate dehydratase (EC 4.2.1.91) and arogenate dehydrogenase (EC 1.3.1.43), re-spectively (IUBMB 2003). In animals, which lack this pathway, direct hydroxylation of phe-nylalanine to tyrosine (and of tyrosine to DOPA) may be achieved. This reaction is catalysed by (6R)-5,6,7,8-tetrahydro-L-biopterin (L-erythro -5,6,7,8-tetrahydrobiopterin, BH4)-dependent hy-droxylase enzyme (phenylalanine hyhy-droxylase, EC 1.14.16.1)4, the hydroxyl oxygen being derived from molecular oxygen. The second oxygen atom
is reduced to water (Figure 19). The rationalised reaction mechanism is given in Figure 20 (CARR et al. 1995; IUBMB 2001).
3.2 Tryptophan
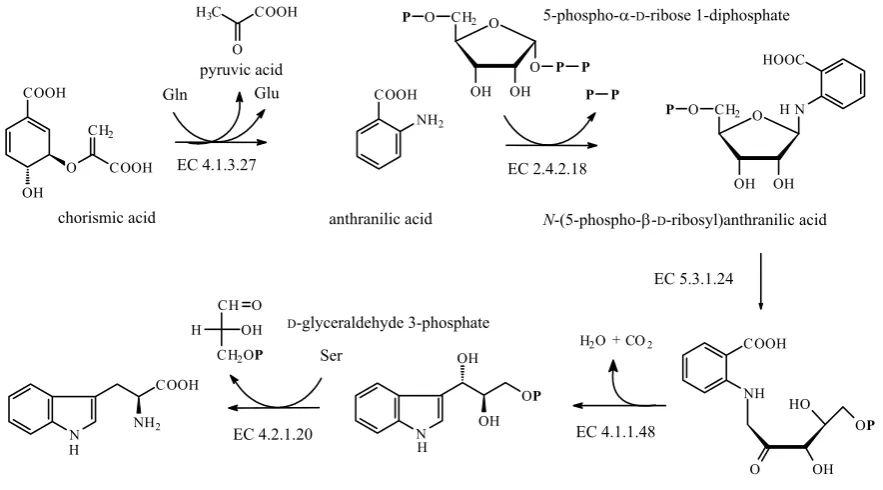
Tryptophan is derived from chorismic acid via anthranilic (2-aminobenzoic) acid in a reaction catalysed by anthranilate synthase (EC 4.1.3.27) (Figure 21). In some organisms this enzyme is a part of a multifunctional protein having more other enzymatic activities for the biosynthesis of tryp-tophan, i.e. anthranilate phosphoribosyltransferase (EC 2.4.2.18), phosphoribosylanthranilate isomer-ase (EC 5.3.1.24), indole-3-glycerol-phosphate synthase (EC 4.1.1.48), and tryptophan synthase (EC 4.2.1.20) activity (IUBMB 2001).
Chorismic acid is first aminated at C-2 by L-glu- tamine to give 2-amino-2-deoxyisochorismic acid,
HO COOH
NH2
COOH
NH2
L-phenylalanine L-tyrosine
H NH2 COOH
O H
H
COOH
NH2
O
H H
H
4The product of tetrahydrobiopterin reduction is 4a-hydroxytetrahydrobiopterin. It can dehydrate 6,7-dihydrobiopterin,
[image:10.595.117.494.88.168.2]both spontaneously and by the action of 4a-hydroxytetrahydropterin dehydratase (EC 4.2.1.96). The 6,7-dihydrobiopterin can be enzymatically reduced back to tetrahydrobiopterin by 6,7-dihydropteridine reductase (EC 1.5.1.34), or spontaneously rearranges to the more stable 7,8-dihydrobiopterin.
Figure 18
Figure 19
Figure 20
5,6,7,8-tetrahydrobiopterin 7,8-dihydrobiopterin
N N
N
N NH2
O H3C
OH OH
H 1 2
3 4 5 6 7 8
4a
8a N N
NH
N NH2
O H3C
OH
OH H
H
HO
COOH
NH2
COOH
NH2
L-phenylalanine
EC 1.14.16.1
L-tyrosine
+ +
which yields anthranilic acid by elimination of the C-4 hydroxyl as water and C-3 substituent as pyruvic acid (Figure 22). The transformation in-volves an SN2'-type of reaction, incoming ammonia, generated from glutamine, acts as a nucleophile attacking the diene system.
5-Phospho-α-D-ribose 1-diphosphate is the ac-tivated form of ribose. It forms from either AMP in a reaction catalysed by adeninephosphoribosyl transferase (EC 2.4.2.7) or from D-ribose 5-phos-phate (forming in pentose phos5-phos-phate pathway) and ATP by ribose-phosphate diphosphokinase
pyruvic acid
N
COOH
NH2
H
O
OH OH
O P P
CH2
O
P
COOH NH2
P P
5-phospho-�-D-ribose 1-diphosphate
EC 2.4.2.18
O
OH OH CH2
O
P N
HOOC
H
L-tryptophan (1S,2R)-1-(indol-3-yl)glycerol 3-phosphate 1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate COOH
OH
O COOH
CH2
Gln Glu
EC 4.1.3.27
N-(5-phospho-�-D-ribosyl)anthranilic acid
chorismic acid anthranilic acid
EC 5.3.1.24
H2O+CO2
EC 4.1.1.48
D-glyceraldehyde 3-phosphate
EC 4.2.1.20 Ser
H3C COOH
O
CH O
CH2OP
H OH
COOH
NH HO
O OH
OP
N H
OP
OH
[image:11.595.70.512.83.328.2]OH
Figure 21
Figure 22
o
OOH OH
PO
OPP
O
OH OH
PO
OH
[image:11.595.75.526.484.713.2]AMP
adenine
D-ribose 5-phosphate
5-phospho-�-D-ribose 1-diphosphate
ATP AMP
EC 2.4.2.7 EC 2.7.6.1
PP
Figure 23
pyruvic acid
H3C COOH
O H2C COOH
OH
HOOC COOH
NH2
COOH NH2
O
H2N COOH
NH2
COOH
OH
O COOH CH2
chorismic acid 2-amino-2-deoxyisochorismic acid anthranilic acid
COOH
NH2
CH2
COOH O
56
(EC 2.7.6.1) (Figure 23). It has also a key posi-tion in the biosynthesis of histidine and purine nucleotides (IUBMB 2003).
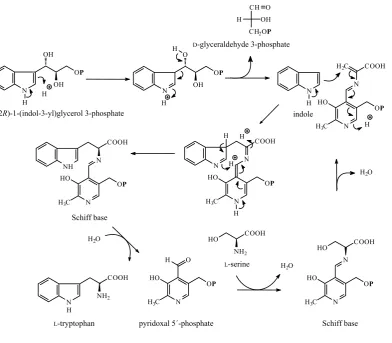
The tryptophan synthase multienzyme complex (EC 4.2.1.20) catalyses the final step of tryp-tophan biosynthesis in its two subunits. In the first enzyme subunit, (1S,2R)-1-(indol-3-yl)glycerol 3-phosphate splits off D-glyceraldehyde 3-phos-phate and yields indole. Indole moves to the sec-ond enzyme subunit where it reacts with the dehydrated Schiff base of L-serine yielding the tryptophan-PLP Schiff base, which is then hy-drolysed to the parent compounds, tryptophan and PLP (Figure 24).
3.3 Histidine
The biosynthetic pathway of L-histidine is unu-sual in that five of its carbon atoms are produced from 5-phospho-α-D-ribose 1-diphosphate, the key intermediate of tryptophan biosynthesis. One carbon atom comes from ATP (Figure 25).
The early stages of histidine biosynthesis involve condensation of 5-phospho-α-D-ribose 1-di-phosphate with ATP, followed by elimination of diphosphoric acid, which is catalysed by ATP phosphoribosyltransferase (EC 2.4.2.17). Elimi-nation of diphosphoric acid from this product, N1-5'-phosphoribosyl-ATP, is catalysed by phos-phoribosyl-ATP diphosphatase (EC 3.6.1.31) and yields N'-5'-phosphoribosyl-AMP. The pyrimidine ring of this compound opens by phosphoribo-syl-AMP cyclohydrolase (EC 3.5.4.19) under the formation of N'-5'-phosphoribosylformimino-5-ami- no-imidazole-4-carboxamide ribonucleotide. Then 1-(5-phosphoribosyl)-5-[(phosphoribosyl amino)methylene amino] imidazole-4-carboxa-mide isomerase (EC 5.3.1.16) splits the ribose attached at N1 to form 5-amino-4-carboxamide ribonucleotide and the intermediate fragment, which reacts with ammonia from glutamine to form 3-(imidazol-4-yl)-1-glycerol phosphate. The reaction catalysed by imidazoleglycerol-phosphate dehydratase (EC 4.2.1.19) converts this compound
HO COOH
NH2
indole
L-tryptophan
(1S,2R)-1-(indol-3-yl)glycerol 3-phosphate
N H
OP
OH
OH H
CH
CH2OP
H OH
O
N
OP
O
OH H
H
N H
N HO
H3C
OP
N COOH H2C
H D-glyceraldehyde 3-phosphate
N H
N HO
H3C
OP
COOH
N
H H
H
NH
COOH
N
OP
H3C HO
N
H2O
N
COOH
NH2 H
H2O
N HO
H3C
OP
H O
H2O
N HO
H3C
OP
N COOH HO
pyridoxal 5´-phosphate Schiff base Schiff base
L-serine
to 3-(imidazol-4-yl)-2-oxopropyl phosphate, which yields, in a transamination reaction catalysed by a PLP protein histidinol-phosphate transaminase (EC 2.6.1.9), L-histidinol phosphate. Histidinol phosphate is hydrolysed to L-histidinol by his-tidinol-phosphatase (EC 3.1.3.15) and histidinol is finally oxidised to L-histidine by the NAD-de-pendent histidinol dehydrogenase (EC 1.1.1.23) (IUBMB 2001).
CH2
OH OH
O
PO
PO
OH OH
O
N N N N
NH
H CH2
OH OH
O
PO
N N N NH2
O N O OH OH
PO H
CH2
OH OH
O
PO
N N N NH2
O N OH
PO OH O NH
H
OPP PO
OH OH
O
CH2
OH OH
O
PPPO N
N N N
CH2
OH OH
O
PPPO
PO
OH OH
O
N N N N
NH
EC 2.4.2.17
PP H2O PP
EC 3.6.1.31
H2O
EC 3.5.4.19
EC 5.3.1.16
CH2
OH OH
O
PO
N N NH2
O H2N
H N
OH
PO OH O
H O
Gln
Glu
ATP
5-phospho-�-D-ribose
1-diphosphate
N
OH OP
NH N NH
OP
O
NH
N OP
NH2
NH
N OH
NH2
NH N
COOH NH2
NH
N OP
OH OH
H
EC 4.2.1.19
3-(imidazol-1-yl)-2-oxopropylphosphate
L-glutamic acid
2-oxoglutaric acid
L-histidinol phosphate L-histidinol
L-histidine
EC 2.6.1.9
EC 3.1.3.15 EC 1.1.1.23
H2O
P
2 NAD 2 NADH + H
EC (Enzyme Commission) numbers and some common abbreviations
EC (Enzyme Commission) numbers, assigned by IUPAC-IUBMB, were taken from KEGG: Kyoto Ency-clopedia of Genes and Genomes, http://www.biologie. uni-hamburg.de. In many structures, the abbreviation P is used to represent the phosphate group and PP the diphosphate group. At physiological pH, these
[image:13.595.68.525.88.590.2]58
and some other groups will be ionized, but in pictures the unionised forms are depicted to simplify the structures, to eliminate the need for counter-ions, and to avoid the mechanistic confusion.
ADP – denosine 5'-diphosphate AMP – adenosine 5'-monophosphate APS – adenosine-5'-phosphosulfuric acid ATP – adenosine 5'-triphosphate
CoA – coenzyme A as a part of a thioester FMN – flavin mononucleotide
GSH – glutathione (reduced) GSSG – glutathione (oxidised)
NADH – nicotinamide adenine dinucleotide NADPH – nicotinamide adenine dinucleotide phos-
phate
P – phosphoric acid
PAPS – 3'-phosphoadenosine-5'-phosphosulfate PLP – pyridoxal 5'-phosphate
PP – diphosphoric acid
References
BENDER S.L., MEHDI S., KNOWLES J.R. (1989): Dihyd-roquinate synthase: the role of divalent metal cations and of nicotinamide adenine dinucleotide in catalysis. Biochemistry, 28: 7555–7560.
BORNEMANN S., LOWE D., THORNELEY R.N. (1996): The transient kinetics ofEscherichia coli chorismate synthase: substrate consumption, product formation, phosphate dissociation, and characterization of a flavin intermediate. Biochemistry, 35: 9907–9916.
BORNEMANN S., THEOCLITOU M.E., BRUNE M., WEBB M.R., THORNELEY R.N., ABELL C.A. (2000): A secondary
β deuterium kinetic isotope effect in the chorismate
syn-thase reaction. Bioorganic Chemistry, 28: 191–204. CARR R.T., BALASUBRAMANIAN S., HAWKINS P.C.,
BEN-KOVIC S.J. (1995): Mechanism of metal independent hydroxylation by Chromobacterium violaceum phenyl-alanine hydroxylase. Biochemistry, 34: 7525–7532. CLARK T., STEWART J.D., GANEM B. (1990):
Transiti-on-state analogue inhibitors of chorismate mutase. Tetrahedron, 46: 731–748.
FLOSS H.G., ONDERKA D.K., CARROLL M. (1972): Stereo-
chemistry of the 3-deoxy-D-arabino-heptulosonate
7-phosphate synthetase reaction and the chorismate synthetase reaction. Journal of Biological Chemistry,
247: 736–744.
GOURLEY D.G., SHRIVE A.K., POLIKARPOV I., KRELL T., COGGINS J.R., HAWKINS A.R., ISAACS N.W., SAWYER L. (1999): The two types of 3-dehydroquinase have dis-tinct structures but catalyze the same overall reaction. Nature Structural Biology, 6: 521–525.
IUBMB (2001): http://www.chem.qmul.ac.uk/iubmb/en-zyme/reaction/.
IUBMB (2002): http://www.chem.qmul.ac.uk/iubmb/en-zyme/reaction/.
IUBMB (2003): http://www.chem.qmul.ac.uk/iubmb/en-zyme/reaction/.
JAKEMAN D.L., MITCHELL D.J., SHUTTLEWORTH W.A., EVANS J.N. (1998): On the mechanism of 5-enolpyru-vylshikimate-3-phosphate synthase. Biochemistry, 37: 12012–12019.
JOST R., BERKOWITZ O., WIRTZ M., HOPKINS L., HAWKES-FORD M.J., HELL R. (2000): Genomic and functio-nal characterization of the oas gene family encoding
O-acetylserine(thiol)lyases, enzymes catalyzing the final step in cysteine biosynthesis in Arabidopsis thaliana. Gene, 253: 237–247.
LEWIS J., JOHNSON K.A., ANDERSON K.S. (1999): The catalytic mechanism of EPSP synthase revised. Bio-chemistry, 38: 7372–7379.
MARTÍ S., ANDRÉS J., MOLINER V., SILLA E., TUŇÓN I., BERTRÁN J. (2003): Conformational equilibrium of chorismate. A QM/MM theoretical study combining statistical simulations and geometry optimistations in gas phase and in aqueous solution. Journal of Molecular
Structure (Theochem),632: 197–206.
OSBORNE A., THORNELEY R.N., ABELL C., BORNEMANN S. (2000): Studies with substrate and cofactor analo-gues provide evidence for a radical mechanism in the chorismate synthase reaction. Journal of Biological Chemistry, 275: 35825–35830.
SENGBUSCH P.: Botany Online – The Internet Hypertext-
book. http://biologie.uni-hamburg.de/b_online/e00/
default.htm.
Received for publication June 13, 2005 Accepted after corrections September 26, 2005
Corresponding author:
Prof. Ing. JAN VELÍŠEK, DrSc., Vysoká škola chemicko-technologická v Praze, Fakulta potravinářské a biochemické technologie, Ústav chemie a analýzy potravin, Technická 5, 166 28 Praha 6, Česká republika